一個(gè)高粘彈的陰離子蠕蟲(chóng)膠束體系
謝丹華 趙劍曦,* 劉 琳 游 毅 魏西蓮
(1福州大學(xué)化學(xué)化工學(xué)院,膠體與界面化學(xué)研究所,福州350108;2聊城大學(xué)化學(xué)化工學(xué)院化學(xué)系,山東聊城252059)
1 Introduction
Upon the addition of salts,cationic cetyltrimethylammonium halides(C16TAX)in aqueous solution can self-assembly into flexible threadlike(wormlike)micelles with the length even on the order of a few microns.1,2At high concentrations these wormlike micelles entangle each other to form a transient network,which sharply increases the viscoelasticity of the solution.Comparatively,anionic wormlike micelles have advantage over cationic counterparts in many applications,including enhanced oil recovery.3Moreover,anionic systems tend to be biodegradable and less toxic compared with cationic ones.But the formation of anionic wormlike micelles is not common since the addition of salts often interacts strongly with anionic surfactant and results in precipitate.Therefore,only a few anionic wormlike micelles were formed by conventional singlechain surfactants such as sodium alkyl sulfate4-9or sodium dodecyltrioxylene sulfate10-14in the presence of additives.What?s more,these anionic micelles were not as long as the cationic ones and hence the anionic systems often followed a poor property(low viscoelasticity).For example,in the reported anionic wormlike micellar system consisted of SDS,the zero-shear viscosity of the solution only had a very low value of~1 Pa·s.5
Many efforts have been made to overcome this difficulty,in which the most valuable test was for the gemini surfactants.For example,Acharya et al.15tried to construct the wormlike micelles using a carboxylate gemini surfactant that has no spacer(designated as GS)mixed with the nonionic surfactants with a short poly(oxyethylene)chain(CmEn,where m,n=12,3;12,4;16,4;respectively).They found that C12E3very effectively promoted the formation of long wormlike micelles and as a result,the maximum viscosity of the solution attained about 104Pa·s.Recently,we reported a novel anionic wormlike micelle system constructed by carboxylate gemini surfactants with an azobenzene spacer(designated as Cm(azo)Cm,where m represented the number of the carbon atoms in each alkyl tail and was 10,12,and 14,respectively,see Scheme 1(a)).16,17The azobenzene spacer has a stretched length of more than 1 nm,which makes the head-group of Cm(azo)Cmincluding two carboxyl groups and a spacer quite large.This seems to say that Cm(azo)Cmis hard to form threadlike aggregates with a low surface curvature according to the molecular packing theory.18Very interestingly,however,all these surfactants formed wormlike micelles.The mechanism has been attributed to the rigid characteristic of the azobenzene spacer,which restrained the two alkyl tails within a gemini molecule drawing close and yielded the pseudo volume between them and thus increased the packing parameter,P.16,17This finding strongly suggests that the gemini surfactants with a long rigid spacer may be good candidates for forming anionic wormlike micelles.

Scheme 1 Chemical structures of carboxylate gemini surfactants with long rigid spacer
Very recently,we synthesized a new family of carboxylate gemini surfactants also with a long aromatic spacer,O,O?-bis(sodium 2-alkylcarboxylate)-p-dibenzenediol,referred to as Cm?2Cm(m=10,12,14,Scheme 1(b)).Surprisingly,we found that C12?2C12followed a new mechanism of aggregation different from general core-shell micelle formation.19In dilute solution,C12?2C12formed large network-like aggregates.This behavior was attributed to an extending configuration of C12?2C12with the two alkyl tails stretching towards the solution due to the rigidity of the long spacer.Thus the large network-like aggregate formation was an inevitable outcome of spontaneously reducing the energy of the system.Due to the columnar-like molecular geometry of C12?2C12,the network-like aggregates were very easily transformed into rod-like micelles with slightly increasing surfactant concentration and finally the wormlike micelles were formed.By this mechanism of aggregation,a new approach to constructing highly viscoelastic anionic wormlike micellar systems is perhaps revealed.In this paper,we report the wormlike micellar solution formed by C14?2C14that has the longest alkyl tails in this family so as to further understand this new constructing way.
2 Experimental
2.1 Materials
Sodium bromide(NaBr,purity>99%,Beijing Chemical Reagents Co.)was used as received.The water used was Milli-Q grade with a resistivity of 18.2 MΩ·cm.
O,O?-bis(sodium 2-tetradecylcarboxylate)-p-dibenzenediol(referred to as C14?2C14)was synthesized in our laboratory according to the following routes(Scheme 2).All reagents used were purchased from Sinopharm Chemical Reagent Co.,Ltd.(China).
Methyl 2-bromotetradecanoate was prepared according to our previous work.17
Synthesis of MC14?2C14M.The potassium carbonate(9.7 g,70 mol)was added to a three-necked flask with 20 mL N,N-dimethylformamide(DMF).4,4?-Biphenol(3.7 g,20 mmol)was dissolved in 10 mL DMF,and then this solution was added to the mixture dropwise.Methyl 2-bromotetradecanoate(19.3 g,60 mmol)was added into the mixture secondly.The mixture was reacted under stirring at 80°C overnight.The mixture was poured into 70 mL ice-water and extracted with petroleum ether(3×60 mL).The combined organic layers were washed with deionized water.The petroleum ether was removed under reduced pressure.The crude product was recrystallized from petroleum ether three times to give MC14?2C14M as white powder.

Scheme 2 Synthetic route of C14?2C14
Synthesis of C14?2C14.A mixture of MC14?2C14M(6.4 g,9.6 mmol)and sodium hydroxide(0.92 g,23 mmol)in 500 mL of 95%ethanol was refluxed overnight.The resulting mixture was cooled to room temperature and centrifuged to afford the white precipitate.The precipitate was washed with ethanol several times and dried in vacuum to give the target compound as white powder.The final yield was 36.6%according to the quality of 4,4?-biphenol.
1H NMR(400 Hz,D2O)for C14?2C14: δ,7.56(d,J=7.6 Hz,4H,H-Ar),6.92(d,J=7.6 Hz,4H,H-Ar),4.47(br,2H,CH),1.90(m,4H,CH2),1.43-1.19(m,4H,CH2),1.05-0.82(m,36H,CH2),0.43(t,J=6.4 Hz,6H,CH3).
Anal.Calcd.for(C40H60Na2O6)(%):C,70.35;H,8.86;Found:C,69.99;H,8.65.
2.2 Rheological measurements
Rheological measurements were performed on a stress controlled rheometer(AR2000ex,TAinstruments,USA)with conical concentric cylinders.The cone was made of standard ETC steel with the diameter of 40 mm and cone angle of 2°.The gap between the center of the cone and plate was 50 μm.At a designed temperature,the sample was kept for 5 min on the plate to reach the equilibrium before testing.A strain sweep was performed at a frequency of 6.28 rad·s-1(1 Hz)before the test.A strain value was then decided to make sure of the sample in the linear viscoelastic region during the following oscillatory measurements.
2.3 Cryogenic transmission electron microscopy(cryo-TEM)
Cryo-TEM samples were prepared in a controlled environment vitrification system(CEVS)at 28°C.A micropipet was used to load 5 μL surfactant solution onto a lacey support TEM grid,which was held by tweezers.The excess solution was blotted with a piece of filter paper and the thin film was suspended on the mesh hole.After waiting for about 10 s to relax any stresses induced during the blotting,the samples were quickly plunged into a reservoir of liquid ethane(cooled by the nitrogen)at its melting temperature.The vitrified samples were then stored in the liquid nitrogen until they were transferred to a cryogenic sample holder(Gatan 626)and examined with a JEM 2200FS TEM(200 keV)at about-174°C.The phase contrast was enhanced by underfocus.The images were recorded on a Gatan multiscan CCD and processed with Digital Micrograph.
2.4 Dynamic light scattering
Dynamic light scattering(DLS)of micellar solutions was measured with a Brookhaven Instrument which was composed of a BI-200SM goniometer,a BI-9000AT digital correlator(522 channels)and a photomultiplier detector.The He-Ne laser with 15 mW power and 632.8 nm wavelength was used as the light source.The measurement temperature was controlled by a thermostatic circulator(Poly-sceience,USA)with an accuracy of ±0.01 °C.All solutions were filtered through 0.22 μm Millipore filters into cylindrical light-scattering cells(od=25 mm).
The intensity-intensity time correlation function G(2)(t,q)in the self-beating mode was measured,where t is decay time and q is scattering vector and equals to 4πn/λsin(θ/2).The G(2)(t,q)was transformed into the electric field-electric field time correlation function g(1)(t,q)by Siegert formula:

where A is the baseline,β is a parameter depending on the coherence of the detection.The g(1)(t,q)was further related to the characteristic line-width(Γ)distribution G(Γ)by

The G(Γ)can be obtained by a Laplace inversion of g(1)(t,q)using the CONTIN program.The average line-width<Γ> was calculated according to

Furthermore,the apparent translational diffusive coefficient Dappcan be related to <Γ> as Dapp=<Γ>/q2.Thus,the apparent hydrodynamic radius of aggregate(Rh,app)was obtained from the Stokes-Einstein equation

where kBis the Boltzmann constant,T is absolute temperature,and η0is the solvent viscosity.
3 Results and discussion
3.1 Characteristic of wormlike micelles at 25°C

Fig.1 (a)Appearance of C14?2C14(140 mmol·L-1)/NaBr(100 mmol·L-1)aqueous system at 25 °C,(b)the cryo-TEM image,and(c)its variations of G?(filled symbols),G?(open symbols)with sweep frequency ω
The aqueous system of C14?2C14(140 mmol·L-1)/NaBr(100 mmol·L-1)at 25 °C has a gel-like appearance(Fig.1a).This is due to the formation of wormlike micelles,which entangle each other into a transient network as seen in the cryo-TEM image(Fig.1b).The result of frequency sweep measurement(Fig.1c)shows high elasticity,where elastic modulus G?exceeds viscous modulus G?at sweep frequency ω>0.1 rad·s-1.This system very well follows the Maxwell fluid behavior with a single stress relaxation time(τR)20as fitted(solid lines in Fig.1c)by the equations:

The corresponding Cole-Cole plot,i.e.,elastic modulus G? against viscous modulus G?(Fig.1c,insert),shows a perfect semicircular shape at low and medium frequencies,which is another indication for the Maxwell fluid behavior.
The τRis estimated as ωc-1,where ωcis the frequency at which two moduli are equal.21On the increase in ω,the G?attains a limiting value called a plateau modulus,G?∞.The living polymer model proposed by Granek and Cates20revealed two time scales of stress relaxation,namely,reptation time(τrep),which corresponds to the curvilinear diffusion of a chain of the mean length along its own contour,and breaking time(τb).When breaking occurs over the time scale of reptation(τb<<τrep),as in a typical wormlike micellar system,the chain undergoes many breakages and recombinations before a chain segment relaxes by reptation.Thus the stress relaxation is characterized by a new time scale given by τR=(τbτrep)1/2,and the solution behaves as a Maxwell fluid with single relaxation time τR.The average scission time for the micelle,τb,is approximately equal to the inverse of ω corresponding to the minimum of G??minin the high-frequency region.For the present system,the estimated τbis ~0.105 s.This value allows us to estimate τrep~1.35×103s using τR(11.9 s),satisfying the expectation of τb<<τrep.20
The micellar contour length(L)can be estimated by the relation20

where leis the average length between two entanglement points and G??minis the minimum at G?at high-frequency region.For a given le,L is inversely proportional to the ratio G??min/G?∞.Thus,a smaller G??min/G?∞r(nóng)esults in a longer L.So far,the smallest G??min/G?∞r(nóng)eported was 0.014 yielded in the mixed system of carboxylate gemini surfactant with no spacer(GS)and C12E3.15In this case,Acharya et al.15have stressed that the added C12E3played a very important role in promoting micellar growth.Another small G??min/G?∞was 0.016 obtained from the system of traditional cationic surfactant having an unsaturated tail as long as 22 carbon atoms.22For the present system,the G??min/G?∞is about 0.022.Even though this value is not as low as those mentioned above,it is produced in an anionic wormlike micellar system only upon the addition of simple salt and hence is very rare.
As a comparison,a typical value of le(80-150 nm)for wormlike micelles can be adopted15and thus L corresponds to roughly 3.6-6.8 μm for the present system at 25 °C.This length is indeed greatly longer than that of cetyltrimethylammonium bromide(C16TABr)micelle in the presence of 1.5 mol·L-1NaBr.23
3.2 Zero-shear viscosity at 25°C
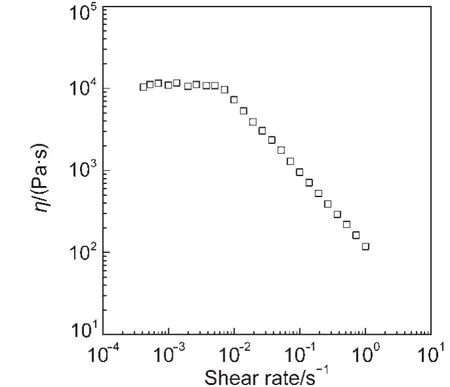
Fig.2 Variations of viscosity(η)with the shear rate at 25 °C
Fig.2 shows the viscosity versus steady shear rate curve for the aqueous system of C14?2C14(140 mmol·L-1)/NaBr(100 mmol·L-1).The viscosity keeps unchanged at low shear rate re-gion and then decreases with increasing the shear rate(the shear thinning behavior)after a critical shear rate,rc.The shear thinning behavior can be taken as an evidence for the formation of wormlike micelles,24which well corresponds to the indication of dynamic viscoelastic measurements.According to the Carreau model,the zero-shear viscosity η0is obtained to be 1.10×104Pa·s,meaning the relative viscosity of the wormlike micellar solution(ηr,given by η0/ηs,where ηsis the viscosity of the solvent,water)to be as high as 107.This is a very large value comparable with that obtained from the mixed system of gemini surfactant(GS)and C12E3.15
3.3 Mechanism of wormlike micelle formation
As seen in Sections 3.1 and 3.2,C14?2C14can form wormlike micelles in aqueous solution,which is similar to the behavior of C14(azo)C14as reported previously.17
Fig.3(left row)shows the intensity-fraction distribution of C14?2C14measured by DLS,in which the aggregates at 5 and 10 mmol·L-1show quite large size(~100 nm in apparent hydrodynamic radius,Rh,app)and narrow distribution.With increasing the surfactant concentration(C),the mean Rhincreases obviously at C≥20 mmol·L-1,indicating the rapid growth of micelles.This behavior is very analogous to that of C12?2C12which is another member of this family.Thus,as revealed in our previous work,19C14?2C14must form large network-like aggregates after the critical micelle concentration(cmc),and these network-like aggregates can be very easily transformed into rod-like micelles with slightly increasing the surfactant concentration and finally the wormlike micelles are formed.
However,it is very surprising that C14(azo)C14shows the aggregation behavior different from C14?2C14,although this surfactant also has a long aromatic spacer in its molecular structure.Fig.3(right row)shows the scattering intensity of C14(azo)C14only distributes at small Rhvalues(0.1-15 nm)but a very wide range(polydispersity in size).The two cases(C14?2C14and C14(azo)C14)reflect the complicacy of gemini surfactants in self-assembly and sometimes these surfactants show completely different behaviors although their molecular structures are similar.A good understanding for their mechanisms needs further and deep investigations.
3.4 Effect of temperature

Fig.3 Intensity-fraction distributions measured at a detector angle θ=90°and analyzed by CONTIN model for C14?2C14(left row)and C14(azo)C14(right row)at different concentrations
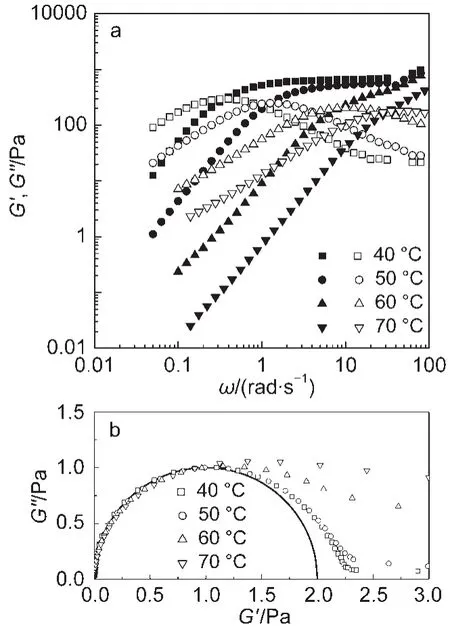
Fig.4 Viscoelastic spectra(a)and the normalized Cole-Cole plots(b)at different temperatures
Fig.4a shows the viscoelastic spectra of this system at different temperatures.On the raise in temperature,the rheological behavior still shows semicircular shape in the Cole-Cole plots(Fig.4b)over the range of low and medium frequencies.Similarly,the characteristic parameters described above can be obtained according to living polymer model.20For those cases where the G?does not give a constant limiting value,the G?∞may be estimated from the modulus value at ωc,using the relation G?∞=2G?max,where G?maxis the viscosity modulus at shear frequency ωc.21As seen from Table 1,the τRmonotonically decreases over the entire temperature range,but G?∞shows an initial enhancement at low temperatures followed by a subsequent decrease at higher temperatures.Since τRassociates with the micellar length,15the decrease in τRindicates the wormlike micelles become shorter with increasing temperature.This is consistent with the estimation for the length from the ratio of G?min/G?∞,which gives out the values of gradual decrease in L(Table 1).For the wormlike micellar system undergoing stress relaxation by reptation,G?∞generally depends on the number density of the aggregates.15The initial increase in G?∞over the temperature range of 25-40°C suggests that the enhanced thermal motion promotes the contact between the micelles and thus yields more entanglements,which makes the system more viscoelastic.The following decrease in G?∞above 40 °C must be due to the shorter micelles as discussed above.
The zero-shear viscosity drown from Fig.5 has a value of 17.6 Pa·s at 70 °C,namely,its relative viscosity is 1.8×104.In general,with increasing temperature to 60 °C,the ηrof wormlike micellar solution falls to a value below 104.There are few,if any,examples of micellar solutions showing high viscosities(ηr>104)above 60 °C.The typical cases are the wormlike micel-lar solutions formed by cationic surfactants,erucyl bis(hydroxyethyl)methylammonium chloride(EHAC)and erucyl trimethylammonium chloride(ETAC),as reported by Raghavan and Kaler.22The both surfactants bear a quite long(C22)tail.Relying on the help of sodium salicylate or sodium chloride,the both systems retain high viscosity(ηr>104)up to ca 90 °C.Compared with EHAC or ETAC,C14?2C14only has short tails(12 carbon atoms in each tail,Scheme 1(b)).Even so,the relative viscosity ηrstill attains a rather large value at 70 °C.This is very rare for the anionic wormlike micelle systems.

Table 1 Characteristic parameters of the wormlike micellar systems of C14?2C14(140 mmol·L-1)/NaBr(100 mmol·L-1)at different temperatures

Fig.5 Variations of viscosity(η)with the shear rate at different temperatures
3.5 Flow activation energy
The variation of η0and τRwith temperature can be empirically described by Arrhenius relationships,indicating exponential reductions in these quantities:25,26

where Eais the flow activation energy,R is the gas constant,and A is a pre-exponential factor.Semilogarithmic plots of η0and τRvs 1/T(Fig.6)fall on straight lines within experimental error,being consistent with Eqs.(8)and(9).The slope gives out the flow activation energy Eato be(141±5)kJ·mol-1.This value is comparable to that reported for other wormlike micelles.22,24
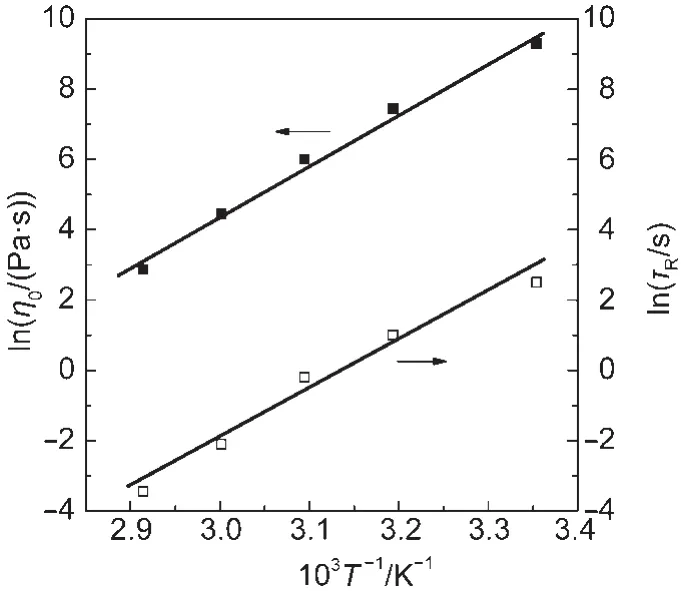
Fig.6 Arrhenius plots of the zero-shear viscosity(η0)and the relaxation time(τR)vs 1/T
4 Conclusions
The present study exhibited an excellent anionic wormlike micelle system formed by carboxylate gemini surfactant C14?2C14.This result again demonstrates that the gemini surfactants are very good candidates for the construction of wormlike micelles and also strongly suggests that based on the gemini surfactants,an effective approach may be developed to construct the anionic wormlike micelle systems with excellent viscoelastic properties.
(1) Dreiss,C.A.Soft Matter 2007,3,956.and references therein.doi:10.1039/b705775j
(2) Yang,J.Curr.Opin.Colloid Interface Sci.2002,7,276.and references therein.doi:10.1016/S1359-0294(02)00071-7
(3) Maitland,G.C.Curr.Opin.Colloid Interface Sci.2000,5,301.doi:10.1016/S1359-0294(00)00069-8
(4) Magid,L.J.;Li,Z.;Butler,P.D.Langmuir 2000,16,10028.doi:10.1021/la0006216
(5) Hassan,P.A.;Raghavan,S.R.;Kaler,E.W.Langmuir 2002,18,2543.doi:10.1021/la011435i
(6) Arleth,L.;Bergstrom,M.;Pedersen,J.S.Langmuir 2002,18,5343.doi:10.1021/la015693r
(7) Nakamura,K.;Shikata,T.Langmuir 2006,22,9853.doi:10.1021/la061031w
(8)Acharya,D.P.;Sato,T.;Kaneko,M.;Singh,Y.;Kunieda,H.J.Phys.Chem.B 2006,110,754.doi:10.1021/jp054631x
(9)Lu,T.;Xia,L.G.;Wang,X.D.;Wang,A.Q.;Zhang,T.Langmuir 2011,27,9815.doi:10.1021/la2018709
(10) Mu,J.H.;Li,G.Z.Chem.Phys.Lett.2001,345,100.doi:10.1016/S0009-2614(01)00799-0
(11) Mu,J.H.;Li,G.Z.Colloid Polym.Sci.2001,279,872.doi:10.1007/s003960100508
(12)Mu,J.H.;Li,G.Z.;Jia,X.L.;Wang,H.X.;Zhang,G.Y.J.Phys.Chem.B 2002,106,11685.doi:10.1021/jp014096a
(13) Mu,J.H.;Li,G.Z.;Wang,Z.W.Rheol.Acta 2002,41,493.doi:10.1007/s00397-002-0246-y
(14)Mu,J.H.;Li,G.Z.;Wang,Z.W.;Zheng,L.Q.;Liao,G.Z.;Huang,L.J.Disper.Sci.Technol.2001,22,421.doi:10.1081/DIS-100107851
(15)Acharya,D.P.;Kunieda,H.;Shiba,Y.;Aratani,K.J.Phys.Chem.B 2004,108,1790.
(16) Song,B.L.;Hu,Y.F.;Zhao,J.X.J.Colloid Interface Sci.2009,333,820.doi:10.1016/j.jcis.2009.02.030
(17) Song,B.L.;Hu,Y.F.;Song,Y.M.;Zhao,J.X.J.Colloid Interface Sci.2010,341,94.
(18) Israelachvili,J.N.;Mitchell,D.J.;Ninham,B.W.Journal of the Chemical Society-Faraday Transactions II 1976,72,1525.doi:10.1039/f29767201525
(19) Xie,D.H.;Zhao,J.X.Langmuir 2013,29,545.
(20) Granek,R.;Cates,M.E.J.Chem.Phys.1992,96,4758.doi:10.1063/1.462787
(21) Oda,R.;Narayanan,J.;Hassan,P.A.;Manohar,C.;Salkar,R.A.;Kern,F.;Candau,S.J.Langmuir 1998,14,4364.doi:10.1021/la971369d
(22) Raghavan,S.R.;Kaler,E.W.Langmuir 2001,17,300.doi:10.1021/la0007933
(23) Khatory,A.;Lequeux,F.;Kern,F.;Candau,S.J.Langmuir 1993,9,1456.doi:10.1021/la00030a005
(24) Shrestha,R.G.;Shrestha,L.K.;Aramaki,K.J.Colloid Interface Sci.2007,311,276.doi:10.1016/j.jcis.2007.02.050
(25) Candau,S.J.;Hirsch,E.;Zana,R.;Delsanti,M.Langmuir 1989,5,1225.doi:10.1021/la00089a018
(26) Fischer,P.;Rehage,H.Langmuir 1997,13,7012.doi:10.1021/la970571d

