Brain remodeling after chronic median nerve compression in a rat model
Bing-Bo Bao , Dan-Qian Qu , Hong-Yi Zhu Tao Gao Xian-You Zheng
1 Department of Orthopedic Surgery, Shanghai Jiao Tong University, Affiliated Sixth People’s Hospital, Shanghai, China
2 Yueyang Hospital of Intergrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai,China
Introduction
Carpal tunnel syndrome (CTS) is the most clinically common compressive neuropathy of the upper extremities,and affects many individuals (Stapleton, 2006). Specifically, it has a prevalence of 3–5% in the general population and 6% in females over the age of 40 years (Grace et al.,2010). It causes altered sensation, chronic pain, and partial thenar atrophy, which can lead to hand dysfunction(Kleopa, 2015; Padua et al., 2016; Dec and Zyluk, 2018).Previous studies have shown that CTS, accompanied by chronic nerve compression with compressive neuropathy,can induce changes in the structure and microvasculature of peripheral nerves (Bai et al., 2016; Chen et al., 2016).Further, CTS is also characterized by structural (Maeda et al., 2013, 2016) and functional (Druschky et al., 2000; Napadow et al., 2006; Dhond et al., 2012; Maeda et al., 2014)neuroplasticity in the primary somatosensory cortex (S1)of the brain.
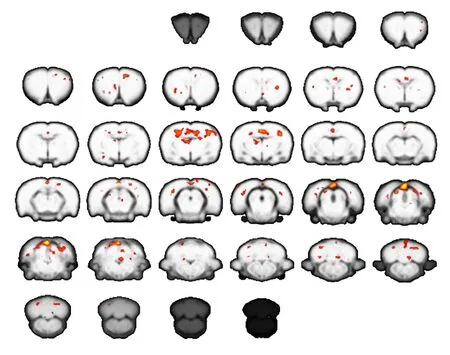
Figure 1 Brain activation map for normal control rats(functional magnetic resonance imaging).
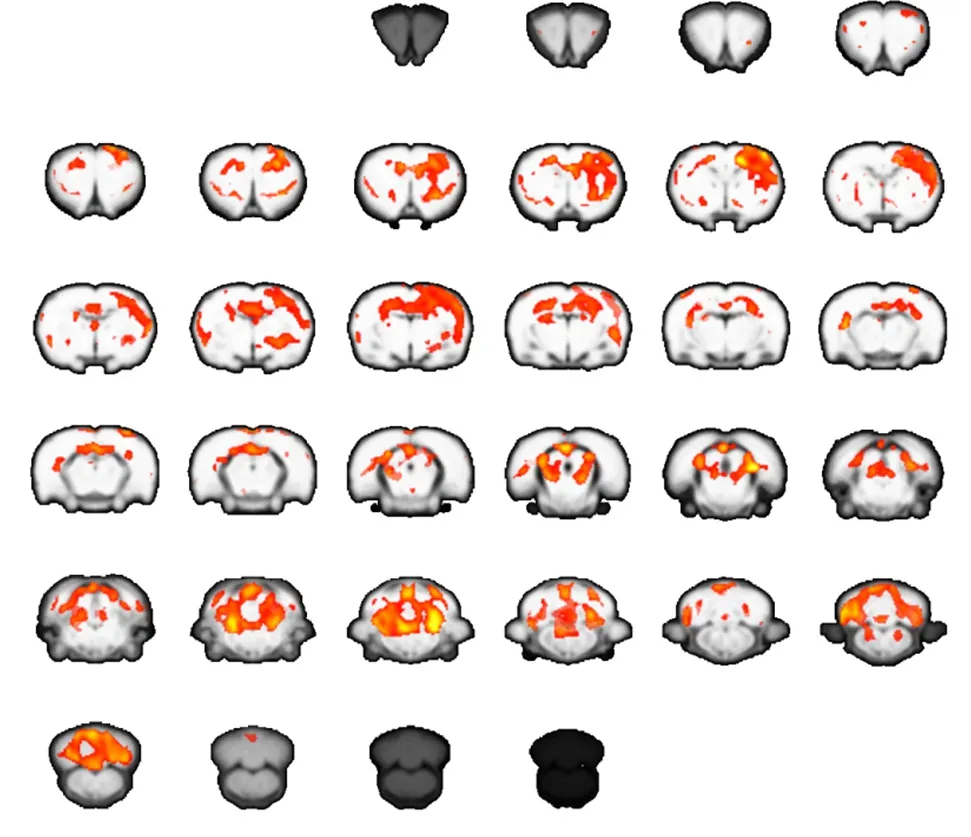
Figure 2 Brain activation map for carpal tunnel syndrome rats at 2 weeks after operation (functional magnetic resonance imaging).
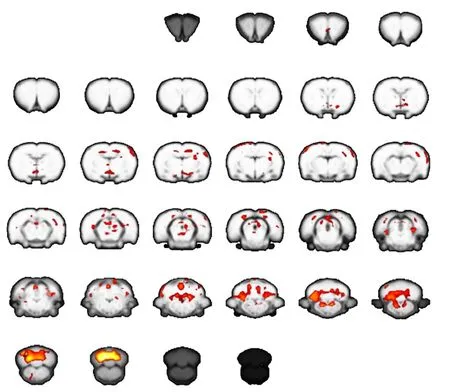
Figure 3 Brain activation map for carpal tunnel syndrome rats at 2 months after operation (functional magnetic resonance imaging).
CTS leads to altered afferent processing throughout the somatosensory system (involving both the peripheral and central nervous systems), as measured by somatosensory evoked potentials in the spinal cord, brainstem, and primary sensorimotor cortex (Maeda et al., 2013b, 2017).The finger and toe digits occupy a significant portion of the human somatotopic map in the primary somatosensory cortex, and are represented in consecutive order along the postcentral gyrus, with digit 1 (D1) being most ventrolateral and digit 5 (D5) most dorsomedial (Maeda et al., 2014, 2016, 2017). Although chronic nerve compression is a peripheral neuropathy, neuroimaging data suggests that irregular afferent signals of CTS produce maladaptive central neuroplasticity (Napadow et al.,2007). For example, spinal amplification of event-related potentials to ulnar nerve stimulation of the CTS-affected hand is thought to represent unmasking of secondary inputs that are normally silent in median nerve signaling. Similarly, studies have recently reported that during stimulation of median nerve-innervated digits, early cortical amplification can evoke responses and alter S1 digit somatotopy. Corroboratively, these findings have been verified by functional magnetic resonance imaging (fMRI)(Khosrawi et al., 2012; Beissner et al., 2013; Kim et al.,2015).
Nonetheless, there are limited longitudinal studies on plasticity in the somatosensory cortex, as it is difficult to acquire multi-point neuroimaging data clinically. Thus,to address this, we developed a rat model of CTS and investigated cerebral plasticity using small animal fMRI.
Materials and Methods
Animals
Forty female Sprague-Dawley rats weighing from 250 g to 300 g and aged 8 weeks were provided by the Animal Center of the Medical College of Shanghai Jiao Tong University, China (license No. SYXK (Hu) 2016-0020). All rats were housed at 20–25°C and 50 ± 5% humidity with free access to food and water in 12-hour light/dark cycles.All procedures and animal experiments were approved by the Animal Care and Use Committee of Shanghai Jiao Tong University, China (approval No. 2017-0124). Rats were randomly divided into a CTS group (n= 20; chronic median nerve compression) and normal group (n= 20).
Rat model establishment
Rats were intraperitoneally anesthetized with pentobarbital sodium (40 mg/kg; Shanghai Longsheng Chemical Co., Ltd., Shanghai, China). A dorsal gluteal splitting approach was used to expose the right median nerve of each rat. The right median nerve at the wrist level was mobilized and a 10-0 prolene suture used for median nerve ligation. This entire procedure was performed using a microscope (Shanghai Eder Medical Technology Inc.Shanghai, China) at 10× magnification. Finally, the incision was closed across all layers, with a tension-free skin closure performed in accordance with previously published methods (Atroshi et al., 1999b; Grace et al., 2010).
MRI acquisition
All fMRI scans were performed using a 7.0T horizontal-bore Bruker scanner (Bruker Corporation, Karlsruhe,Germany), which was equipped with a gradient system of 116 mm inner diameter and maximum gradient strength of 400 mT/m. fMRI scanning was performed to investigate cortical plasticity. Rats were anesthetized by sevoflurane inhalation (3% in oxygen) (Shanghai Longsheng Chemical Co., Ltd.), and then fixed on the scanner with the necessary ventilator support. A single transmitting and receiving surface coil consisting of a single copper-wire loop was used. For functional imaging, an interleaved single-shot echo planar imaging (EPI) sequence was applied with the following parameters: flip angle, 90°;slice thickness, 0.5 mm; repetition time, 3,000 ms; echo time, 20 ms; number of averages, 1; and field of view, 32 mm × 32 mm with 64 × 64 points. EPI fMRI volumes covered a relatively restricted area centered approximately on bregma point. The whole scan began with a dummy epoch of 8 seconds, which was automatically discarded by the system. Both “ON” and “OFF” epochs lasted for 30 seconds and these two epochs sequentially formed one cycle. A total of six cycles were performed in one stimulation session, during which only one side was stimulated with electric needles in the palm position.
Imaging preprocessing
There are several preprocessing steps that must be performed before data analysis. All images had their pixel dimensions scaled up by a factor of 10 in the Nifti header to avoid scale-dependent issues using standard FSL software(Oxford University, Oxford, UK). Apart from brain extraction and band-pass filtering, all steps were performed using the MELODIC graphical user interface. Preprocessing steps included:
(1) Brain extraction: brain extraction was manually performed. Specifically, masks were manually created by masking all slices from the first volume of each individual rat to generate a mask file. These mask files were then applied to all volumes in each functional image.
(2) Band-pass filtering: functional images were band-pass filtered between 0.01 and 0.1 Hz.
(3) Slice timing correction: because each slice was acquired in interleaved order (0, 2, 4, 6 …1, 3, 5, 7 …), interleaved slice timing correction was used.
(4) Spatial smoothing: functional data were spatially smoothed to minimize minor registration imperfections.Because we were interested in large-scale networks across the whole brain of a young rat, Gaussian kernel full width at half maximum (FWHM) of 0.7 mm was used to preprocess data and identify relatively large areas of coherent activity.
(5) Normalization to standard space: animals slightly differ in brain size, which must be taken into account.Therefore, before brain network analysis, individual brains were registered to a standardized anatomical image (see below). Registration of fMRI data to a standard space (in-house adult anatomical rat brain template) was performed using FSL’s flirt, with a freedom affine transformation of 12° and resampling resolution of 0.4 mm.Consequently, for registration, affine transformation was used to ensure proper alignment of each individual rat to the adult rat brain atlas. This step is a pre-requisite for group analysis to identify common networks across all animals. Common expected minimal artifacts were detected across all animals in the brain.
(6) Post analysis: higher-level analysis was performed using a general linear model. One-samplet-test was first performed in each group for determining the significant area within the group (false discovery rate, FDR correction,P< 0.05). The significant area in each group was extracted and combined into one binary mask. Subsequently, a two-samplet-test was performed within the boundary of the previously-generated mask (FDR correction,P< 0.05). MRIcroGL software (Bonilha et al., 2016)was used to visualize the results.
Results
Intragroup differences in the sensory stimulus task at 2 weeks after operation
In control rats, stimulation to either forepaw generated significant activation of the contralateral sensorimotor cortex. However, in rats with CTS, stimulation to the affected right forepaw at 2 weeks after operation generated a strong signal change in the contralateral primary motor area (M1) and sensory cortex. Additional activation was observed in the cerebellum and thalamus.
Intergroup differences in the sensory stimulus task at 2 weeks and 2 months after operation
The extent of activation in the brain was greater in CTS rats than normal control rats at 2 weeks. However, activation in the contralateral primary motor area (M1) and sensory cortex at 2 months was much weaker compared with normal control rats. These results suggest there is dynamic plasticity in the sensorimotor cortex of CTS rats(Figures 1–3).
Discussion
Peripheral entrapment neuropathies are common sources of pain and paraesthesia (Neal and Fields, 2010) in clinical practice (Atroshi et al., 1999a; Wilson d’Almeida et al., 2008; Foley and Silverstein, 2015). Entrapment of the median nerve at the wrist, called CTS, accounts for 90% of such neuropathies (Papanicolaou et al., 2001;Kleopa, 2015). Here, we demonstrate a dynamic plastic process of cortical reorganization in CTS rats using a long-term study. Our results show that the sensory map of the affected forepaw expands at the early stage,and then shrinks at the later stage. This suggests a compensatory process in the brain of CTS rats. Similarly,previous neuroimaging studies have shown that while CTS results from compression of the median nerve at the wrist, it is also characterized by structural and functional neuroplasticity in the brain (Tecchio et al., 2002; Maeda et al., 2017). Specifically, CTS patients show decreased primary somatosensory cortex (S1) gray matter volume and cortical thickness contralateral to the affected hand,which is pronounced in paraesthesia dominant symptom subgroups and associated with aberrant median nerve conduction. Further, fMRI shows reduced separation between S1 cortical representations of adjacent median nerve-innervated fi ngers, digits 2 and 3 (D2/D3), which is a reproducible finding in different CTS cohorts using both fMRI and magnetoencephalography. Reduced D2/D3 separation in S1 is associated with median sensory nerve conduction latency, symptom severity, reduced fi ne motor performance, and diminished sensory discrimination accuracy, demonstrating that functional brain neuroplasticity is indeed maladaptive (Baraban et al., 2016;Maeda et al., 2016, 2017).
In our present study, block-design stimulation of the affected forepaw generated significant activation in the contralateral sensorimotor cortex in normal control rats.However, the same stimulation in CTS rats at the early stage generated extended activation in the contralateral hemisphere, including the primary sensorimotor cortex,cerebellum, and thalamus. This suggests that the brain attempts to compensate for sensory loss after median nerve entrapment by enlarging central representation in the sensorimotor cortex and related brain regions of sensorimotor networks. Interestingly, brain activation decreased remarkably in CTS rats at the later stage. This suggests a maladaptive process in the brain after median nerve entrapment. Possibly with continuously decreased sensory input, the brain is unable to maintain control of the affected forepaw.
A limitation of our study is that the sensory nerve action potential test was difficult to perform in the rat model. Consequently, we were unable to obtain enough clinical neurophysiology data. Indeed, there were only two time-points in the follow-up investigation. In further studies, we would overcome this limitation by performing more investigations.
In conclusion, our results strongly support a dynamic plastic process after median nerve entrapment. Cortical reorganization is the foundation of sensorimotor func-tion recovery and may be a treatment biomarker. Our future study will quantify the functional differences so as to objectively compare the temporal changes.
Author contributions:XYZ was in charge of study design and paper writing. BBB and TG performed animal experiments. BBB and DQQ were responsible for fMRI data collection and analysis. BBB and HYZ participated in the revision of the paper. XYZ supervised the work. All authors discussed the results and commented on the paper, and approved the final version of the paper.
Conflicts of interest:The authors declare that they have no conflicts of interest.
Financial support:This work was supported by the National Natural Science Foundation of China, No. 81371965, 81672144; and a grant from the Shanghai Pujiang Program of China, No. 16PJD035. The funding bodies played no role in the study design, collection, analysis and interpretation of data, the writing of the paper, or the decision to submit the paper for publication.
Research ethics:The study was approved by the Ethics Committee of Affiliated Sixth People’s Hospital of Shanghai Jiao Tong University of China (approval No. 2017-0124). The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23,revised 1985).
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:José M. Ferrandez, Universidad Politecnica de Cartagena, Cartagena, Spain.
Additional file:open peerreview report 1.
Atroshi I, Gummesson C, Johnsson R, Sprinchorn A (1999a) Symptoms, disability, and quality of life in patients with carpal tunnel syndrome. J Hand Surg 24:398-404.
Atroshi I, Gummesson C, Johnsson R, Ornstein E, Ranstam J, Rosen I (1999b) Prevalence of carpal tunnel syndrome in a general population. JAMA 282:153-158.
Bai J, Xu YB, Xia L, Zhou HZ (2016) The clinical efficacy and safety of endoscopic release versus mini-open release for carpal tunnel syndrome. Zhongguo Zuzhi Gongcheng Yanjiu 20:5009-5016.
Baraban M, Mensch S, Lyons DA (2016) Adaptive myelination from fish to man. Brain Res 1641:149-161.
Beissner F, Meissner K, Bar KJ, Napadow V (2013) The autonomic brain: an activation likelihood estimation meta-analysis for central processing of autonomic function. J Neuroscience 33:10503-10511.
Bonilha L, Gleichgerrcht E, Nesland T, Rorden C, Fridriksson J(2016) Success of anomia treatment in aphasia is associated with preserved architecture of global and left temporal lobe structural networks. Neurorehabil Neural Repair 30:266-279.
Chen Y, Zhao CQ, Ye G, Liu CD, Xu WD (2016) Low-power laser therapy for carpal tunnel syndrome: effective optical power. Neural Regen Res 11:1180-1184.
Dec P, Zyluk A (2018) Bilateral carpal tunnel syndrome - A review.Neurol Neurochir Pol 52:79-83.
Dhond RP, Ruzich E, Witzel T, Maeda Y, Malatesta C, Morse LR, Audette J, Hamalainen M, Kettner N, Napadow V (2012) Spatio-temporal mapping cortical neuroplasticity in carpal tunnel syndrome.Brain 135:3062-3073.
Druschky K, Kaltenhauser M, Hummel C, Druschky A, Huk WJ,Stefan H, Neundorfer B (2000) Alteration of the somatosensory cortical map in peripheral mononeuropathy due to carpal tunnel syndrome. Neuroreport 11:3925-3930.
Foley M, Silverstein B (2015) The long-term burden of work-related carpal tunnel syndrome relative to upper-extremity fractures and dermatitis in Washington State. Am J Ind Med 58:1255-1269.
Grace PM, Hutchinson MR, Manavis J, Somogyi AA, Rolan PE (2010)A novel animal model of graded neuropathic pain: utility to investigate mechanisms of population heterogeneity. J Neurosci Methods 193:47-53.
Khosrawi S, Moghtaderi A, Haghighat S (2012) Acupuncture in treatment of carpal tunnel syndrome: a randomized controlled trial study. J Res Med Sci 17:1-7.
Kim J, Loggia ML, Cahalan CM, Harris RE, Beissner FDPN, Garcia RG, Kim H, Wasan AD, Edwards RR, Napadow V (2015) The somatosensory link in fibromyalgia: functional connectivity of the primary somatosensory cortex is altered by sustained pain and is associated with clinical/autonomic dysfunction. Arthritis Rheumatol 67:1395-1405.
Kleopa KA (2015) In the Clinic. Carpal Tunnel Syndrome. Ann intern Med 163:ITC1.
Maeda Y, Kettner N, Lee J, Kim J, Cina S, Malatesta C, Gerber J, Mc-Manus C, Im J, Libby A, Mezzacappa P, Morse LR, Park K, Audette J, Napadow V (2013) Acupuncture evoked response in contralateral somatosensory cortex reflects peripheral nerve pathology of carpal tunnel syndrome. Med Acupunct 25:275-284.
Maeda Y, Kettner N, Holden J, Lee J, Kim J, Cina S, Malatesta C, Gerber J, McManus C, Im J, Libby A, Mezzacappa P, Morse LR, Park K,Audette J, Tommerdahl M, Napadow V (2014) Functional deficits in carpal tunnel syndrome reflect reorganization of primary somatosensory cortex. Brain 137:1741-1752.
Maeda Y, Kettner N, Kim J, Kim H, Cina S, Malatesta C, Gerber J,McManus C, Libby A, Mezzacappa P, Mawla I, Morse LR, Audette J, Napadow V (2016) Primary somatosensory/motor cortical thickness distinguishes paresthesia-dominant from pain-dominant carpal tunnel syndrome. Pain 157:1085-1093.
Maeda Y, Kim H, Kettner N, Kim J, Cina S, Malatesta C, Gerber J,McManus C, Ong-Sutherland R, Mezzacappa P, Libby A, Mawla I, Morse LR, Kaptchuk TJ, Audette J, Napadow V (2017) Rewiring the primary somatosensory cortex in carpal tunnel syndrome with acupuncture. Brain 140:914-927.
Napadow V, Kettner N, Ryan A, Kwong KK, Audette J, Hui KK (2006)Somatosensory cortical plasticity in carpal tunnel syndrome-a cross-sectional fMRI evaluation. NeuroImage 31:520-530.
Napadow V, Kettner N, Liu J, Li M, Kwong KK, Vangel M, Makris N,Audette J, Hui KK (2007) Hypothalamus and amygdala response to acupuncture stimuli in carpal tunnel syndrome. Pain 130:254-266.
Neal S, Fields KB (2010) Peripheral nerve entrapment and injury in the upper extremity. Am Fam Physician 81:147-155.
Padua L, Coraci D, Erra C, Pazzaglia C, Paolasso I, Loreti C, Caliandro P, Hobson-Webb LD (2016) Carpal tunnel syndrome: clinical features, diagnosis, and management. Lancet Neurol 15:1273-1284.
Papanicolaou GD, McCabe SJ, Firrell J (2001) The prevalence and characteristics of nerve compression symptoms in the general population. J Hand Surg 26:460-466.
Stapleton MJ (2006) Occupation and carpal tunnel syndrome. ANZ J Surg 76:494-496.
Tecchio F, Padua L, Aprile I, Rossini PM (2002) Carpal tunnel syndrome modifies sensory hand cortical somatotopy: a MEG study.Human brain mapp 17:28-36.
Wilson d’Almeida K, Godard C, Leclerc A, Lahon G (2008) Sickness absence for upper limb disorders in a French company. Occup Med (Lond) 58:506-508.
 中國(guó)神經(jīng)再生研究(英文版)2018年4期
中國(guó)神經(jīng)再生研究(英文版)2018年4期
- 中國(guó)神經(jīng)再生研究(英文版)的其它文章
- Use of curcumin in diagnosis, prevention, and treatment of Alzheimer’s disease
- Alpha-7 nicotinic acetylcholine receptor agonist treatment in a rat model of Huntington’s disease and involvement of heme oxygenase-1
- Compound of icariin, astragalus, and puerarin mitigates iron overload in the cerebral cortex of Alzheimer’s disease mice
- Structural neural connectivity of the vestibular nuclei in the human brain: a diffusion tensor imaging study
- Intracerebroventricularly-administered 1-methyl-4-phenylpyridinium ion and brain-derived neurotrophic factor affect catecholaminergic nerve terminals and neurogenesis in the hippocampus, striatum and substantia nigra
- Induced dural lymphangiogenesis facilities soluble amyloid-beta clearance from brain in a transgenic mouse model of Alzheimer’s disease
