Compound of icariin, astragalus, and puerarin mitigates iron overload in the cerebral cortex of Alzheimer’s disease mice
Yu Zhang, Wei-Na Kong, Xi-Qing Chai, ,
1 Department of Neurology, the First Hospital of Hebei Medical University, Shijiazhuang, Hebei Province, China
2 Hebei Chemical and Pharmaceutical College, Shijiazhuang, Hebei Province, China
Introduction
Alzheimer’s disease (AD) is an irreversible and progressive neurodegenerative brain disorder, and the most common cause of dementia in the elderly. Its progression is characterized by deterioration of cognitive function and memory. The main histological hallmarks of AD brains are senile plaques,neurofibrillary tangles, and extensive neuronal loss (Katzman and Saitoh, 1991). However, the precise mechanisms underlying AD have not been completely elucidated.
Previous studies have suggested that iron is involved in progression of AD (El Tannir El Tayara et al., 2006), and that altered iron homeostasis may play an crucial role in disease pathogenesis (Crapper McLachlan et al., 1991). Nevertheless, the mechanism of iron overload in AD brain remains unclear. During the last decade, many studies have shown that metabolic imbalance in iron and the resulting oxidative stress play important roles in disease pathogenesis (Crapper McLachlan et al., 1991). In a state of toxic overload, iron is perceived as an endogenous toxin in traditional Chinese medicine (Lu and Black, 2016). Indeed, abnormally high levels of brain iron constitute an endogenous toxin that is important to AD pathogenesis. Iron accumulation is also thought to be highly correlated with abnormal function of other organs including the kidney, liver, heart, and spleen(Crapper McLachlan et al., 1991; El Tannir El Tayara et al.,2006). Excessive iron levels were previously shown to lead to increased oxidative stress using Fenton chemistry (Rolston et al., 2009). Therefore, therapeutic intervention to decrease iron accumulation may be an effective way of improving AD pathology.
Figure 1 Chemical structures of icariin,astragaloside IV, and puerarin.
Iron chelators, such as deferoxamine (DFO), significantly relieve the symptoms of AD patients, and consequently exert neuroprotective effects on the disease (Wan et al.,2006; Hishikawa et al., 2008). However, chemical drugs have drawbacks such as side effects and poor oral availability, and there is a need for research into alternative therapies. In the last decades, many traditional Chinese medicines have been studied, with benefits shown using different AD experimental models and clinical trials (Xian et al., 2012). Accordingly,components of Epimedium, Astragalus, and Puerariae may overcome these disadvantages. Icariin is extracted from Epimedium (Chen et al., 2005), and studies have shown that icariin improves learning and memory abilities in aged rats(Cheng et al., 2007; Hagemeier et al., 2012). Astragalus polysaccharide has antioxidative (Ko et al., 2005), immunoregulatory, and antiviral effects (Huang et al., 2013), antitumor activities (Du et al., 2012), and cardiac protective properties(Dang et al., 2009; Dai et al., 2014). While Puerariae is reported to have anti-inflammatory effects (Lim et al., 2013).Furthermore, these compounds have effects on reducing inflammation, free radical scavenging capacity, altering multiple visceral organ functions (Gao et al., 2013), and reducing brain iron load (Chen and Huang, 2008). Further, previous research in our laboratory suggests that these active components reduce spatial learning and memory impairments in the APP/PS1 mouse model, and inhibit iron overload in the cerebral cortex in a mouse model of AD (Dong et al., 2015),although the mechanism is still not clear.
Thus, the aim of this study was to investigate the mechanism of the active components extracted from Astragalus,Icariin, and Puerariae on brain iron overload in APP/PS1 transgenic mice.
Materials and Methods
Ethics statement and animals
All animal handling procedures were in accordance with the Guidelines of Animal Experiments from the Committee of Medical Ethics, Ministry of Health of China. Experiments were approved by the Ethics Committee for Animal Experiments of Hebei Medical University of China (approval No.HEBMU-2010-10). Precautions were taken to minimize suffering and the number of animals used in each experiment.Seven male six-month-old C57BL/6J (C57) mice and forty-two male six-month-old APPswe/PS1ΔE9 (APP/PS1)mice (weighing 28.8 ± 5 g) were obtained from Beijing HFK Bioscience Co., Ltd., Beijing, China (SCXK (Jing) 2014-0004). The APP/PS1 transgenic mouse model of AD overexpresses the Swedish (K594M/N595L) mutation of APP, and with presenilin 1 (PS1) deleted in exon 9, on a C57 genetic background. APP/PS1 mice were genotyped by polymerase chain reaction. Mice were housed in specific-pathogen-free conditions on a 12-hour light/dark cycle with free access to water and food.
Compound preparation
The compound comprised purified extracts from three different herbs used in Chinese medicine, namely Epimedium, Astragalus, and Puerariae. The individual components extracted from Epimedium, Astragalus, and Puerariae are icariin, astragaloside, and puerarin, respectively (Figure 1). These components were purchased from China Nanjing Zelang Medical Technology Co., Ltd. (Nanjing, China). Drug purity was > 98%. Icariin, astragaloside IV, and puerarin were dissolved in distilled water at a ratio of 3:2:2 (w/w).
Drug treatment
APP/PS1 transgenic mice were randomly divided into six groups, with seven mice in each group. Mice in the AD model group were intragastrically administered 1 mL normal saline. Mice in the compound group were intragastrically administered compound containing icariin (120 mg/kg),astragalus (80 mg/kg), and puerarin (80 mg/kg). The DFO group received 30 mg/kg DFO by intraperitoneal injection.The puerarin group received 80 mg/kg puerarin by intragastric administration. The astragalus group received 80 mg/kg astragalus by intragastric administration. The icariin group received 120 mg/kg icariin by intragastric administration.All groups were given treatments once a day for 3 consecutive months. In addition to the six groups, seven male C57 mice were included as a normal control group, and were intragastrically administered 1 mL normal saline.
Tissue preparation
After 3 consecutive months, mice were deeply anesthetized with sodium pentobarbital (50 mg/kg) by intraperitoneal injection, and immediately perfused through the heart with 0.9% NaCl. Brains were rapidly removed and divided into separate hemispheres on an ice-cold surface. The cerebral cortex was dissected from one hemisphere and stored at–80°C for enzyme-linked immunosorbent assay (ELISA) and biochemistry. The other cerebral cortex was dried at 110°C overnight and used for detecting iron content by flame atomic absorption spectroscopy.
Flame atomic absorption spectroscopy
The dried cerebral cortex was weighed and treated with 1 mL concentrated nitric acid and 0.5 mL perchloric acid, then dissolved and placed in a fume hood at 90°C for 1 hour until the moisture evaporated. Dissolved tissues were diluted with deionized water to 3 mL. Absorbance values were measured at 248.3 nm using the Varian Spectra AA-10 Spectrophotometer(Agilent Technologies, Santa Clara, CA, USA). Standard iron curves were prepared from commercially available standards.
ELISA
ELISA was used to determine levels of interleukin-1β (IL-1β)and interleukin-6 (IL-6), and tumor necrosis factor alpha(TNF-α). Tissues were weighed and homogenized in an icecold protease and phosphatase inhibitor cocktail. Homogenates were centrifuged at 13,000 r/min for 20 minutes at 4°C.Supernatants were collected for analysis using mouse IL-1β,IL-6, and TNF-α ELISA kits (Raybio, Norcross, GA, USA).Protein concentration of supernatants was determined by the Lowry method using bovine serum albumin as a standard.IL-1β ELISA assay used an antibody specific for mouse IL-1β, which was coated on 96-well plates. Standards and samples were pipetted into the wells and IL-1β present bound to wellsviaimmobilized antibody. The wells were washed and biotinylated antibody added. After washing away unbound biotinylated antibody, horseradish peroxidase-conjugated streptavidin was added. The wells were washed again, and a tetramethylbenzidine substrate solution added. Color developed in proportion to the amount of bound IL-1β: stop solution changed the color from blue to yellow, and the color intensity was measured at 450 nm. The IL-6 and TNF-α assay procedures were the same as for IL-1β. All assays were performed as outlined by the manufacturer’s protocols.
Colorimetry of glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD) activities andmalondialdehyde (MDA) content
Tissues were weighed and 10% (w/v) buffered homogenates prepared. Homogenates were centrifuged at 2500 r/min for 10 minutes at 4°C, and the supernatants used for biochemical analyses. The protein concentration of supernatants was determined by the Lowry method using bovine serum albumin as the standard. SOD activity in homogenates was examined using a commercially available kit (Jian Cheng Biological Engineering Institute, Nanjing, China) based on auto-oxidation of hydroxylamine. The developed blue color of the reaction was measured at 550 nm.
Activity of GSH-Px was determined by the velocity method using a GSH-Px kit (Jian Cheng Biological Engineering Institute). The reaction was initiated by addition of hydrogen peroxide. A series of enzymatic reactions was activated by GSH-Px in homogenates, which subsequently led to conversion of GSH (reduced glutathione) to GSSG (oxidized glutathione). The change in absorbance during conversion of GSH to GSSG was recorded spectrophotometrically at 412 nm. The procedure was performed according to the manufacturer’s instructions.
MDA concentration was determined in homogenates using a commercially available kit (Jian Cheng Biological Engineering Institute) based on thiobarbituric acid reactivity.Trichloracetic acid was mixed with homogenate and centrifuged, then the supernatant removed and thiobarbituric acid added. The developed red color of the resulting reaction was measured at 532 nm using a spectrophotometer. The rest of the procedure was performed as outlined by the manufacturer’s instructions.
Statistical analysis
Data are expressed as the mean ± SD, and analyzed using repeated measures analysis of variance. For single dependent variables assays, univariate analysis of variance was performed using group as the between-subject factor. Following a significant omnibus analysis of variance, Bonferronipost hoccomparisons were performed. All analyses were performed using the SPSS 17.0 for Windows (SPSS Inc., Chicago, IL, USA). A value ofP< 0.05 was considered statistically significant.
Results
Effect of icariin, astragalus, and puerarin on iron levels in the cerebral cortex of APP/PS1 transgenic mice
To determine whether icariin, astragalus, and puerarin reduce iron levels in APP/PS1 mice, total iron levels in the cortex were detected using a flame atomic absorption spectrometry method. Compared with the normal control group, iron levels were higher in the AD model group (P<0.05). Compared with the AD model group, iron levels were reduced in the compound group and DFO group (P< 0.05).There was no significant difference in iron levels between the compound and DFO groups (P> 0.05). Iron levels in the puerarin, astragalus, and icariin groups were higher compared with the compound group, and lower compared with the AD group (P< 0.05; Figure 2).
Effect of icariin, astragalus, and puerarin on expression of proinflammatory factors in the cerebral cortex of APP/PS1 transgenic mice
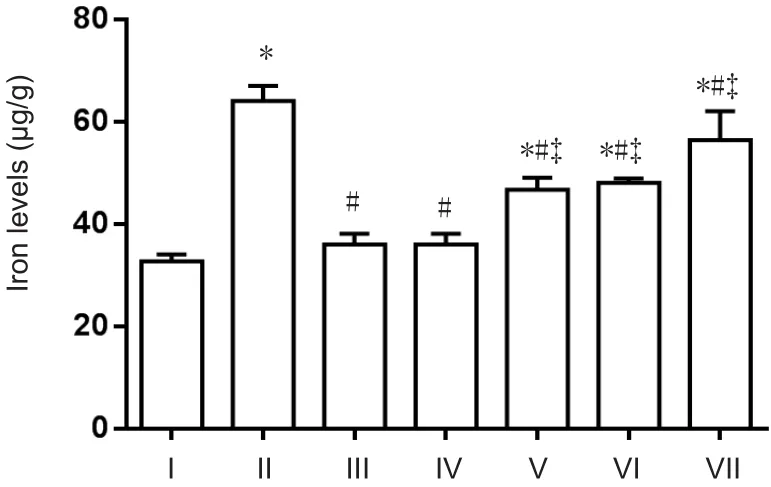
Figure 2 Total iron levels in the cerebral cortex of APP/PS1 transgenic mice (Flame atomic absorption spectroscopy).
ELISA was used to investigate proinflammatory factors including IL-1β, IL-6, and TNF-α. Compared with the normal control group, IL-1β, IL-6, and TNF-α expression was higher in the AD model group (P< 0.05). Compared with the AD model group, IL-1β, IL-6, and TNF-α expression was significantly reduced in the compound group (P< 0.05).DFO-treated mice showed a significant reduction compared with AD model mice (P> 0.05). IL-6 expression was higher in the puerarin group than the normal control and lcariin groups. IL-6 expression was increased in the puerarin group compared with the AD model group. There was no difference in IL-1β expression between the puerarin and normal control groups. IL-1β expression was increased in the icariin andPuerarin groups compared with the AD model and DFO groups. Expression of TNF-α was higher in the icariin group than the control group (P< 0.05). TNF-α expression was higher in the astragalus and puerarin groups compared with the AD model group. No significant differences were found in TNF-α expression between the astragalus and puerarin groups and DFO group (Figure 3).
Effect of icariin, astragalus, and puerarin on MDA content and SOD and GSH-Px activity in the cerebral cortex of APP/PS1 transgenic mice
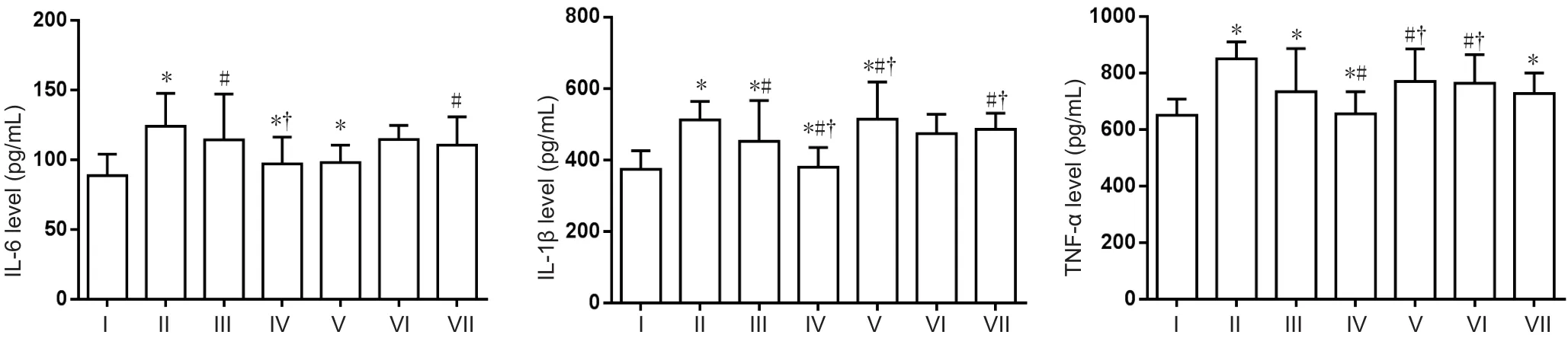
Figure 3 Effect of a compound containing icariin, astragalus, and puerarin on IL-6, IL-1β, and TNF-α levels in the cerebral cortex of AD model mice (ELISA assay).
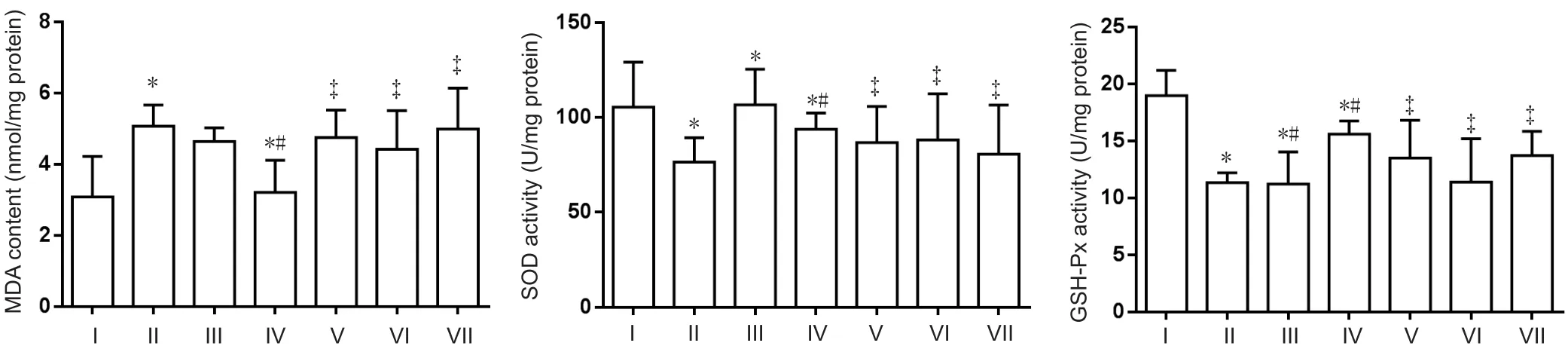
Figure 4 Effect of a compound containing icariin, astragalus, and puerarin on MDA content and SOD and GSH-Px activity in the cerebral cortex of AD model mice.
Compared with the normal control group, MDA content was significantly enhanced in the AD model group (P<0.05). While compared with the AD model group, excess iron-mediated enhancement of MDA content was significantly inhibited in the compound group (P< 0.05). Furthermore, SOD and GSH-Px activity was significantly decreased in the AD model group compared with the normal control group (P< 0.05). SOD activity was increased in compoundand DFO-treated mice (P< 0.05). No difference in GSH-Px was determined between effective component-treated and DFO-treated mice (Figure 4). MDA content and SOD activity were increased in the icariin, astragalus, and puerarin groups compared with the compound group. GSH-Px activity was decreased in the icariin and astragalus groups (P< 0.05).
Discussion
Abnormal iron accumulation is thought to occur early in the onset of AD, appearing before the formation of neurofibrillary tangles and senile plaques. Brain iron content was previously shown to be associated with degree of dementia,with increasing iron related to more severe disease (Zhu et al., 2009). Excessive brain iron levels may play an important role in progression of AD by initiating a cascade that eventually leads to neuronal death (Zhu et al., 2009). Consequently,iron chelation may be an effective therapeutic intervention for AD (Whitnall and Richardson, 2006). Indeed, the icariin,astragalus, and puerarin compounds may overcome these shortcomings in iron chelation. They scavenge free radicals,decrease inflammation, and alter multiple organ functions,which may ease iron overload on central nervous system function. Here, we have attempted to demonstrate and explain this using contemporary methods and transgenic animal models of AD. In our present study, we first investigated the neuroprotective effect of effective extracts of Epimedium,Astragalus, and Puerariae. We evaluated brain iron levels using flame atomic absorption spectroscopy,and found they were increased in the cerebral cortex of the APP/PS1 mouse model, but could be relieved by compound treatment. Thus,the compound may have a similar mechanism to DFO, and act by chelating and clearing free iron ion to reduce brain deposition (Genlain et al., 2007).
Oxidative stress induced by iron overload is not only a crucial mechanism in AD, but also in development of neurodegenerative diseases (Qian and Shen, 2001) and cerebral ischemia (Yoo et al., 2016). Oxidative stress produces oxygen free radicals, which promote generation of amyloid-beta(Aβ). Moreover, Aβ promotes generation of oxygen free radicals, which leads to neuronal damage and further positive feedback (Misonou et al., 2000). Iron overload causes an increase in lipid peroxidation (Galleano and Puntarulo, 1997).As a major product of lipid peroxidation (Lee et al., 2011),MDA indirectly reflects the extent of damage (Parks et al.,1994). SOD and GSH are enzymatic antioxidants that scavenge harmful reactive oxygen species, forming the first line of defense against free radicals, which convert toxic superoxide into the less toxic hydrogen peroxide (Geisser, 1997).Our results provide evidence that the icariin, astragalus, and puerarin compounds exert effective protection against lipid peroxidation induced by iron overload. Furthermore, our results show that MDA content is significantly increased in the brain of AD model mice. The response of the cerebral cortex to excess toxic iron was acceleration in lipid peroxidation accompanied by increased MDA content, as well as decreased SOD and GSH activity. Treatment with compounds of purified herbal extracts may increase antioxidant defenses by enhancing GSH and SOD activity, consequently decreasing lipid peroxidation and MDA content. The resulting improvement in antioxidant defenses effectively protects the brain from tissue damage due to free radicals induced by iron overload. This increase in antioxidant activity may be an important mechanism in protective effects of the compound against brain damage.
During AD, aggregation of Aβ peptide induced by iron overload in the brain usually leads to glial cell activation,which initiates a neuroinflammatory response involving inflammatory cytokines (including IL-6, IL-1β, and TNF-α)(Mucke, 2009; Vetrivel and Thinakaran, 2010; Tang et al.,2013), as well as reactive oxygen intermediates.In vitrostudies have shown that iron deposition on glial cells in the cortex, cerebellum, substantia nigra, and hippocampus is associated with neuroinflammation in AD (Fu et al., 2013).Inflammatory cytokines activate cell apoptosis, stimulate plaque-associated microglia, and increase lipid peroxidation (Griffin, 2006; Hoozemans et al., 2006). Here, we found significantly decreased IL-1β, IL-6, and TNF-α expression in the compound-treated AD group compared with the untreated AD group. In contrast, levels were increased in the AD model group compared with the normal control group.These results suggest that treatment with the effective component of the herbs Astragalus, Puerariae, and Icariin may attenuate damage and slow progression of AD by inhibiting the inflammatory response. It has been shown that increased inflammation contributes to progression of AD (Swardfager et al., 2010). Therefore, it is hypothesized that inflammatory components might be involved in microglial activation. Infl ammation may augment disease progression by facilitating brain iron deposition during progression of AD. Moreover,released inflammatory cytokines can disrupt the blood-brain barrier (Blasko et al., 2001; Qiao et al., 2001; Sastre et al.,2003), increasing vascular permeability, and allowing iron to enter and accumulate in the brain .
Our results demonstrate that the icariin, astragalus, and puerarin compounds remarkably ameliorate pathological changes in iron overloaded mice. These remarkable protective effects against injury caused by excess iron may be attributed to prevention of iron deposition, inhibition of neuronal apoptosis induced by oxidative stress, and inflammatory factors. Therefore, the compounds are effective in clearing heat, removing toxin, strengthening the brain, and developing intellect. Although there is still a long way to go,icariin, astragalus, and puerarin compounds may have potential benefits for AD treatment.
Author contributions:YZ provided data and ensured the integrity of the data, analyzed the data and wrote the paper. WNK participated in study concept and design. XQC served as a principle investigator and obtained the funding. All authors approved the final version of the paper.
Conflicts of interest:None declared.
Financial support:This study was supported by the National Natural Science Foundation of China, No. 81273983. The conception, design execution, and analysis of experiments, as well as the preparation of and decision to publish this manuscript, were made independent of this funding organization.
Research ethics:All animal handling procedures were in accordance with the Guidelines of Animal Experiments from the Committee of Medical Ethics, Ministry of Health of China, and experiments were approved by the Ethics Committee for Animal Experiments of Hebei Medical University of China (approval No. HEBMU-2010-10).
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Blasko I, Apochal A, Boeck G, Hartmann T, Grubeck-Loebenstein B,Ransmayr G (2001) Ibuprofen decreases cytokine-induced amyloid beta production in neuronal cells. Neurobiol Dis 8:1094-1101.
Chen GF, Huang WF (2008) Progress in pharmacological effects of compositions of Astragalus membranaceus. Zhongguo Xinyao Zazhi 17:1482-1485.
Chen KM, Ge BF, Ma HP, Liu XY, Bai MH, Wang Y (2005) Icariin, a flavonoid from the herb Epimedium enhances the osteogenic differentiation of rat primary bone marrow stromal cells. Pharmazie 60:939-942.
Cheng S, Qiu F, Wang S, He J (2007) HPLC analysis and pharmacokinetics of icariin in rats. J Sep Sci 30:1307-1312.
Crapper McLachlan DR, Dalton AJ, Kruck TP, Bell MY, Smith WL, Kalow W, Andrews DF (1991) Intramuscular desferrioxamine in patients with Alzheimer’s disease. Lancet 337:1304-1308.
Dai H, Jia G, Liu X, Liu Z, Wang H (2014) Astragalus polysaccharide inhibits isoprenaline-induced cardiac hypertrophy via suppressing Ca(2)(+)-mediated calcineurin/NFATc3 and CaMKII signaling cascades.Environ Toxicol Pharmacol 38:263-271.
Dang SS, Jia XL, Song P, Cheng YA, Zhang X, Sun MZ, Liu EQ (2009)Inhibitory effect of emodin and Astragalus polysaccharide on the replication of HBV. World J Gastroenterol 15:5669-5673.
Dong XH, Bai JT, Kong WN, He XP, Yan P, Shao TM, Yu WG, Chai XQ,Wu YH, Liu C (2015) Effective components of Chinese herbs reduce central nervous system function decline induced by iron overload.Neural Regen Res 10:778-785.
Du X, Zhao B, Li J, Cao X, Diao M, Feng H, Chen X, Chen Z, Zeng X(2012) Astragalus polysaccharides enhance immune responses of HBV DNA vaccination via promoting the dendritic cell maturation and suppressing Treg frequency in mice. Int Immunopharmacol 14:463-470.
El Tannir El Tayara N, Delatour B, Le Cudennec C, Guégan M, Volk A,Dhenain M (2006) Age-related evolution of amyloid burden, iron load,and MR relaxation times in a transgenic mouse model of Alzheimer’s disease. Neurobiol Dis 22:199-208.
Fu JT, Wang P, Guo C (2013) Disturbance of iron metabolism in brain and Alzheimer’s disease. Jiepou Kexue Jinzhan 19:79-82.
Galleano M, Puntarulo S (1997) Dietary alpha-tocopherol supplementation on antioxidant defenses after in vivo iron overload in rats. Toxicology 124:73-81.
Gao J, Inagaki Y, Liu Y (2013) Research progress on flavonoids isolated from traditional Chinese medicine in treatment of Alzheimer’s disease.Intractable Rare Dis Res 2:3-10.
Geisser P (1997) Iron therapy and oxidative stress. Met Based Drugs 4:137-152.
Genlain M, Godaux E, Ris L (2007) Involvement of hyperpolarization-activated cation channels in synaptic modulation. Neuroreport 18:1231-1235.
Griffin WS (2006) Inflammation and neurodegenerative diseases. Am J Clin Nutr 83:470S-474S.
Hagemeier J, Geurts JJ, Zivadinov R (2012) Brain iron accumulation in aging and neurodegenerative disorders. Expert Rev Neurother 12:1467-1480.
Hishikawa T, Ono S, Ogawa T, Tokunaga K, Sugiu K, Date I (2008) Effects of deferoxamine-activated hypoxia-inducible factor-1 on the brainstem after subarachnoid hemorrhage in rats. Neurosurgery 62:232-240; discussion 240-241.
Hoozemans JJ, Veerhuis R, Rozemuller JM, Eikelenboom P (2006) Neuroinflammation and regeneration in the early stages of Alzheimer’s disease pathology. Int J Dev Neurosci 24:157-165.
Huang WM, Liang YQ, Tang LJ, Ding Y, Wang XH (2013) Antioxidant and anti-inflammatory effects of Astragalus polysaccharide on EA.hy926 cells. Exp Ther Med 6:199-203.
Katzman R, Saitoh T (1991) Advances in Alzheimer’s disease. FASEB J 5:278-286.
Ko JK, Lam FY, Cheung AP (2005) Amelioration of experimental colitis by Astragalus membranaceus through anti-oxidation and inhibition of adhesion molecule synthesis. World J Gastroenterol 11:5787-5794.
Lee CH, Yan B, Yoo KY, Choi JH, Kwon SH, Her S, Sohn Y, Hwang IK,Cho JH, Kim YM, Won MH (2011) Ischemia-induced changes in glucagon-like peptide-1 receptor and neuroprotective effect of its agonist, exendin-4, in experimental transient cerebral ischemia. J Neurosci Res 89:1103-1113.
Lim DW, Lee C, Kim IH, Kim YT (2013) Anti-inflammatory effects of total isoflavones from Pueraria lobata on cerebral ischemia in rats.Molecules 18:10404-10412.
Lu Q, Black SM (2016) Iron metabolism, oxidative stress, and neonatal brain injury. Neural Regen Res 11:725-726.
Misonou H, Morishima-Kawashima M, Ihara Y (2000) Oxidative stress induces intracellular accumulation of amyloid beta-protein (Abeta)in human neuroblastoma cells. Biochemistry 39:6951-6959.
Mucke L (2009) Neuroscience: Alzheimer’s disease. Nature 461:895-897.
Parks RR, Huang CC, Haddad J, Jr. (1994) Evidence of oxygen radical injury in experimental otitis media. Laryngoscope 104:1389-1392.
Qian ZM, Shen X (2001) Brain iron transport and neurodegeneration.Trends Mol Med 7:103-108.
Qiao X, Cummins DJ, Paul SM (2001) Neuroin flammation-induced acceleration of amyloid deposition in the APPV717F transgenic mouse.Eur J Neurosci 14:474-482.
Rolston RK, Perry G, Zhu X, Castellani RJ, Dwyer BE, Lee HG, Petersen RB, Smith MA (2009) Iron: A pathological mediator of Alzheimer disease? Agro Food Ind Hi Tech 19:33-36.
Sastre M, Dewachter I, Landreth GE, Willson TM, Klockgether T, van Leuven F, Heneka MT (2003) Nonsteroidal anti-inflammatory drugs and peroxisome proliferator-activated receptor-gamma agonists modulate immunostimulated processing of amyloid precursor protein through regulation of beta-secretase. J Neurosci 23:9796-9804.
Swardfager W, Lanct?t K, Rothenburg L, Wong A, Cappell J, Herrmann N (2010) A meta-analysis of cytokines in Alzheimer’s disease. Biol Psychiatry 68:930-941.
Tang J, Wu L, Huang H, Feng J, Yuan Y, Zhou Y, Huang P, Xu Y, Yu C(2013) Back propagation artificial neural network for community Alzheimer’s disease screening in China. Neural Regen Res 8:270-276.
Vetrivel KS, Thinakaran G (2010) Membrane rafts in Alzheimer’s disease beta-amyloid production. Biochim Biophys Acta 1801:860-867.
Wan S, Hua Y, Keep RF, Hoff JT, Xi G (2006) Deferoxamine reduces CSF free iron levels following intracerebral hemorrhage. Acta Neurochir Suppl 96:199-202.
Whitnall M, Richardson DR (2006) Iron: a new target for pharmacological intervention in neurodegenerative diseases. Semin Pediatr Neurol 13:186-197.
Xian YF, Lin ZX, Mao QQ, Ip SP, Su ZR, Lai XP (2012) Protective effect of isorhynchophylline against beta-amyloid-induced neurotoxicity in PC12 cells. Cell Mol Neurobiol 32:353-360.
Yoo DY, Yoo KY, Park JH, Kwon HJ, Jung HY, Kim JW, Choi GM, Moon SM, Kim DW, Yoon YS, Won MH, Hwang IK (2016) Time- and celltype specific changes in iron, ferritin, and transferrin in the gerbil hippocampal CA1 region after transient forebrain ischemia. Neural Regen Res 11:924-930.
Zhu WZ, Zhong WD, Wang W, Zhan CJ, Wang CY, Qi JP, Wang JZ, Lei T (2009) Quantitative MR phase-corrected imaging to investigate increased brain iron deposition of patients with Alzheimer disease.Radiology 253:497-504.
- 中國神經(jīng)再生研究(英文版)的其它文章
- Use of curcumin in diagnosis, prevention, and treatment of Alzheimer’s disease
- Alpha-7 nicotinic acetylcholine receptor agonist treatment in a rat model of Huntington’s disease and involvement of heme oxygenase-1
- Structural neural connectivity of the vestibular nuclei in the human brain: a diffusion tensor imaging study
- Intracerebroventricularly-administered 1-methyl-4-phenylpyridinium ion and brain-derived neurotrophic factor affect catecholaminergic nerve terminals and neurogenesis in the hippocampus, striatum and substantia nigra
- Induced dural lymphangiogenesis facilities soluble amyloid-beta clearance from brain in a transgenic mouse model of Alzheimer’s disease
- Brain remodeling after chronic median nerve compression in a rat model

