Two Copper Complexes Based on Pyrazole- 3-carboxylic Acid as Heterogeneous Catalysts for Highly Selective Oxidation of Alkylbenzenes①
JIANG Xiu-Yn RONG Nin-Xin QIAN Rui QIU Tin-Tin YAO Qing-Xi HUANG Xin-Qing
?
Two Copper Complexes Based on Pyrazole- 3-carboxylic Acid as Heterogeneous Catalysts for Highly Selective Oxidation of Alkylbenzenes①
JIANG Xiu-YanaRONG Nian-XinbQIAN RuibQIU Tian-TianbYAO Qing-Xiab②HUANG Xian-Qiangb②
a(257061)b(252059)
Two new copper complexes based on pyrazole-3-carboxylic acid (H2pca) ligand, Cu(Hpca)2(H2O)2·2H2O (1) and Cu2(pca)2(H2O)4(2)have been synthesized and fully characterized by single-crystal X-ray diffraction (SXRD), infrared spectroscopy (IR), thermal gravity analysis (TGA), powder X-ray diffraction (PXRD) and elemental analyses. Complex1 is mononuclear whilecomplex 2 shows a dinuclear structure. Complex1 crystallizes in the monoclinic system, space group21/with= 2,= 6.5591(5),= 21.696(2),= 4.9486(2) ?,= 680.94(9) ?3,(000) = 366,D= 1.745 g/cm3,= 1.650 mm-1, the final= 0.0340 and= 0.0792. Complex2 crystallizes in the monoclinic system, space group21/with= 2,= 5.1935(4),= 9.6052(7),= 12.7347(9) ?,= 634.44(8) ?3,(000) = 420,D= 2.195 g/cm3,= 3.404 mm-1, the final= 0.0305 and= 0.0653.The three-dimensional frameworks of two complexes are formed by the O?H···O and N?H···O hydrogen bonding interactions. Notably, two copper complexes are further used as catalysts in the oxidation of alkylbenzenes using-butylhydroperoxide (TBHP) as the oxidant and they exhibit excellent catalytic performance (Conv. up to 98.9%, Sele. up to 98.7%).
copper complexes, crystal structure, oxidation of alkylbenzenes;
1 INTRODUCTION
In recent decades, transition-metal complexes have obtained long-lasting attention owing to their special structures and extensive applications in catalysis, adsorption, electric conducting materials, magnetic and optical materials, and so on[1-4]. Among transi- tion metals, copper is an important element in coordination chemistry, catalysis chemistry and a microelement necessary to life[5-8]. Its flexible coor-dination modes make it easy to form mononuclear, dinuclearand multinuclear complexes[9, 10]. Pyrazole carboxylates as a kind of multifunctional ligands play an important role in generating excellent architectures because of some advantages: firstly, the multidentate organic ligands possess potential coor- dination nodes and the strong coordination ability, and they exhibit diverse chelating and bridging modes; secondly, pyrazole carboxylates also act as the multiple proton donors and acceptors, and may build high-dimensional supramolecular frameworks by hydrogen-bonding orstacking interactions.However, few examples are reported with the transi- tion-metal complexes based on 1-pyrazole-3-car- boxylic acid owning to only one carboxyl group in the ligand[11-13].And it remains a significant challenge to synthesize such pyrazole-3-carboxylic acid complexes and develop their catalytic activities in some organic reactions.
Herein, we report the syntheses and structural characterizations of the mononuclear copper complex 1 and dinuclear copper complex 2, which were obtained by theroom temperature conditions or hydrothermal method. The two copper complexes have been fully characterized by SXRD, PXRD, elemental analyses, TGA and FT-IR spectroscopy. Complexes 1 and 2 were further investigated as catalysts for the oxidation of alkylbenzenes with TBHP as an oxidant.
2 EXPERIMENTAL
2. 1 Instruments and reagents
All reagents and solvents were purchased from commercial sources and used without further puri- fication. PXRD patterns of the samples were analy- zed with monochromatized Cu-(= 1.54178 ?) incident radiation by a Shimadzu XRD-6000 instrument operating at 40 kV voltage and 50 mA current, and PXRD patterns were recorded from 4° to 50° (2) at 298 K. The C, H and N elemental analyses were conducted on a Perkin-Elmer 240C elemental analyzer. The FT-IR spectra were recorded from KBr pellets in the range of 4000~400 cm-1on a Nicolet 170 SXFT/IR spectrometer. The GC analyses were performed on Shimadzu GC-2014C with a FID detector equipped with an Rtx-1701 Sil capillary column.TGA experiments were carried out on a Perkin-Elmer TGA 7 analyzer at a heating rate of 10 °C/min from the room temperature to 600 °C under nitrogen atmosphere.
2. 2 Synthesis ofcomplex Cu(Hpca)2(H2O)2·2H2O (1)
A solution containing Cu(ClO4)2·6H2O (0.074 g, 0.2 mmol) in 5 mLof distilled water was slowly added to a solution containing H2pca (0.0448 g, 0.4 mmol) and NaOH (0.1 mL, 1 M in H2O) under stirring in 5 mL of distilled water. The reaction mixture was stirred for 1 h at room temperature and filtrated. The blue block crystals of 1 were obtained by slow evaporation of the filtrate at room tempera- ture over a period of three weekswith the yield of 32% based on Cu. IR (KBr, cm-1): 3484(s), 3363(s), 1660(s), 1555(m), 1507(m), 1475(m), 1382(m), 1355(s), 1263(s), 1232(w), 1133(m), 1068(s), 1014(m), 942(m), 898(m), 839(m), 785(m), 649(m), 502(w). Anal. calcd forC8H14CuN4O8: 26.86, H 3.94, N 15.66%. Found: C, 26.91; H, 3.97; N, 15.54%.
2. 3 Synthesis of complex Cu2(pca)2(H2O)4 (2)
A mixture of H2pca (0.0448 g, 0.4 mmol), CuCl2·2H2O (0.068 g, 0.4 mmol), NaOH (0.2 mL, 1 M in H2O) and distilled water (10 mL) was sealed in a 23 mL Teflon-lined steel vessel and heated at 150 °C for 72 h, and then cooled to room tempera- ture at a rate of 0.1°C/min. The resulting blue block crystals of 2 were obtained and washed with dis- tilled water with a yield of 31% based on Cu. IR (KBr, cm-1): 3485 (s), 1659 (s), 1557 (w), 1512 (m), 1475 (m), 1382 (s), 1356 (s), 1263 (s), 1232 (w), 1133 (m), 1068 (m), 1014 (m), 942 (m), 898 (m), 838 (m), 785 (m), 649 (w), 497 (w). Anal. calcd for C8H12Cu2N4O8: C, 22.91; H, 2.88; N, 13.36%. Found: C, 22.98; H, 2.95; N, 13.27%.
2. 4 Procedure for the catalytic oxidation of alkylbenzenes
Alkylbenzenes (0.25 mmol), copper complex (5 mol%), 70% TBHP (0.625 mmol) and benzonitrile (2 mL) were added to a 10 mL flask, and the catalytic reaction was performed at 70 °C for 24 h. After the reaction was completed, the resulting mixture was analyzed by GC-MS and GC.
2. 5 Reuse experiments
The reuse experiments were carried out for the oxidation of diphenylmethane under the optimum conditions. After the reaction was completed, the catalyst was retrieved by filtration (5.8 mg, 96% recovery), washed with MeOH (ca. 3* 5 mL), and air-dried prior to being used for the reuse experi- ment. The PXRD spectrum of the retrieved catalyst was identical to that of the fresh catalyst (Fig. 6). In addition, the retrieved catalyst could be reused for the second run of oxidation of diphenylmethane. After the second run reaction was completed, the catalyst was retrieved by filtration (5.5 mg, 95% recovery), washed with MeOH (ca. 3* 5mL), and air-dried prior to being used for the third run experiment. The experiment of the third run was prepared in the same way as that for the second run, and finally the 95.7% conversion of diphenyl- methane was also determined by GC. The PXRD spectrum of the third run retrieved catalyst was identical to that of the fresh catalyst (Fig. 6).
2. 6 Structure determination
Single-crystal X-ray diffraction data forcom- plexes 1and2 were performed with Moradia- tion (= 0.71073 ?) on a Bruker-AXS CCD diffractometer equipped with multi-scan technique at 296 K. The structures were solved by direct methods and refined through full-matrix leastsqua- res techniques method on2using the SHELXTL 97 crystallographic software package[14, 15]. The final refinements included anisotropic displacement parameters for all atoms. The selected bond lengths, bond angles and hydrogen bond parameters of 1and2 are shown in Tables 1 and 2, respectively.

Table 1. Selected Bond Lengths (?) and Bond Angles (°) for Complexes 1 and 2
Symmetry code: a: –+1, –+1, –3

Table 2. Hydrogen BondLengths (?) and Bond Angles (°) for Complexes 1 and 2
Symmetry codes: a:–+1, –+1, –+2; b:,,–1; c: –, –+1, –+3; d: –, –+1, –+3; e:, –+3/2,+1/2
3 RESULTS AND DISCUSSION
3. 1 Structure description of Cu(Hpca)2(H2O)2·2H2O (1)
The single-crystal X-ray diffraction reveals thatcomplex 1 contains one crystallographically inde- pendentCu2+ion, one Hpca?anion and one coor- dination water molecule. As shown in Fig. 1a, the Cu2+ion is surrounded by two oxygen atoms, two nitrogen atoms (O(1), O(1A), N(1) and N(1A)) from two Hpca?anions, and two oxygen atoms (O(3), O(3A)) from two coordinated water mole- cules, and it exhibits a distorted {CuO4N2}octahe- dral geometry. The bond distances of the Cu?O and Cu?N are 1.9901(19)~2.508(2) and 1.976(2) ?, respectively, which are similar with those of repor- ted copper complexes[16]. The bond angles around Cu2+ion are in the range of 81.44(8)~180.0.00(5)°. The Hpca?anion adopts a chelate coordination mode: one oxygen atom and one nitrogen atom of Hpca?anion act as coordinationatoms connecting theadjacent Cu2+ions, forming a five-membered ring. The adjacent mononuclear structures are connected by two kinds of hydrogen bonding interactions to form a 1D supramolecular chain, in which the first hydrogen bonding interaction is formed by the oxygen atoms (O(3)) of coordinated water molecules and the carboxylic oxygen atoms (O(1)) of Hpca?anions with the O(3)?H(3C)···O(1) distance of 2.737(5) ?, and the second hydrogen bonding interaction is formed by oxygen atoms (O(3)) and nitrogen atoms (N(2)) of pyrazole rings from Hpca?anions with the N(2)?H(2)···O(3) distance of 2.733(4) ? (Fig. 1b). The coordinated water molecules and carboxylic oxygen atoms (O(2)) of the adjacent supramolecular chains are linked by the O(3)?H(3D)···O(2) (2.687(0) ?) hydrogen bonding interactions to construct a 2D supramo- lecular layer (Fig. 1c). It is noteworthy that there are 1D water chains formed by O(4)?H(4D)···O(4) (2.814(3) ?) hydrogen bonding interactions. The existence of hydrogen-bonding interactions (O(4)? H(4C)···O(2), 2.888(7) ?) between carboxylic oxygen atoms (O(2)) of the 2D supramolecular layers and the 1D water chains which further stabi- lize the whole structure leads to a 3D supramo- lecular structure (Fig. 1d).
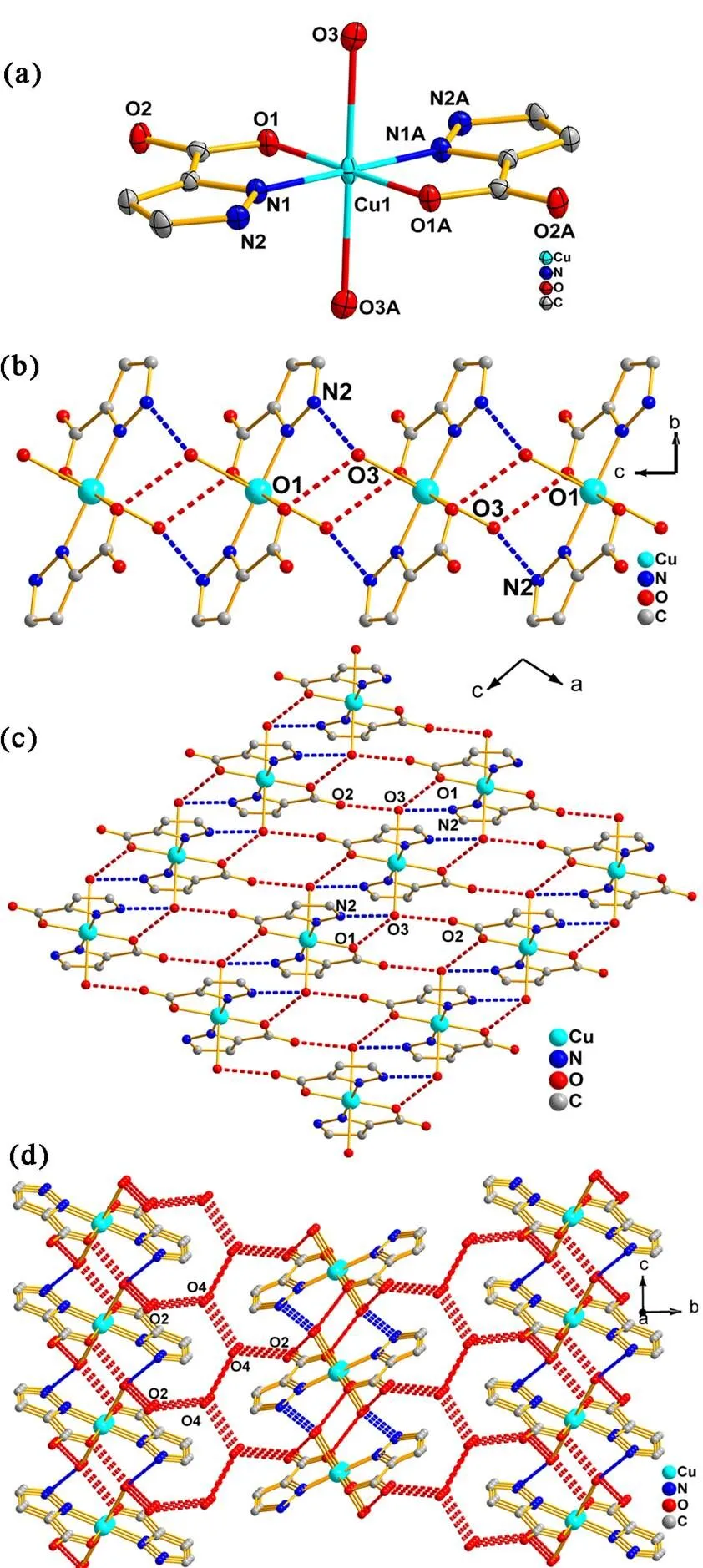
Fig. 1. (a) Coordination environment of the Cu2+ions in 1 (Hydrogen atoms and lattice water molecules are omitted for clarity). (b) 1D supramolecular chain of 1 formed by hydrogen bonds in-axis. (c) 2D supramolecular layer of 1 formed by hydrogen bonds in theplane. (d) 3D supramolecular framework of 1 consisting of 1D water chains
3. 2 Crystal structure of Cu2(pca)2(H2O)4 (2)
The single-crystal X-ray data reveal the asym- metric unit of 2 consists of one Cu2+cation, one pca2-anion and two coordination water molecules (Fig. 2a). The Cu2+ion is five-coordinated by one oxygen atom (O(1)) and one nitrogen atom (N(1)) from one pca2-anion, one nitrogen atom (N2) from the other pca2-anion and two coordination water molecules (O(3), O(4) to give the {CuO3N2}tetragonal pyramidal geometry. The bond distances of Cu?O and Cu?N are 1.9684(17)~2.3624(18) and 1.9521(19)~1.9628(19) ?, respectively. The bond angles around Cu2+ion are in the range of 81.98(7)~172.52(8)°. The pca2-anion adopts the monodentate-chelating coordination mode connecting Cu2+ions to generate the dinuclear structure, in which two pyrazole rings from the adjacent pca2-anion are parallel with a dihedral angle of 0o, and the distance of Cu???Cu is 3.9060 ?. Four pyrazole nitrogen atoms and two Cu2+ions almost locate in a plane and construct a six-membered ring. The dinuclear structures are further connected to form a 1D supramolecular chain by the O–H???O hydrogen bonding interactions, which occur between the oxygen atoms (O(3)) from coordination water molecules and the carboxyl oxygen atoms (O(1)) from pca2-anions with the distance of 2.720(2) ? (Fig. 2b). The final 3D supramolecular architecture is formed through O(4)?H(8)···O(3), O(3)?H(6)···O(2) and O(4)? H(7)···O(2) hydrogen bonding interactions between the neighboring 1D supramolecular chains with the distances of 2.729(2), 2.749(2) and 2.736(3) ?, respectively (Fig. 2c).

Fig. 2. (a) Coordination environment of the Cu2+ions in 2 (Hydrogen atoms are omitted for clarity). (b) 1D supramolecular chain of 2 formed by hydrogen bonds in-axis. (c) 3D supramolecular framework of 2 formed by hydrogen-bonding interactions
3. 3 PXRD analysis
The experimental and simulated PXRD patterns of complexes 1and2 are shown in Fig. 3. Their peak positions were in good agreement with each other, indicating the phase purity of complexes 1 and 2.
3. 4 Thermal stability analysis
In order to study the thermal stabilities of com- plexes 1 and 2, their TGA were performed. The TGA curves of complexes 1 and 2 are shown in Fig. 4. For 1, the weight loss from room temperature to 105 °C is 19.98% (calculated 20.12%), which corresponds to the loss of lattice water and coordinated water molecules. Then the network began to decompose totally at 410 °C, and the residual is CuO. Complex 2 displayed the first weight loss of 16.69% at the temperature of 90 °C (calculated 17.17%), which was due to the departure of coordinated water molecules, and then it kept stable until 225 °C. When the temperature was above 395 °C, the framework collapsed totally.
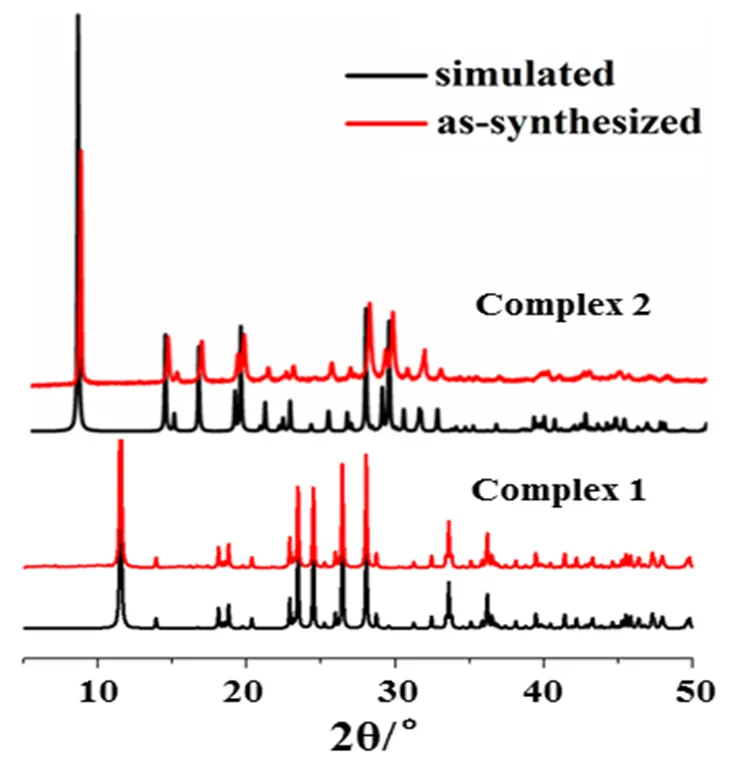
Fig. 3. Powder X-ray diffraction patterns of complexes 1 and 2
Fig. 4. TGA curves for complexes 1 and 2
3. 5 Selective oxidation of alkylbenzenes catalyzed by complexes 1 and 2
The oxidation of C?H bonds of alkylbenzenes, for instance, is a powerful tool to generate high value chemical feedstock from less expensive raw mate- rials such as alkyl aromatics, and great efforts have been made in exploring new catalysts[17]. In this regard, the means to convert benzylic hydrocarbons into valuable compounds have received considerable attention in recent years[18]. Herein, we report the oxidation of alkylbenzenes with TBHP as the oxidant using two copper complexes as catalysts.
To investigate the effectiveness of complexes 1 and 2 in the oxidation of alkylbenzenes, the oxide- tion of diphenylmethane was first examined as a standard substrate with 70% TBHP inbenzonitrile at 80 oC for 24 h (Scheme 1). The conversion and selectivity of each reaction were summarized and illustrated in Fig. 5. After the preliminary optimiza- tion, we noted that complex 2 was active and more selective for the oxidation of diphenylmethane than other catalysts tested.
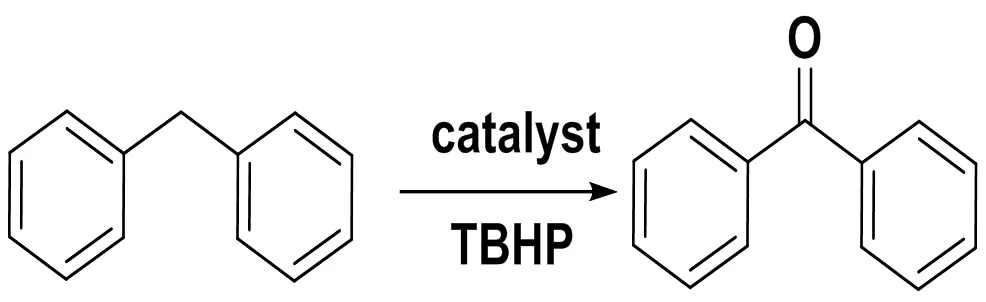
Scheme 1. Oxidation of diphenylmethane to benzophenone with TBHP
As the excellent performance, complex2was selected to examine the long-term stability in a heterogeneous system. After completion of the oxidation reaction, the catalyst can easily be separa- ted from the reaction mixture by filtration. The recovered catalyst was re-activated by washing with methanol and further reused directly in the subsequent oxidation reactions. The experiment results displayed that after three runs no obvious loss of activity (Conv. 96.2% (1st), 96.0% (2nd), 95.7% (3rd)) was observed (Fig. 5). The PXRD spectra of 2 collected before and after the catalytic reactions indicatedthat the structure was maintained under turnover conditions(Fig. 6).
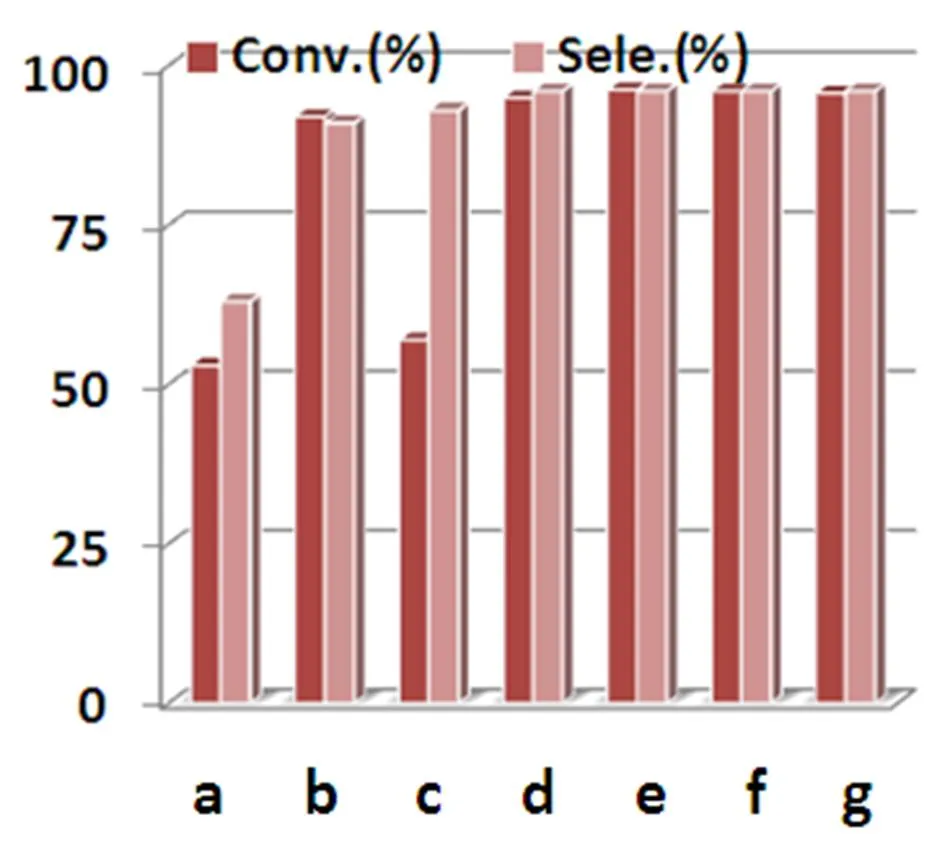
Fig. 5. Conversion of diphenylmethane to benzophenone with different catalysts. Reaction condtions: diphenylmethane (0.25 mmol), catalysts (5 mol%), 353 K, TBHP (0.625 mmol), benzonitrile (2 mL), 24 h. a) Blank; b) CuCl2(10 mol%);c) complex 1 (10 mol%); d) complex 2 (5 mol%); e) 1strun; f) 2ndrun; g) 3rdrun
Fig. 6. PXRD spectra of comlpex 2 after three runs of catalytic cycles
The mild reaction conditions, excellent stability, and high yield for the transformation of diphenyl- methane to benzophenone prompted us to extend the scope of 2 as heterogeneous catalyst for other benzylic hydrocarbons. As shown in Table 3, complex 2 exhibits excellent catalytic activity for oxidation of diphenylmethane, 9H-xanthenes, fluo- rene and derivates of fluorene to the corresponding aryl ketones with up to 97.6% yield (Table 3, entries 1-5). By comparison with previous reports, we found that 2 outperformed some heterogeneous catalysts, such as polyoxometalates[19], MOFs[20]in the oxidation of diphenylmethane, fluorene and 9H-xanthene (Table 3).
4 CONCLUSION
In summary, by controlling the reaction con-ditions, we have synthesized twopyrazole-3-car- boxylic acidcomplexes 1 and 2. The introduction of Cu2+cations make 1 and 2 more stable and can be used as heterogeneous catalysts in the selective oxidation of alkylbenzenes with high catalytic activities. Specifically, complex2 can convert alkylbenzenes to corresponding aromatic ketones efficiently and can be reused by filtration without the loss of its activity. Investigations on the use of these complexes for other potential catalytic reac-tions are in progress.
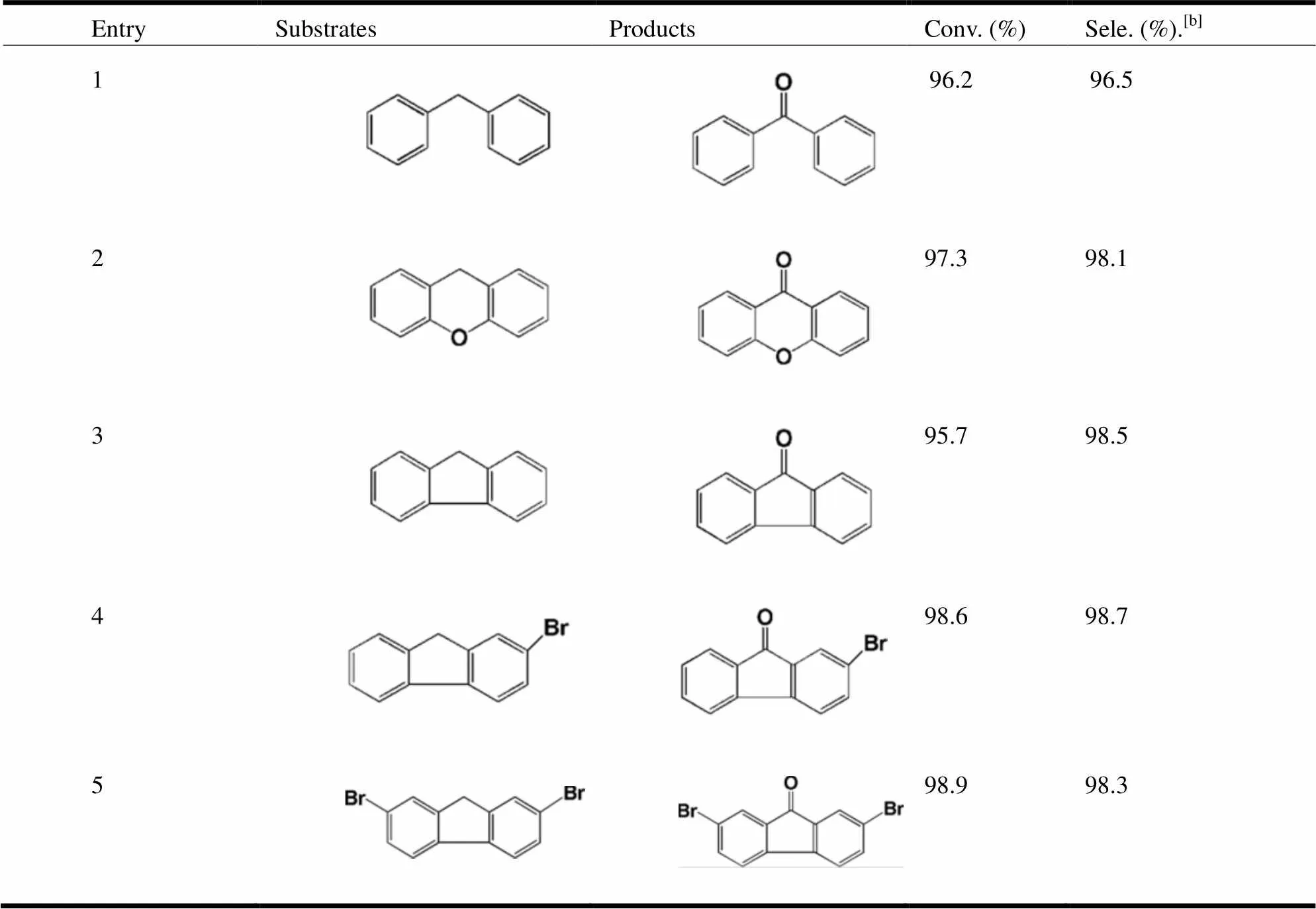
Table 3. Results of Selective Oxidation of Benylic Compounds Catalyzed by Complex 2 Using TBHP Oxidant[a]
[a] Reaction conditions: substrates (0.25 mmol), complex 2 (5 mol%), TBHP (0.625 mmol), benzonitrile (2 mL), 80 oC, 24 h.
[b] Selectivity to ketones was analyzed by GC using the naphthalene as internal standard.
The by-products are corresponding alcohols, which were analyzed by GC-MS.
(1) (a) Zhang, J. P.; Zhang, Y. B.; Lin, J. B.; Chen, X. M. Metal azolate frameworks: from crystal engineering to functional materials.2012, 112, 1001-1033; (b) Jiang, X. Y.; Rong, N. X.; Wang, G. D.; Cui, C. S.; Huang, X. Q.An imidazole-functionalized dioxovanadium complex with the highly selective oxidation of sulphides.2017, 36, 429-437.
(2) (a) Yin, Y.; Tan, Z.; Hu, L.; Yu, S.; Liu, J.; Jiang, G.Isotope tracers to study the environmental fate and bioaccumulation of metal-containing engineered nanoparticles: techniques and applications.2017, 117, 4462-4487; (b) Han, C. B.; Wang, Y. L.; Liu, Q. Y.Crystal structure and magnetic properties of a dinuclear terbium compound Tb2(2-anthc)4(anthc)2(1,10-phen)2.2017, 36, 705-710.
(3) Paskevicius, M.; Jepsen, L. H.; Schouwink, P.; ?erny, R; Ravnsb?k, D. B.; Filinchuk, Y.; Dornheim, M.; Besenbacher, F.; Jensen T. R.Metal borohydrides and derivatives – synthesis, structure and properties.2017, 46, 1565-1634.
(4) Zhao, Y.; Li, Z.; Sharma, U. K.; Sharma, N.; Song, G.; Eycken, E. V. V. Copper-catalyzed alkylarylation of activated alkenes using isocyanides as the alkyl source:an efficient radical access to 3,3-dialkylated oxindoles.2016, 52, 6395-6398
(5) ?ili?, D.; Rakvin, B.; Mili?, D.; Paji?, D.; ?ilovi?, I. Crystal structures and magnetic properties of a set of dihalo-bridged oxalamidato copper(II) dimers.2014, 43, 11877-11887.
(6) Teong, S. P.; Yu, D.; Sum, Y. N.; Zhang, Y. Copper catalysed alkynylation of tertiary amines with CaC2sp3C–H activation.2016, 18, 3499-3502.
(7) Tirsoaga, A.; Cojocaru, B.; Teodorescu, C.; Vasiliu, F.; Grecu, M. N.; Ghica, D.; Parvulescu, V. I.; Garcia, H. C–N cross-coupling on supported copper catalysts: the effect of the support, oxidation state, base and solvent.2016, 341, 205-220.
(8) Niu, M.; Li, Z.; Li, X.; Huang, X.Two chiral alkanolamine Schiff base Cu(II) complexes as potential anticancer agents: synthesis, structure, DNA/protein interactions, and cytotoxic activity.2016, 6, 98171-98179.
(9) He, J.; Yin, Y. G.; Wu, T.; Li, D.; Huang, X. C. Design and solvothermal synthesis of luminescent copper(I)-pyrazolate coordination oligomer and polymer frameworks.2006, 2845-2847.
(10) Fernandes, T. A.; Santos, C. I. M.; Andre?, V.; K?ak, J.; Kirillova, M. V.; Kirillov, A. M. Copper(II) coordination polymers self-assembled from aminoalcohols and pyromellitic acid: highly active precatalysts for the mild water-promoted oxidation of alkanes.2016, 55, 125-135.
(11) Artetxe, B.; Reinoso, S.; Felices, L. S.; Vitoria, P.; Pache, A.; Martín-Caballero, J.; Gutie?rrez-Zorrilla, J. M. Functionalization of krebs-type polyoxometalates with N,O-chelating ligands: a systematic study.2015, 54, 241-245.
(12) López-Viserasa, M. E.; Fernández, B.; Hilfiker, S.; González, C. S.; González, J. L.; Calahorro, A. J.; Colacio, E.; Rodríguez-Diéguez, A. In vivo potential antidiabetic activity of a novel zinc coordination compound based on 3-carboxy-pyrazole.2014, 131, 64-67.
(13) Liu, G. N.; Zhu, W. J.; Chu, Y. N.; Li, C. C. Three10metal coordination compounds based on pyrazole-3-carboxylic acid showing mixed-ligand characteristic: syntheses, crystal structures, and photoluminescent properties.2015, 425, 28-35.
(14) Sheldrick, G. M.. University of G?ttingen, Germany 1997.
(15) Brese, N. E.; O’Keeffe, M. Bond-valence parameters for solids.1991, 47, 192-197.
(16) Li, B.; Zhao, J. W.; Zheng, S. T.; Yang, G. Y. Hydrothermal synthesis and structure of di-copper(II)-complex substituted monovacant polyoxotungstate with a 1D chain structure.2008, 11, 1288-1291.
(17) Chen, H.; Deng, Y.; Yu, Z.; Zhao, H.; Yao, Q.; Zou, X.; Ba?ckvall, J. E.; Sun, J. 3D Open-framework vanadoborate as a highly effective heterogeneous pre-catalyst for the oxidation of alkylbenzenes.2013, 25, 5031?5036.
(18) Shi, D.; Ren, Y.; Jiang, H.; Lu, J.; Cheng, X. A new three-dimensional metal-organic framework constructed from 9,10-anthracene dibenzoate and Cd(II) as a highly active heterogeneous catalyst for oxidation of alkylbenzenes.2013, 42, 484-491.
(19) Yang, X. L.; Xie, M. H.; Zou, C.; He, Y.; Chen, B.; O’Keeffe, M.; Wu, C. D. Porous metalloporphyrinic frameworks constructed from metal 5,10,15,20-tetrakis(3,5-biscarboxylphenyl)porphyrin for highly efficient and selective catalyticoxidation of alkylbenzenes.2012, 134, 10638-10645.
(20) He,Q. T.; Li, X. P.; Chen, L. F.; Zhang, L.; Wang, W.; Su, C. Y. Nanosized coordination cages incorporating multiple Cu(I) reactive sites: host-guest modulated catalytic activity.2013, 3, 1-9.
23 May 2017;
12 October 2017 (CCDC 1055760 for 1 and 1055762 for 2)
10.14102/j.cnki.0254-5861.2011-1731
①This project was supported by the NNSFC (Nos. 21401094 and 21501086), the Project of Shandong Province Higher Educational Science and Technology Program (No. J16LC53), and the College Students' Science and Technology Innovation Fund (No. CXCY2017028 and CXCY2017037)
②E-mails: hxq@lcu.edu.cnand yaoqx_666@163.com
- 結構化學的其它文章
- Synthesis, Crystal Structure and Photoluminescent Property of a New Zn(II) Complex Based on 3,4-Bis(2-pyridyl)-5-(4-pyridyl)-1,2,4-triazole①
- Transitional Area of Ce4+ to Ce3+ in SmxCayCe1-x-yO2-δ with Various Doping and Oxygen Vacancy Concentrations: A GGA + U Study①
- A New Dinuclear Zinc Polymer Based on 3-Methoxy-2-hydroxybenzaldehyde:Synthesis, Structure, Spectral Characterization and Hirshfeld Surface Analysis①
- Fabrication of WO3/TiO2 Heterostructures for Efficiently Photocatalytic Gaseous Hydrocarbons Degradation: Origin of Photoactivity and Revisit the Role of WO3 Decoration①
- Synthesis, Crystal Structure and Cytotoxic Activities of Oxazolidin-2-one Derivatives①
- Crystal Structures, Thermal Behaviors and Biological Activities of Acylhydrazone Compounds Containing Pyrazine Rings and Halogen Atoms①

