The Separation of Catechol from Carbofuran Phenol by Extractive Distillation*
ZHANG Jianyu (張建宇), HU Aixi (胡艾希)**, WANG Yu (王宇), XIAO Xuhui (肖旭輝), GUO Jiabin (郭家斌) and LUO Xianfu (羅先福)
?
The Separation of Catechol from Carbofuran Phenol by Extractive Distillation*
ZHANG Jianyu (張建宇)1,2, HU Aixi (胡艾希)1,**, WANG Yu (王宇)1,2, XIAO Xuhui (肖旭輝)2, GUO Jiabin (郭家斌)1and LUO Xianfu (羅先福)1
1College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082, China2Hunan Haili Chemical Industry Co., Ltd, Changsha 410007, China
In this study, extractive distillation has been applied to separate catechol (CAT) from carbofuran phenol (CFP) with high purity and yield. The relative volatility of CFP to CAT was measured, and the choice of separating agents was investigated. The experimental results indicated that CFP/CAT is an azeotropic system with an azeotropic point at 93.40°C/0.400 kPa and an azeotropic mixture containing 49.96% of CFP and 50.04% of CAT. Data from the determination of the relative volatility have shown that separating agents such as diglycol and 4-butylcatechol (4-TBC) are able to increase the relative volatility up to 1.90. In one shot process batch extractive distillation of CFP mixture with 3% (by mass) diglycol as separating agent, the purity and yield of the obtained CFP was 99.0% and 95.0%, respectively, while the distillation without separating agent provided a purity and yield of only 98.0% and 90.0%, respectively. There was no residual separating agent found in the product.
carbofuran phenol, catechol, azeotrope, extractive distillation
1 INTRODUCTION
With the rapid development of fine chemical industry, the application of special distillation methods has attracted considerable interest. The most common used special distillation methods include azeotropic distillation, extractive distillation, steam distillation, and salt distillation [1]. Among these methods, extractive distillation is defined as a distillation in the presence of a miscible, high boiling, relatively non-volatilesolvent, which forms no azeotrope with the components in the mixture. It has been applied for the distillation of the mixtures with no or small difference in volatility for the components [2-6]. In general, these mixtures cannot be well separated by simple distillation because of the similar volatility of the components in the mixture. However, once a certain solvent is added to the mixture, the solvent will interact with the components in the mixture leading to a change of the relative volatility of the components, and will enable the separation of the components in the mixture to be achieved by normal distillation.
Carbofuran phenol (CFP), 2,3-dihydro-2,2- dimethyl-7-benzofuranol, which is an important intermediate for the preparation of carbamates, such as carbofuran, benfuracarb, carbosulfan, and furathiocarb, is most often prepared from catechol (CAT)[7-10]. The synthetic route can be described as follows:
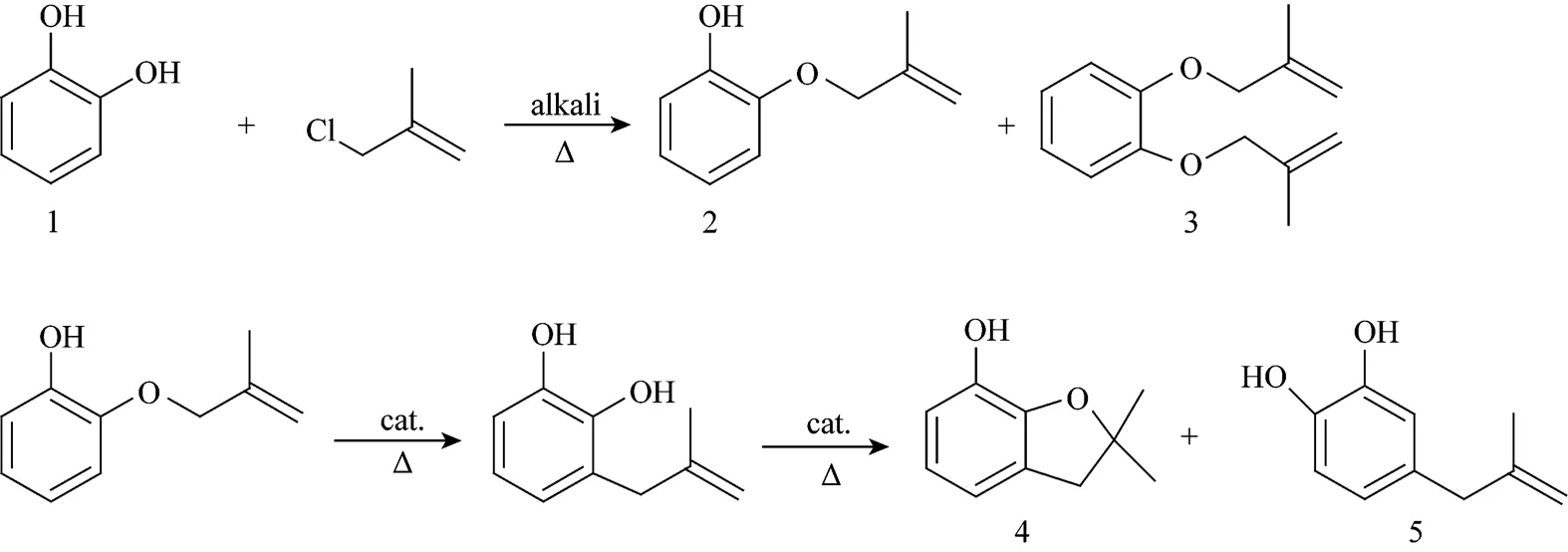
To prepare CFP through the above synthetic route,there are three main steps including etherification, rearrangement & cyclization, and purification. Following is a general description of the synthetic process.
In this step, CAT was dissolved in a solvent at a temperature of 80-120°C, and then methallyl chloride (MAC) was added to this solution dropwise in the presence of the alkali. CAT (1) reacted with MAC to give 2-methallyoxyphenol (2) (.. monoether) as the main product and-dimethallyoxybenzene (3) (.. diether) as the by product.
Product (2) obtained from the etherification step was dissolved in a solvent at a temperature of 160-250°C to carry the Claisen rearrangement and cyclization reaction to form CFP (4) in the presence of acidic catalyst. 4-Methylallyl-1,2-dihydroxybenzene was the main by product, which appeared from the Cope rearrangement followed by Claisen rearrangement of product (2) [11].
Owing to the presence of 2%-3% CAT in the reaction mixture, the product purity can be only 98% with a content of CAT up to 1.5% through simple vacuum distillation. Moreover, the presence of CAT in the reaction mixture causes a lower efficiency.
It is recognized that the purity and yield are very crucial for the production of CFP; however, there is no report for other purification method except simple vacuum distillation. In this study, one shot process batch extractive distillation was performed on the reaction mixture of CFP, and the target was to select a suitable separation agent for this type of distillation, which is chemically inert, not corrosive to reactors, and can be easily separated from distillation residues.
2 EXPERIMENTAL
2.1 Materials and instrumentation
Distillation raw material (CFP 49.66%, CAT 3.39%, and xylene 2.2%) was kindly offered by Hunan Haili Chemical Industry Co., Ltd; CFP was purchased from FMC US (Contents 99.2%); CAT was purchased from Rhodia Company France (Contents 99.5%). Glycol, diglycol, 4-butylcatechol, and 3, 4′-dichlorodiphenyl ether (DCDPE) were of analytical grade and purchased from Tian Hong Chemical Company, China. All chemicals were used without further treatment.
A rectifying tower with an inner diameter of 40 mm, a height of 700 mm, and glass springs as filler was used for the vacuum distillation. Analyses were performed on an American High-Tech instrument (High-Tech, America) using a mixture of solvents (55% of methanol, 44% of water and 1% HOAc) as the eluting solvent at a flow of 1 ml·min-1through a C8column (4.6 mm×150 mm) equipped with an Alltech UV detector. The wavelength of the detector was set at 280 nm, and the external standard method was used.
2.2 Procedure
2.2.1
A 250 ml distillation flask equipped with a condenser was charged with CFP (0-150 g) and CAT (150-0 g). This mixture was heated up after connecting to the vacuum system. Once the system pressure and temperature were stable between 0.133-0.500 kPa and between 75-130°C respectively, the mixture was steadily refluxed for another 2 h, and then the boiling point and the vacuum value of the system were recorded. In the meanwhile, the samples (several drops) from vapor phase condenser and the distillation flask were taken for analysis.
2.2.2
A 500-ml distillation flask equipped with a condenser was charged with 100 g of CFP, certain amount of CAT (3-100 g), and the separating agents (3-100 g). This mixture was heated up after connecting to the vacuum system. Once the system pressure and temperature were stable between 0.133-0.500 kPa and between 80-130°C respectively, the mixture was steadily refluxed for another 2 h, and then the boiling point and the vacuum value of the system were recorded. Meanwhile, the samples (several drops) from vapor phase condenser and the distillation flask were taken for analysis.
The relative volatility of CFP (4) to CAT (1) is calculated using Eq. (1):

where41is the relative volatility of CFP to CAT;4is the mass fraction of CFP in liquid phase; and4is the mass fraction of CFP in gas phase.
2.2.3
In this study, one shot process bath extractive distillation method was adopted. The rectifying tower coupler and condenser were connected to a 1000-ml distilling flask. 500 g of distillation raw material and certain amount of separating agent (0-25 g) were added to the flask, and then this mixture was heated up after connecting to the vacuum system. After steadily refluxing for 0.5 h, the solvent of xylene, front cut, the product cut, and the latter cut were collected in order. The recorded parameters of the system should be stable as follows: top pressure in the range of 0.500-8.000 kPa, top temperature in the range of 60-80°C, bottom temperature in the range of 100-120°C, reflux ratio (1-2)︰1 when xylene is collected; and top pressure in the range of 0.133-0.500 kPa, top temperature in the range of 80-130°C, bottom temperature in the range of 120-160°C, and reflux ratio (3-5)︰1 when product cuts are collected. All cuts were weighed and the samples were analyzed.
3 RESULTS AND DISCUSSION
3.1 Influence of catechol on purification of carbofuran phenol
In the etherification process, because of the competing reaction between monoether and diether, it is difficult to obtain a high conversion of CAT with high selectivity of monoether [12-14]. In general, to have a high selectivity of monoether, a high mole ratio of CAT to MAC (for example 1.2︰1) should be used, and the excessive CAT in the reaction mixture must be separated by repetitious neutralization or extraction leading to a long proceeding with a large amount of wastage. In industry, the CAT conversion rate is kept between 96% and 97%, and about 3% CAT is left in the purification raw material of the crude product CFP.
In the purification process, CFP was distilled out, and its purity was affected seriously by the excess CAT from etherification because of the small difference in the boiling points between CAT and CFP. In most of the cases, the CFP product can be obtained only with a purity of 98.0%, which is not up to the quality requirement (99.0%). In addition, the operation parameters were not consistent; the distillation time was longer while the yield was lower.
The boiling points of CFP and CAT are shown in Fig. 1, and the--curves of the VLE of CFP and CAT under a pressure of 0.400 kPa are shown in Fig. 2.
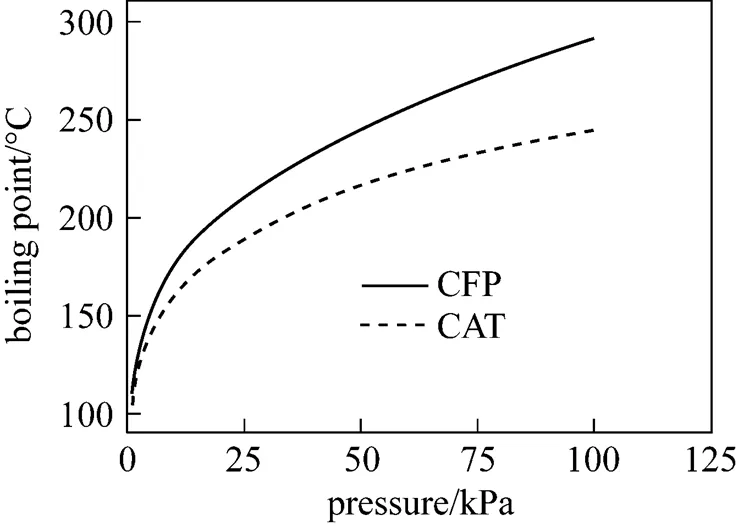
Figure 1 Boiling points of CFP and CAT at different pressures
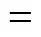
As seen in Fig. 1, the boiling point of CAT is lower than that of CFP under the same pressure. However, the difference is only 3-4°C at the actual operation pressure 0.3 kPa, therefore, it is difficult to separate CAT from CFP in industry with a high efficiency.
As seen in Fig. 2, CFP/CAT is an azeotropic system; the azeotropic point is 93.40°C/0.400 kPa, the azeotropic mixture contents are 49.96% of CFP and 50.04% of CAT, and the calculated relative volatility of CFP to CAT is 1.00.
It is understandable why the product cut continuously contents CAT, and the contents of CAT were gradually increased from the beginning to the end of distillation. Thus, it is not possible using conventional methods such as increasing reflux ratio to improve the product purity. In addition, the mixture of CFP and CAT is viscous with a high boiling point, and CFP is heat-sensitive, and a high vacuum condition is required. To meet all the requirements for the separation of CAT from CFP, extractive distillation should be a promising method.
3.2 Effect of by-products on extractive distillation and the selection of separating agents
The data in Section 3.1 indicate that the boiling point of CAT is lower than that of CFP at the same pressure. In general, CAT should appear first in a normal vacuum distillation; however, it was surprising to find that the concentration of CAT in the distillation of the mixture of CAT and CFP was higher in later period cuts. To understand this “abnormal observation”, LC-MS analysis was performed on the feeding material of distillation. In the feeding material, the main chemicals were CFP (about 50%), CAT (about 3%), and xylene (about 2%). Other compounds were also present as minor part. Following are the structures of these compounds:
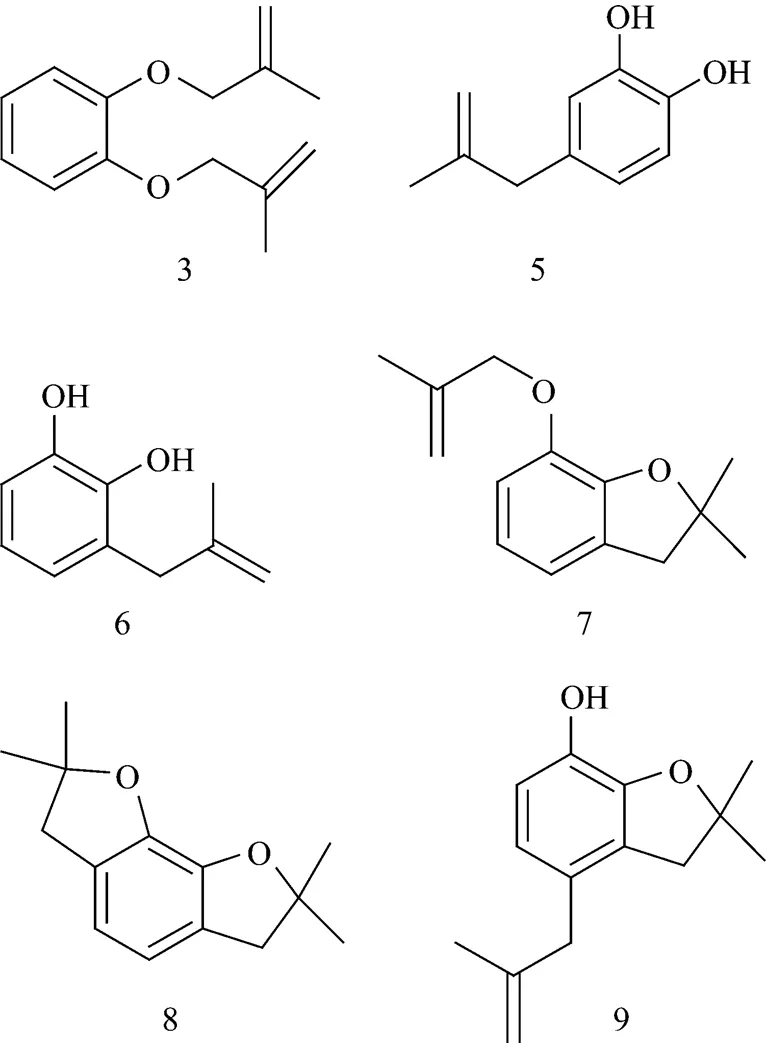

Catechol has two hydroxyls but CFP has a hydroxyl and an ether linkage. The molecular volume of CAT is smaller than that of CFP; therefore, these compounds will form the intermolecular hydrogen bonds with CAT while there are no so strong interactions between these compounds and CFP. These interactions caused the increase of the relative volatility of CFP to CAT, and well answered the question why CAT, whose boiling point is lower, has a high concentrate in the later period cuts in the CFP rectifying process.

3.3 Relative volatility
The effects of glycol, diglycol, 4-TBC, and DCDPE as separating agents on the relative volatility of CFP to CAT at different mass ratios of CFP/CAT and CFP/ separating agent, in which the mass ratio of CFP/CAT equals to that of CFP/separating agent in the same batch, were investigated, and the data are shown in Table 1 and Fig. 3.
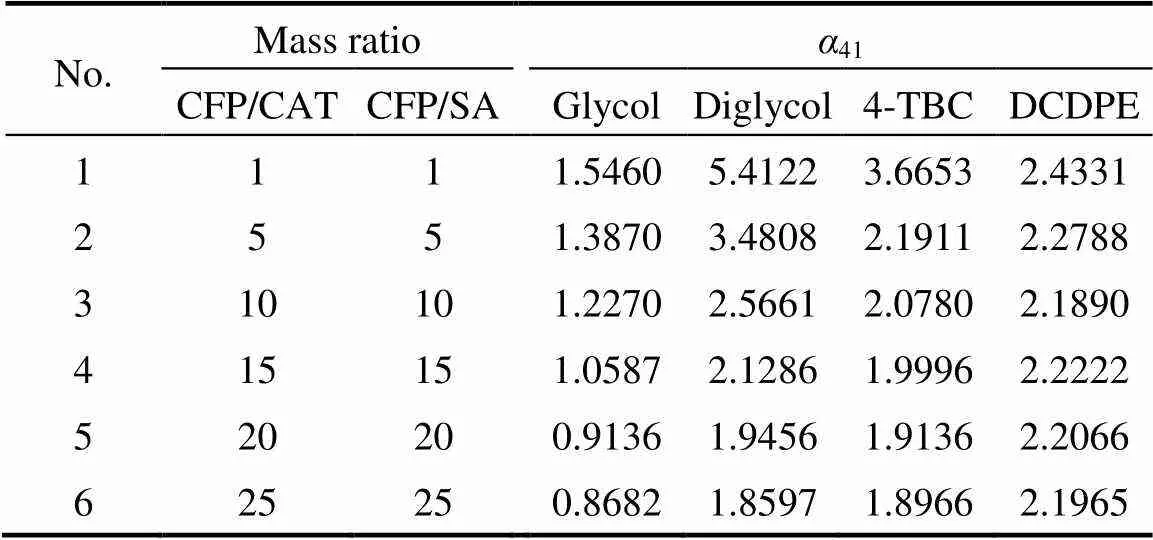
Table 1 Relative volatilities of CFP to CAT (α41) by adding selected separating agents (SA)
It is obvious that41is greater than 1.90 after adding separating agent such as diglycol and 4-TBC. The boiling point of CFP becomes lower than that of CAT owing to the extractive distillation effect of the selected separating agents. Thus, it can be realized that a high-purity CFP will be collected through the full separation of distillation tower with the addition of separating agent.
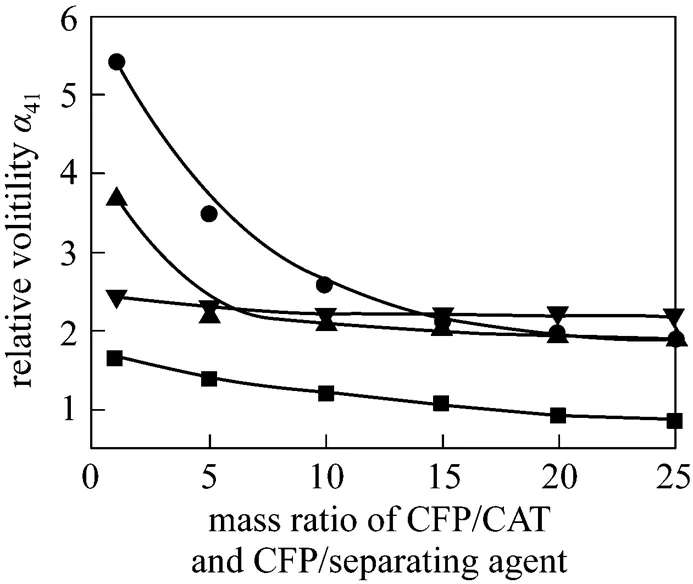
Figure 3 Relative volatility of CFP to CAT
■?glycol; ●?diglycol; ▲?4-TBC; ▼?DCDPE
3.4 Extractive distillation of carbofuran phenol
Extractive distillation experiments were carried on with the addition of separating agent at different mass ratios. The experimental data for the addition of diglycol as separating agent are listed in Table 2.
The data in Table 2 indicate that the purity of CFP can be achieved only in the range of 98.0%-98.5% and the yield in single process is around 66.6% when there is no added separating agent. After adding separating agent, the purity of CFP product can be obtained up to 99.5% and the yield in single process is up to 80.0%.
Experimental data from HPLC analysis of the obtainedproduct indicate that the mass content of separating agent in product is only 0.0025%, and there is no effect on the product quality. In addition, the amount of the separating agent and CAT in the residue is small and the separating agent used in this experimental is cheap, therefore, there is no need to recycle the separating agent.
Comparing to the conventional distillation, the product purity is increased about 1.0% while the yield is increased by 5.2%. The distillation operating time is reduced 25% by applying one shot process batch extractivedistillation technology in the CFP production process with a small amount of diglycol as 3% (by mass).

Table 2 Results of the extractive distillation of CFP

4 CONCLUSIONS
It has been found that the carbofuran phenol/ catechol system is an azeotropic mixture. In the rectification of carbofuran phenol production, catechol with lower boiling point has a high concentration in later period cuts of the carbofuran phenol distillation owing to the weak hydrogen bonding of between by-products and catechol in distillation raw material. To obtain an efficient distillation of a mixture of carbofuran phenol/catechol, the addition of separating agent(s) is required.
1 Lei, Z.G., Li, C.Y., Chen, B.H., “Extractive distillation: A review”,..., 32 (2), 121-213 (2003).
2 Lei, Z.G., Li, C.Y., Chen, B.H., “Behaviour of tributylamine as entrainer for the separation of water and acetic acid with reactive extractive distillation”,...., 11 (5), 515-519 (2003).
3 Hua, C., Li, X.G., Xu, S.M., Bai, P., “Design and operation of batch extractive distillation with two reboilers”,...., 15 (2), 286-290 (2007).
4 Kuipers, N.J.M., Wentinkl, A.E., De Haan, A.B., Scholtz, J., Mulder, H., “Functionalized solvents for olefin isomer purification by reactive extractive distillation”,,:...., 85, 88-99 (2007).
5 Chen, B.H., Lei, Z.G., Li, J.W., “Separation on aromatics and non-aromatics by extractive distillation with NMP”,...., 36 (1), 20-24 (2003).
6 Chen, B.H., Lei, Z.G., Li, Q.S., Li, C.Y., “Application of CAMD in separating hydrocarbons by extractive distillation”,., 51 (12), 3114-3121 (2005).
7 Scharpf, W.G., “Pesticidal carbamates of dihydrobenzofuranols”, U.S.Pat., 3474170 (1969).
8 Richard, F.F.B., “Synthesis of 2,2-dimethyl-7-benzofuranol”, U.S.Pat., 3320286 (1967).
9 Towns, D.L., “Synthesis of 2, 3-dihydro-2, 2-dimethyl-7-benzofuranol”,U.S.Pat., 3419579 (1968).
10 Start, J.F., Towns, D.L., “Synthesis of 2, 2-dimethyl-7-benzofuranol”,U.S.Pat., 3723472 (1973).
11 Guo, J.B., Zhang, J.Y., Hu, A.X., “Separation of the main by-product from 2-(2-methylallyoxy)phenol and its identification”,, 47 (2), 92-93 (2008). (in Chinese)
12 Michel, R., “Process for the selective monoetherification of pyrocatechol”, U.S.Pat., 4250333 (1981).
13 Franko-Filipasic, B., Snyder, J., “Selective removal and recovery of catechol mixed with 2-methallyloxyphenol”, U.S.Pat., 4420642 (1983).
14 Franko-Filipasic, B., Snyder, J., “Single solvent process for preparing 2-methallyloxyphenol”, U.S.Pat., 4851587 (1989).
2008-06-05,
2008-08-26.
the National High Technology Research and Development Program of China (2006AA03Z460).
** To whom correspondence should be addressed. E-mail: axhu0731@yahoo.com.cn
 Chinese Journal of Chemical Engineering2009年1期
Chinese Journal of Chemical Engineering2009年1期
- Chinese Journal of Chemical Engineering的其它文章
- An Improved Fuzzy Predictive Control Algorithm and Its Application to an Industrial CSTR Process*
- Preparation, Characterization and Catalytic Behavior of 12-Molybdophosphoric Acid Encapsulated in the Supercage of Cs+-exchanged Y Zeolite*
- Purification of Sulfuric and Hydriodic Acids Phases in the Iodine-sulfur Process*
- Synthesis and Characterization of Novel Temperature and pH Responsive Hydroxylpropyl Cellulose-based Graft Copolymers
- Electrochemical Performance of Nickel Hydroxide/Activated Carbon Supercapacitors Using a Modified Polyvinyl Alcohol Based Alkaline Polymer Electrolyte*
- Liquid Film Characteristics on Surface of Structured Packing*
