Global trends in diabetic eye disease research from 2012 to 2021
Yuan Yuan ,Shangli Ji ,Yali Song ,Zhaodi Che ,Lu Xiao ,Shibo Tang,,Jia Xiao,,
Abstract Diabetic eye disease refers to a group of eye complications that occur in diabetic patients and include diabetic retinopathy,diabetic macular edema,diabetic cataracts,and diabetic glaucoma.However,the global epidemiology of these conditions has not been well characterized.In this study,we collected information on diabetic eye disease-related research grants from seven representative countries––the United States,China,Japan,the United Kingdom,Spain,Germany,and France––by searching for all global diabetic eye disease journal articles in the Web of Science and PubMed databases,all global registered clinical trials in the ClinicalTrials database,and new drugs approved by the United States,China,Japan,and EU agencies from 2012 to 2021.During this time period,diabetic retinopathy accounted for the vast majority (89.53%) of the 2288 government research grants that were funded to investigate diabetic eye disease,followed by diabetic macular edema (9.27%).The United States granted the most research funding for diabetic eye disease out of the seven countries assessed.The research objectives of grants focusing on diabetic retinopathy and diabetic macular edema differed by country.Additionally,the United States was dominant in terms of research output,publishing 17.53% of global papers about diabetic eye disease and receiving 22.58% of total citations.The United States and the United Kingdom led international collaborations in research into diabetic eye disease.Of the 415 clinical trials that we identified,diabetic macular edema was the major disease that was targeted for drug development (58.19%).Approximately half of the trials(49.13%) pertained to angiogenesis.However,few drugs were approved for ophthalmic (40 out of 1830;2.19%) and diabetic eye disease (3 out of 1830;0.02%) applications.Our findings show that basic and translational research related to diabetic eye disease in the past decade has not been highly active,and has yielded few new treatment methods and newly approved drugs.
Key Words:clinical trials;diabetic cataracts;diabetic eye disease;diabetic glaucoma;diabetic macular edema;diabetic retinopathy;drug development;global research;publication;research grant
Introduction
According to estimates from the International Diabetes Federation in 2021,approximately 537 million adults between the ages of 20 and 79 years worldwide have been diagnosed with diabetes mellitus.This number is expected to increase to 643 million by 2030 and 783 million by 2045 (Sun et al.,2022).Diabetic eye disease (DED) refers a group of eye complications that affect patients with diabetes (Hanineva et al.,2022;So et al.,2022).These mainly include diabetic retinopathy(DR),diabetic macular edema (DME),diabetic cataracts(DC),and diabetic glaucoma (DG),as defined by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health.In 2020,an estimated 103.12 million adults worldwide had DR,representing 22.27% of all adults with diabetes (Teo et al.,2021).Importantly,DR was the only major cause of blindness with a global increase in prevalence from 1990 to 2020 (an increase of 14.9% per 1000 persons),compared with other major eye diseases such as undercorrected refractive error,cataract,age-related macular degeneration,and glaucoma (GBD 2019 Blindness and Vision Impairment Collaborators,2021).The prevalence of DR varies across different regions of the world,with the highest rates in Africa (35.90%) and North America and the Caribbean(33.30%),while the rates are lower in South and Central America (13.37%) and Southeast Asia (16.99%) (Teo et al.,2021).DME can occur at any stage of DR and is characterized by an accumulation of excess fluid in the retinal macular area(Varma et al.,2014).It is the leading cause of vision loss in patients with type 2 diabetes (Romero-Aroca,2011).In the United States,DME affects more than 3.8% of adults aged 40 and above with diabetes,based on 2008 data (Varma et al.,2014;Lundeen et al.,2022).Persistently high blood glucose levels in diabetic patients can cause structural changes to the lens of the eye,accelerating the development of cataracts,also known as diabetic cataracts.Patients with diabetes are up to five times more likely to develop cataracts than individuals without diabetes,particularly at an earlier age (Saxena et al.,2004).Data on the prevalence of DC in the general and diabetic populations are limited.In the United Kingdom,the incidence of cataracts was 20.4 per 1000 person-years in patients with diabetes and 10.8 per 1000 person-years in the general population in 2015 (Becker et al.,2018).Diabetes can cause an obstruction in aqueous outflow and elevate intraocular pressure,which can eventually lead to primary open-angle glaucoma.When patients with diabetes also have blood circulation disorders,this can result in decreased blood flow to the eyes,causing optic nerve damage in glaucoma and normal tension glaucoma.Hyperglycemia can also cause swelling in the lens that can increase intraocular pressure and cause secondary angle-closure glaucoma.The most severe form of glaucoma caused by DR is neovascular glaucoma.Patients with diabetes are twice as likely to develop glaucoma,especially open-angle glaucoma,compared with non-diabetic individuals (Li et al.,2021).The prevalence of glaucoma in patients with diabetes ranges from 4.96% to 14.6%.However,the association between diabetes and DG is significantly influenced by geographic distribution and other factors such as race,age,and sex (Zheng et al.,2010;Zhao and Chen,2017).
Overall,therapeutic options for DED are limited.Managing blood glucose levels and diabetic progression are crucial at all stages of DED (Stitt et al.,2016).Vascular endothelial growth factor (VEGF) inhibitors,including faricimab-svoa,ranibizumab,aflibercept,and bevacizumab,can help inhibit the growth of new blood vessels and decrease fluid buildup in the context of advanced DR and DME.Photocoagulation (or panretinal photocoagulation) can also be used in conjunction with VEGF inhibitors (Wong et al.,2018).Nevertheless,future retinal damage and vision loss are still possible despite drug or laser therapy slowing or halting the progress of DR/DME because diabetes is a chronic disease.Most types of cataracts,including DC,are preferentially treated with phacoemulsification.However,patients with diabetes have been reported to experience a higher rate of complications(e.g.,poorer vision outcomes) after phacoemulsification cataract surgery than non-diabetic (Pollreisz and Schmidt-Erfurth,2010).For patients with diabetes and primary openangle glaucoma,intraocular pressure (IOP)-lowering drugs(e.g.,prostaglandin analogs),laser,and surgery can be used,as for non-diabetic patients (Tang et al.,2023b).Owing to the rapidly increasing global prevalence of diabetes,a higher DED disease burden is expected in the coming decades.Effective,novel Food and Drug Administration (FDA)-approved drugs and therapies are urgently needed for the management of patients with DED.Developing new medications/treatments depends heavily on rigorous basic and clinical research designed to identify novel disease mechanisms and treatment targets,as well as to generate data on the efficacy and safety of new therapies.Thus,comprehensively analyzing global trends in basic and clinical research into DED is essential to devise evidence-based strategies for future research and therapy development.In this study,we analyzed trends(research grants,publications (from the Web of Science and PubMed databases),clinical trials,and newly approved drugs)in DED research from 2012 to 2021 at a global level.
Methods
Data collection
We collected data on the number of funded projects and total funding amounts for DED-related projects from online information systems: The United States (National Institutes of Health,the USA-NIH): https://reporter.nih.gov/,China(National Natural Science Foundation of China,NSFC): https://kd.nsfc.gov.cn/fundingProjectInit,Japan (Grants-in-Aid for Scientific Research,KAKENHI): https://kaken.nii.ac.jp/,the United Kingdom (Research Councils UK,RCUK): https://gtr.ukri.org/,Spain (El Instituto de Salud Carlos III,ISCIII):https://portalfis.isciii.es/es/Paginas/inicio.aspx,Germany(German Research Foundation,DFG): https://gepris.dfg.de/gepris/OCTOPUS?language=en,and France (French National Research Agency,ANR): https://anr.fr/en/funded-projectsand-impact/funded-projects/.We searched project titles using the keywords “diabetic retinopathy,” “diabetic macular edema,” “diabetic cataracts,” and “diabetic glaucoma”(accessed December 13–15,2022).To assess DED-related journal publications,we searched the Web of Science Core Collection (https://apps.webofknowledge.com/) using the same keywords for the time period spanning 2012–2021(accessed December 15,2022).In addition,we downloaded papers related to DED from the PubMed-MeSH database(https://www.ncbi.nlm.nih.gov/mesh) using the same keywords (accessed December 17,2022).We eliminated duplicate papers and re-retrieved the remaining papers from the Web of Science database.For clinical trial information,we searched the ClinicalTrials website (https://clinicaltrials.gov/)using the keywords “diabetic retinopathy,” “diabetic macular edema,” “diabetic cataracts,” and “diabetic glaucoma” in the“condition or disease” tab (accessed December 17,2022).We collected newly approved drug information from the Food and Drug Administration (FDA) for the United States (https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm),the National Medical Products Administration (NMPA) for China (https://www.cde.org.cn/hymlj/index), the Pharmaceuticals and Medical Devices Agency (PMDA) for Japan (https://www.pmda.go.jp/english/review-services/reviews/approved-information/drugs/0002.html), and the European Medicines Agency(EMA) for the European Union (https://www.ema.europa.eu/en/medicines/download-medicine-data#european-publicassessment-reports-(epar)-section) (accessed December 22,2022). We included only new medicinal products and orphan medicinal products, and excluded biosimilar and generic drug information. The authors YY and YS independently analyzed all information on grants, journal papers, clinical trials, and new drugs. Their analyses were later verified by the authors SJ and JX.
Disease and research coding
All research grants, journal papers, and clinical trials were manually assigned a disease code (DR, DME, DC, or DG). If a grant, paper, or trial described two diseases, we labeled it with two codes and counted it as 0.5 for each disease in the statistical analysis. We also manually assigned a research code to grants and journal papers to indicate whether they involved basic research (mechanistic study, therapeutic study, novel animal model study, and bioengineering study)or clinical research (epidemiological study, diagnostic study,observational study, or interventional study). If a grant or paper involved both types of research, we assigned it both codes and counted it as 0.5 for each type of study in the statistical analysis.
Calculation of citations per article, relative citation impact,and closeness centrality of journal papers
The frequency of citations per article is a relative index that can eliminate differences in country sizes and can better reflect the quality or impact of scientific research. The formula used to calculate this value is as follows: the cited frequency of papers in a country (or a research direction) = the cited frequency of papers in a country (or a research direction)/the number of papers in a country (or a research direction).
Relative citation impact (RCI) refers to the ratio of the cited frequency of papers published by the country to the cited frequency of papers published by the world (country) (Grover et al., 2021), and it is used to measure the quality or impact of a country in a specified field. The formula used to calculate this value is as follows:
where Cijrefers to the citation frequency of papers of country i in the discipline field j; Pijrefers to the number of papers of country i in the discipline field j; WCjrefers to the citation frequency of the world’s papers in the discipline field j; and WPjrepresents the number of papers in the world in the subject area j. If the RCI index value is greater than 1, this indicates that the quality or impact of papers in a given field from that country is higher than the average quality or impact of papers in that field from around the world. When the RCI is equal to 1, this means that the quality or impact of papers from that country in that field is equivalent to that of papers from around the world in that same field. Conversely,if the RCI value is less than 1, this indicates that the quality or impact of papers from that country in that field is lower than the average quality or impact of papers from around the world in that same field.
Closeness centrality describes the control power and influence of nodes in a network (Diallo et al., 2016). The closeness centrality of a node measures its average farness (inverse distance) to all other nodes. Nodes with a high closeness score have a short distance to all other nodes. The “absolute overall centrality” of a point is the sum of the shortcut distances between the point and all other points in the graph, and is expressed as:
where dijis the shortcut distance between point i and point j, and n is the total number of network nodes. To eliminate the impact of network size on the overall centrality value of points and enhance the comparability of the overall centrality value of network points of different sizes, we introduced the concept of “relative overall centrality.” Because the absolute overall centrality of the core points of the “star” network can reach the minimal valuen–1, the “absolute overall centrality”can be divided by the minimal overall centrality to obtain the“relative overall centrality” (standardization of the overall centrality), which is expressed as:
wheredijis the shortcut distance between pointiand pointj, andnis the total number of network nodes. The overall centrality value in this study defaults to “relative overall centrality.”
Statistical analysis
Only descriptive analyses were conducted in this study.
Results
DR accounts for the vast majority of global DED research grants
From 2012 to 2021, a total of 2288 research grants for DED were awarded by the governments of the seven representative countries (the United States, China, Japan, the United Kingdom, Spain, Germany, and France). DR accounted for the vast majority of global DED grants (2048.5 out of 2288;89.53%), followed by DME (212 out of 2288; 9.27%). DC- and DG-related grants accounted for only very small percentages of overall DED grants (0.9 out of 100 (0.90%) and 0.31 out of 100 (0.31%), respectively). The number and amount of global DED research funds remained relatively stable in this period,at 203 to 245 grants per year and 87.70 to 103.35 million USD per year. The USA-NIH approved 1748 DED grants with a total value of 901.86 million USD, which exceeded the sum of other countries: China approved 264 grants worth 16.07 million USD, Japan approved 161 grants worth 6.34 million USD, the United Kingdom approved 45 grants worth 31.19 million USD,Spain approved 34 grants worth 4.09 million USD, Germany
approved 26 grants,and France approved eight grants worth 4.18 million USD.This indicated that the United States made the largest investment in this research field during this time period.Only the United States and China funded DC-related grants (12.5 and 8,respectively),and only the United States funded DG-related grants (seven) (Figure 1).
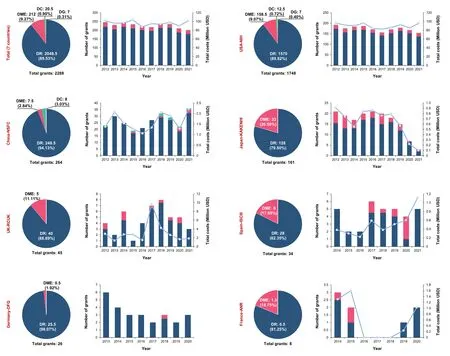
Figure 1 |Proportions of research grants focusing on the four types of diabetic eye disease (DED)diseases,namely diabetic retinopathy (DR),diabetic macular edema (DME),diabetic cataracts (DC),and diabetic glaucoma (DG),funded by the governments of global representative countries,and trends in funding,from 2012 to 2021.
The research objectives of DR and DME grants vary by country
Next,we analyzed the research objectives of all DR-and DMErelated grants.DC-and DG-related grants were not included because there were too few.For DR-related grants,the United States,China,and Japan approved more basic studies than clinical studies,while in European countries (the United Kingdom,Germany,Spain,and France),the numbers of basic and clinical studies were similar (49vs.51;Figure 2A).In the United States,the NIH approved 786 mechanistic studies of DR from 2012 to 2021,followed by 325.5 studies of DR diagnosis and 179 DR clinical observational studies.In China,the NSFC encourages basic/mechanistic studies instead of clinical studies,and thus most of the approved DR grants were for studies that proposed to address molecular mechanisms(64.59%),followed by therapeutic studies conducted in animals (19.32%).Japan approved DR studies in a similar ratio,with mechanistic studies accounting for 57.42% of all DR-related grants,followed by clinical interventional studies(15.23%) and therapeutic studies in animals (10.94%).In European countries,mechanistic DR studies still accounted for the largest proportion of all DR-related grants (37.50%),followed by clinical diagnostic studies (28.50%) and clinical observational studies (12.50%).Of note,the ratio of clinical studies was higher for DME-related grants than that for DRrelated grants (77.5 basic studiesversus81 clinical studies for NIH-approved DME grants;32 basic studies versus 21.5 clinical studies for DME grants approved by the other six countries).Mechanistic studies and clinical interventional studies accounted for more than half of the total DME-related grants(Figure 2B).
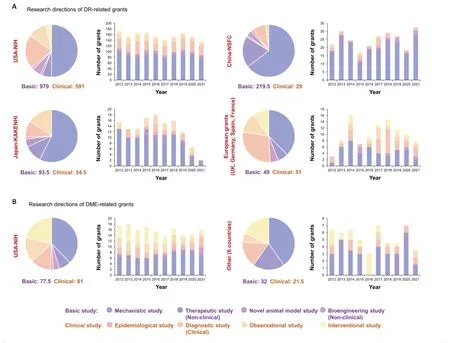
Figure 2 |Proportion of research grants for basic (mechanistic study,non-clinical therapeutic study,novel animal model study,and non-clinical bioengineering study) and clinical (epidemiological study,clinical diagnostic study,observational study,and interventional study) studies addressing (A) diabetic retinopathy (DR) and (B) diabetic macular edema (DME) funded by the governments of global representative countries,and trends in funding,from 2012 to 2021.
DR and clinical observational studies account for the majority of journal papers addressing DED
We analyzed 4697 DED journal papers published between 2012 and 2021 retrieved from the Web of Science and PubMed databases.The number of published papers addressing DED increased steadily from 2012 (241 papers)to 2021 (843 papers),reflecting a growing interest in DED research globally.DR was the most commonly studied disease,accounting for 3342 papers (71.15%),followed by DME(1058;22.52%),DC (169;3.60%),and DG (128;2.73%;Figure 3A).Of these 4697 DED journal papers,3746 were research articles (79.75%),and 951 were review articles (20.25%).DR accounted was the main topic of 2629.5 research articles(70.20% of all research articles),followed by 908.5 DME research articles (24.25%),159 DC research articles (4.24%),and 49 DG research articles (1.31%).The ratio of disease topics addressed in review articles was similar,while the ratio of reviews that addressed DG was higher than that of research articles that focused on DG (8.31%vs.1.31%).Analyzing DED research article type showed that the number of clinical research papers far exceeded the number of basic research papers (2693vs.783 papers).Although DR was the major topic of both basic and clinical research papers,DME was the focus of more clinical papers than basic papers (892vs.17.5;30.10%vs.2.23%;Figure 3B).The majority of DR,DME,and DG research articles were clinical observational studies,whereas therapeutic studies in animals accounted for nearly half of the DC research papers (Figure 3C).
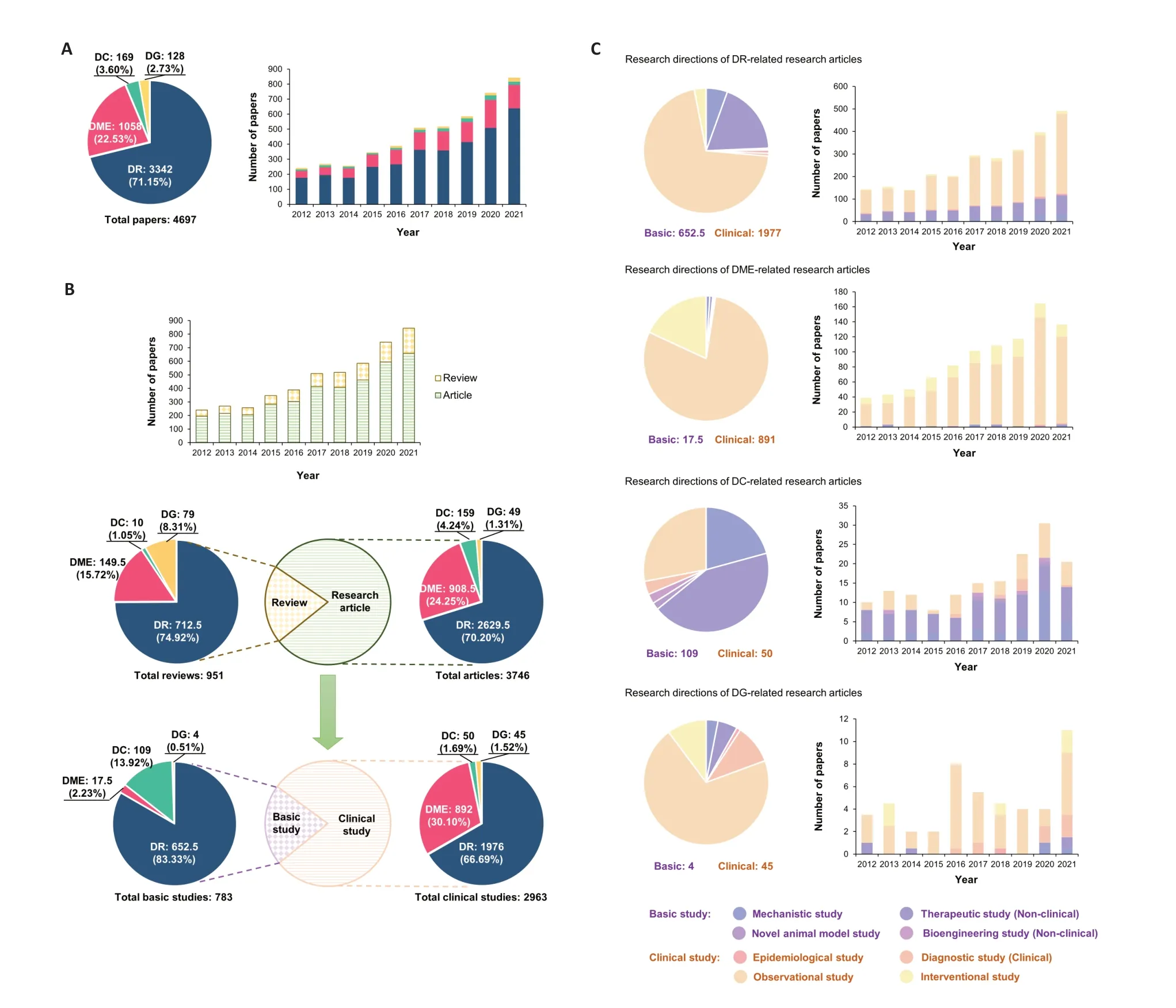
Figure 3 |Research trends of 4697 journal papers reporting on four types of diabetic eye disease (DED),namely diabetic retinopathy (DR),diabetic macular edema (DME),diabetic cataracts (DC),and diabetic glaucoma (DG),listed in the Web of Science and PubMed databases (2012–2021).
The United States dominates research output related to DED
From 2012 to 2021,China and the United States published 19.55% and 17.53% of global DED journal papers,respectively.Other countries (the United Kingdom,India,Japan,Korea,Italy,and Australia) published only 3.41% to 5.95%.DR remained the dominant paper topic for all countries except Italy (Figure 4A).From 2012 to 2021,published DED journal papers from the United States received 22.58% of the citations to all DED papers published globally,far exceeding China(11.55%),the United Kingdom (7.95%),Singapore (5.57%),Italy (5.47%),and Australia (5.23%).Other countries received less than 5% of citations (Figure 4B).Papers published from the Netherlands had the highest average citation frequency(110.76 per paper),followed by South Africa (102.88),Thailand (96.81),Singapore (70.60),Denmark (62.92),Norway (57.83),and Germany (57.18) (Figure 4C).The top 10 countries and the average citation frequency per paper for each DED are listed inTable 1.The Netherlands (135.09),Norway (277.00),Switzerland (44.00),and Mexico (865.00)had the highest average citation frequencies per paper for DR,DME,DC,and DG,respectively.Next,we calculated RCI values to determine the relative position of a country’s papers in a certain discipline worldwide.Nineteen countries had RCI values >1.DED papers from the Netherlands had the highest RCI value (3.61),followed by Ethiopia,South Africa,Thailand,Singapore,Grenada,and Denmark (Figure 4D).

Table 1 |Top 10 countries of average citation frequency per paper of each diabetic eye disease [diabetic retinopathy (DR),diabetic macular edema (DME),diabeticcataract (DC),and diabetic glaucoma (DG)] papers from 2012–2021
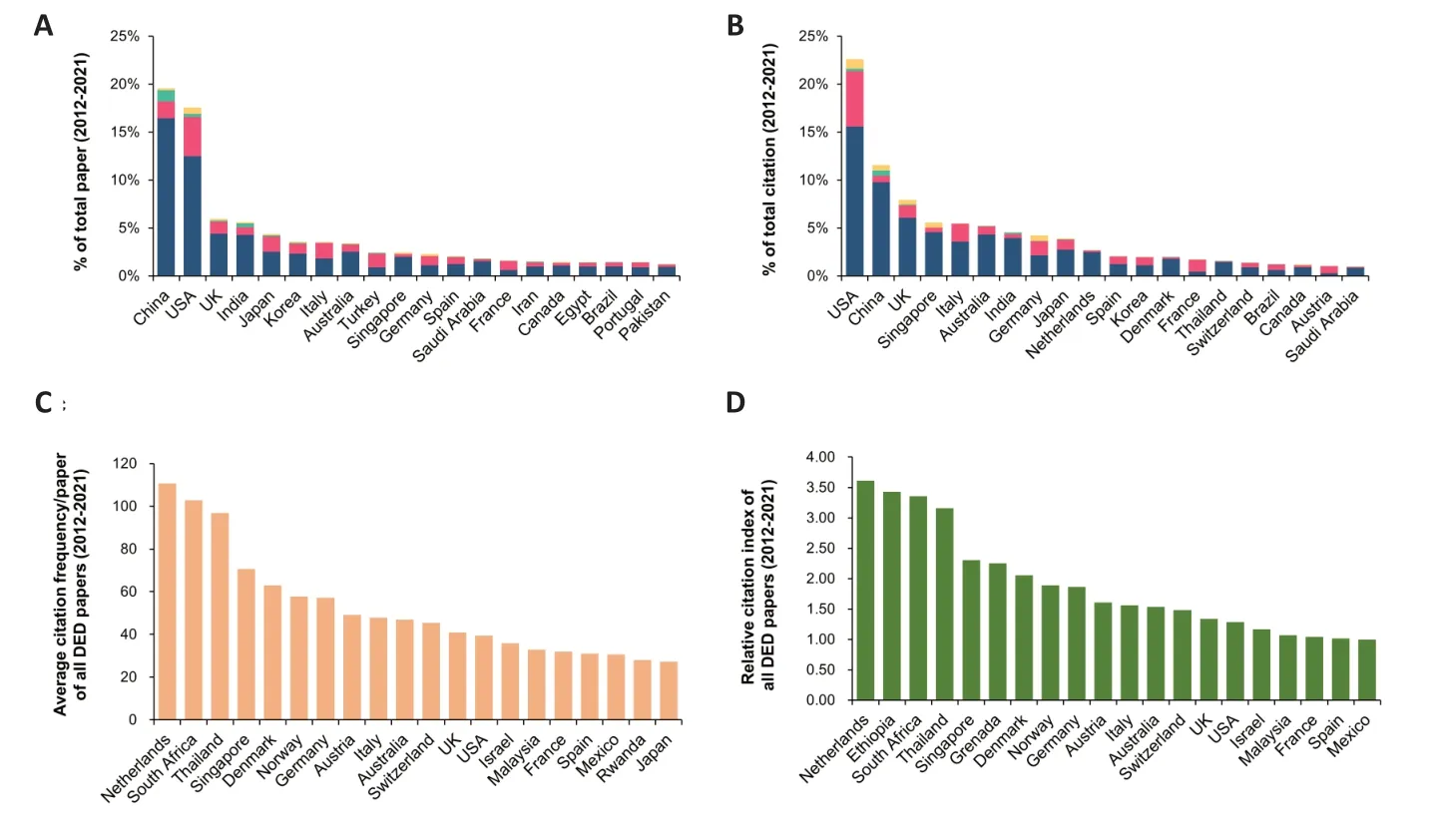
Figure 4 |Published journal papers addressing four types of diabetic eye diseases (DED),namely diabetic retinopathy (DR),diabetic macular edema (DME),diabetic cataracts (DC),and diabetic glaucoma (DG),listed in the Web of Science and PubMed databases.Research output of the top 20 countries,encompassing publication counts,proportion of papers addressing the different disease types,total citation counts,average citations per paper,and relative citation impact (RCI)(2012–2021).
The United States and the United Kingdom lead international collaborations in DED research
International collaboration plays an important role in development of science and technology.Thus,we quantitatively analyzed papers about DED generated by international cooperation in major countries based on bibliometric and cooperation network analyses.The United States and the United Kingdom were at the center of the international cooperation network constructed for all DED papers published from 2012 to 2021 and exhibited close collaborations with China,Germany,Australia,and India.Many other countries,including Singapore,Portugal,Canada,Brazil,France,South Africa,Spain,Italy,Saudi Arabia,Israel,and New Zealand,also closely participated in international DED research collaboration.The United States and the United Kingdom were also the leading countries in international collaboration in DR,DC,and DG research.For DME publications,the United States and Germany exhibited close collaborative relationships with Australia,India,France,Italy,and Spain (Figure 5).We also observed changes in the relative overall centrality ranking of each country overtime.As shown inTable 2andAdditional Table 1,compared with 2012,in 2021 the United Kingdom replaced the United States as the world’s top country in DED research.India and Australia maintained their rankings as third and fourth globally,respectively,while China rose from sixth to fifth place.Singapore’s ranking dropped significantly from fifth to ninth in the world during the same time period.

Table 2 |Degree and centrality of Top 25 countries in the international cooperation network in the field of diabetes eye disease in 2012 and 2021
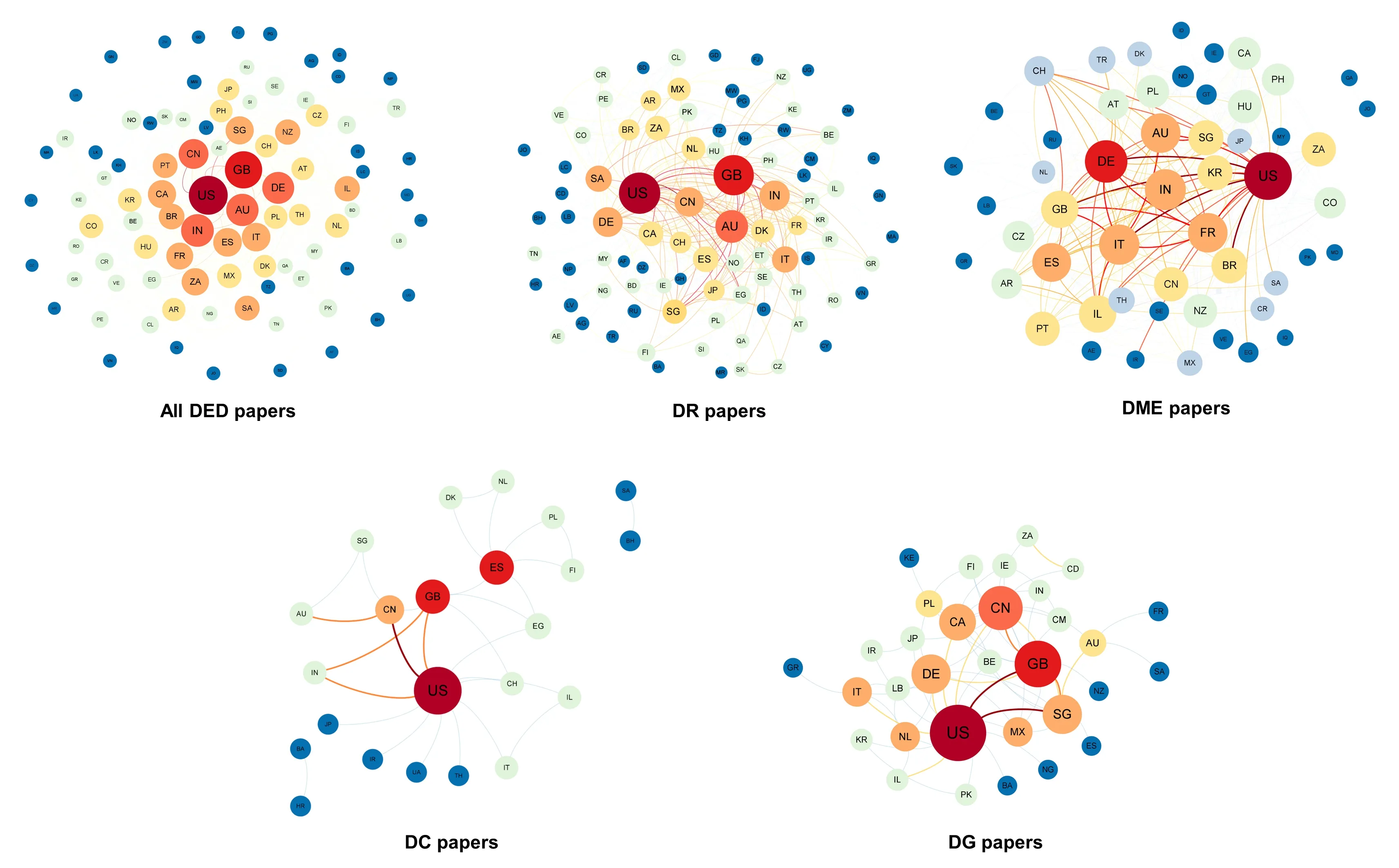
Figure 5 |International collaboration networks in the field of diabetic eye disease (DED) and its four disease types,namely diabetic retinopathy (DR),diabetic macular edema (DME),diabetic cataracts (DC),and diabetic glaucoma (DG),from 2012 to 2021 (based on all published papers listed in the Web of Science and PubMed database).
DME is the major topic of clinical trials investigating DED
The ultimate goal of drug and therapy development is to bring a novel compound or method with proven therapeutic efficacy to market.To analyze new drug and treatment development trends for DED,we identified all DED-related clinical trials registered between 2012 and 2021.A total of 415 clinical trials were conducted during this period,comprising 165 trials for DR (39.76%),241.5 trials for DME (58.19%),5.5 trials for dry eye disease (1.33%),and three trials for dysthyroid eye (DG) (0.72%).The number of annual studies did not change significantly from 2012 to 2021,ranging from 34 to 56 trials per year (Figure 6A).The US registered 157 trials during this time period,followed by China (51),Egypt (21),Switzerland (19),and Canada (14).North American (178),Asian (108),and European (87) countries conducted most of the DED clinical trials (Figure 6B).Of note,a large majority of those trials were either in phase 2 (104 trials;25.06%)or phase 4 (91 trials;21.93%).We observed only a slightly increasing trend in the number of phase 3 trials during this period,in terms of annual trial number (Figure 6C).Next, we assessed the mechanisms of the new drugs tested in the identified clinical trials.Angiogenesis inhibitors accounted for the vast majority,including 85 trials in total,while 65 trials tested VEGF inhibitors.Other drug categories included antiinflammatory drugs (41 trials),metabolic regulators (16 trials),neuroprotective drugs (11 trials),IOP-lowering drugs (nine trials),antioxidant drugs (seven trials),and anti-infectious drugs (four trials).Unfortunately,many of the above drugs were not new drugs,having already been approved by the FDA,and only a small number of newly developed drugs were tested (62 out of 182;34.07%;Figure 6D).
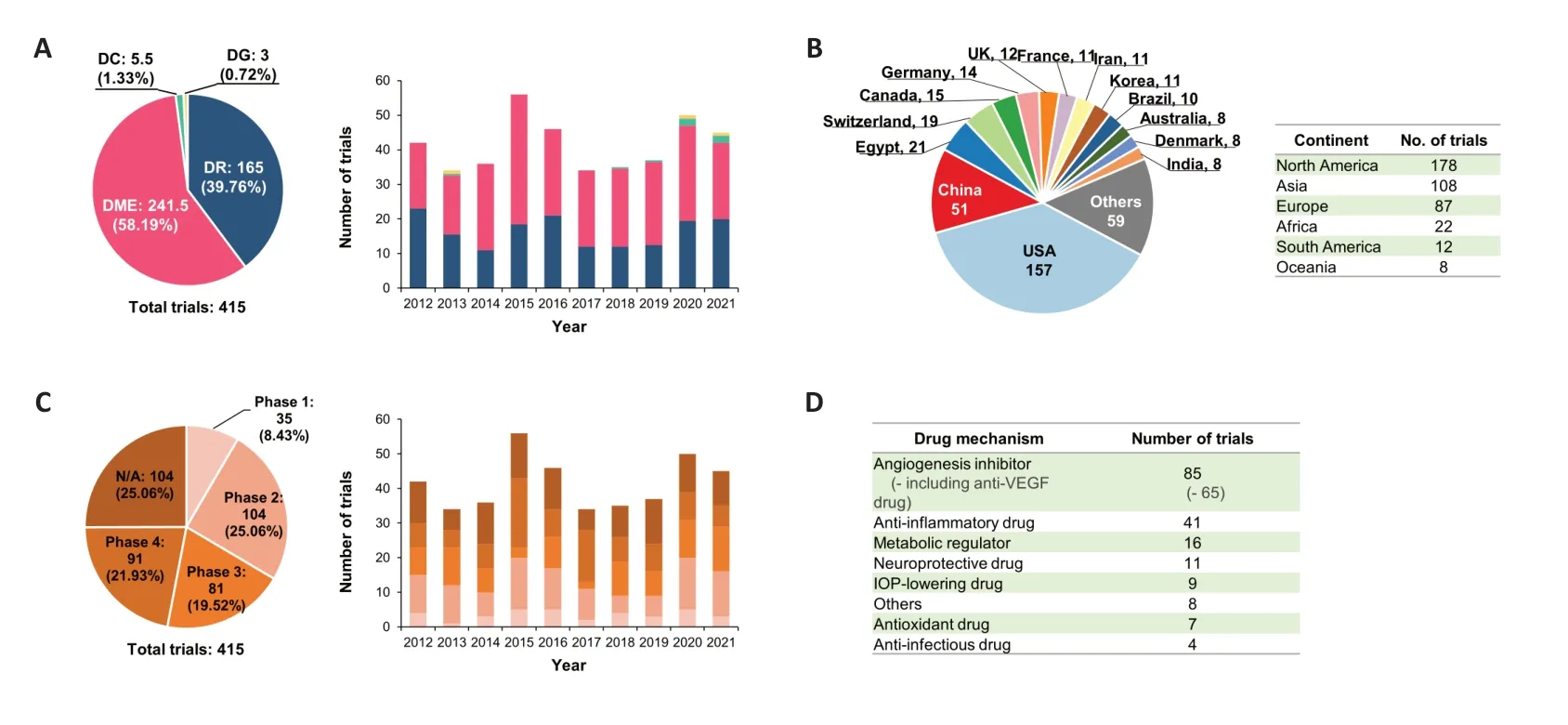
Figure 6 |Clinical trials of treatments for various types of diabetic eye disease (DED) diseases,including diabetic retinopathy (DR),diabetic macular edema (DME),diabetic cataracts (DC),and diabetic glaucoma (DG) (2012–2021).These data show the distribution and temporal trends of these diseases within clinical trials,ranked trials by country and continent,proportions of clinical trial phases,and drug statistics.
Few ophthalmic and DED drugs were newly approved for use
Increased DED diagnosis and disease burden have led to an increased need for novel ophthalmic disease treatments.From 2012 to 2021,the US FDA approved 353 new drugs,of which only five were for ophthalmic diseases (1.42% of all new drugs).During the same period (2012–2021),the National Medical Products Administration (NMPA,China),Pharmaceuticals and Medical Devices Agency (PMDA,Japan),and European Medicines Agency (EMA,European Union) approved 5,15,and 15 new drugs for eye diseases,accounting for 3.45%,2.49%,and 2.06% of each agency’s total drug approvals during the period,respectively (Figure 7A).Of the 40 new ophthalmic drugs approved by the above agencies during this time period,nearly half were for treating glaucoma/ocular hypertension (17 drugs;42.50%),followed by macular diseases (five drugs;12.50%).Of particular note,the EMA approved three new drugs (Eylea,Duloxetine Mylan,and Byooviz) for treating diabetic eye damage (Figure 7B).
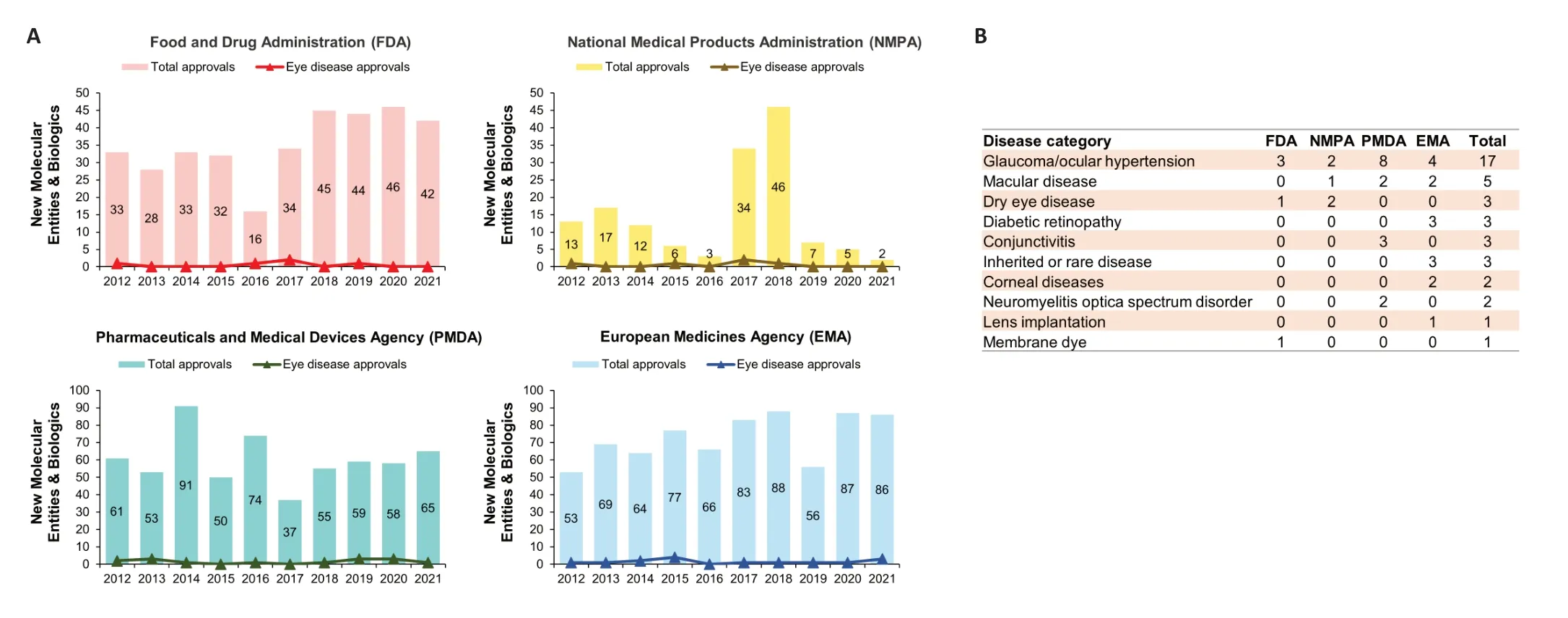
Figure 7 |Comparison of new drug approvals by the Food and Drug Administration (FDA),National Medical Products Administration (NMPA),Pharmaceuticals and Medical Devices Agency (PMDA),and European Medicines Agency (EMA).
Discussion
Our study provides a comprehensive and up-to-date evaluation of global DED research trends.It is the largest bibliometric analysis of DED research to date,encompassing the full course of the scientific research process from research funding to paper publication,clinical trial,and new drug approval.Our findings indicate that DR remained the primary area of interest for global scientists and clinicians in the preclinical stages,including research grants and journal publications,between 2012 and 2021 (Gardner and Chew,2016).The United States led the world in terms of research investment and publication output regarding DED during this time period,while China made significant progress in both grant funding and research output,becoming the secondlargest DED research funder globally,producing the highest number of published papers about DED,and garnering the second-largest number of paper citations in the world over the past decade.In addition,DME emerged as the main focus area of focus for DED clinical trials over the past decade.Although 53.01% of the 415 trials that were registered from 2012 to 2021 were successfully completed,only 7.50% (3 out of 40) of the new drugs that were tested were approved for the treatment of diabetic eye damage.
Currently,DR is the most common complication of diabetes(Oshitari,2022;Tang et al.,2023a).Its molecular pathogenesis is still not fully understood,and its treatment is challenging.Although VEGF inhibitors and other angiogenesis inhibitors have been used for decades to treat patients with DR,and demonstrate remarkable clinical benefits for many patients,a large number of patients still do not achieve clinically significant visual improvement (Wang and Lo,2018).Therefore,a deeper understanding of the pathophysiological processes underlying DR progression is needed to identify novel drug targets.This explains why more than half of the DR research grants funded over the past decade have been for mechanistic studies.To inhibit or reverse the progression of DR,not only in ophthalmological diseases,a large number of the grants and journal articles identified in out study focused on adipose tissue regulation,lipid/glucose re-balance,and organ crosstalk.Moreover,16 clinical trials tested the application of metabolic regulators (e.g.metformin,empagliflozin,and pemafibrate),which target systemic lipid/glucose metabolism.Although the number and funding amount of research grants from the major representative countries remained largely unchanged in the past decade,the number of global research papers on DED has drastically increased,by approximately 3.5 times,from 241 in 2012 to 843 in 2021.DED has gradually become a key focus of ophthalmology research.There are several possible explanations for this trend: (1) researchers and clinicians receiving good funding (e.g.company or other government funding types) to support their studies (Suresh,2012;Woelbert et al.,2021);(2) more efficient use of research funds;and (3) lack of high-impact papers in this field.Although DR was still the major disease type addressed in DED research articles,in contrast to research grants,the article topics shifted from mechanistic studies to clinical observational and interventional studies,implying that researchers are focusing more on translational research and clinical applications for DED-related diseases.
DME is mainly caused by destruction of the blood-retinal barrier (BRB) in the macular region,increased permeability of capillaries and microangiomas,and accumulation of extracellular fluid.DME can occur at any stage of DR,and is an important factor leading to visual impairment in patients with DR (Wong et al.,2016).The number of adults worldwide with clinically significant macular edema is predicted to rise to 28.61 million in 2045.Thus,early disease stages urgently need to be addressed,both in terms of public awareness and in the development of new treatments to supplement the currently available VEGF inhibitor therapy and laser photocoagulation(Teo et al.,2021).Unlike for research grants and published papers,DME was the major topic of clinical trials for DED from 2012 to 2021.This might be the result of a combination of factors.Compared with DR,DC,and DG,(1) the pathogenesis of DME is relatively clear (BRB damage);(2) DME symptoms are relatively distinctive (3) DME treatment efficacy is easy to observe (e.g.OCT and fundus photography);and (4) DME has a significant impact on patient vision (Das et al.,2015;Chakravarthy et al.,2018;Jampol et al.,2020).Of note,many drugs tested in these clinical trials were already on the market,with approval for indications other than DED (120 out of 182;65.93%).Therefore,increasing investment in basic and translational research is urgently needed in order to discover novel therapeutic targets and develop more new drugs for DED.
The global market for ophthalmic disease therapeutics is projected to grow from 35.57 billion USD in 2023 to 54.87 billion USD by 2030,for an overall annual growth rate of 6.40% during the forecast period (Insights,2023).However,we found that only three out of 40 new drugs (7.50%)approved by the FDA/NMPA/PMDA/EMA from 2012 to 2021 were for DR or other diabetic eye complications.Because retinal diseases have dominated the market in the past years,and the DR/DME patient population is growing,companies are investing more resources in new drug development,particularly VEGF inhibitors.For example,Genetech Inc.received FDA and EMA approval for faricimab for DME treatment in January and September 2022,respectively(Shirley,2022).Several new drugs currently in clinical trials are promising for DED treatment.One such drug is a novel αvβ3 inhibitor called OTT-166.This drug has completed a phase 1/2 randomized controlled trial (RCT) for the treatment of DME (NCT02914613).Additionally,a phase 2 RCT is currently underway to assess the safety and efficacy of OTT-166 for treating DR (NCT05409235).Another potential treatment for DR is eye drops containing somatostatin,which is a corticotropin-releasing hormone/growth hormone-releasing hormone inhibitor.A phase 3 clinical trial of this drug for the treatment of DR has been completed (NCT01726075).In subjects with proliferative DR,the administration of oral emixustat hydrochloride (a retinoid isomerohydrolase inhibitor) significantly improved central subfield thickness and total macular volume compared with the control group in a phase 2 RCT study (NCT02753400).Overall,these new drugs offer hope for the future treatment of DED and DR,and ongoing clinical trials will provide further insight into their effectiveness and safety.Aldose reductase inhibitors and antioxidants have been reported to alleviate DC and DG phenotypes in animal models (Pollreisz and Schmidt-Erfurth,2010;Snow et al.,2015;Himori et al.,2021);however,further preclinical studies and clinical trials are needed to demonstrate their suitability for clinical application.In terms of DR diagnosis,the FDA has recently approved a revolutionary EyeArt artificial intelligence system.This system is equipped with multiple cameras and is capable of autonomously detecting DR.The approval of this system is a significant development because it provides primary care clinics with an additional screening option for diabetic populations.
This study had some limitations.Given the retrospective nature of our study,we deliberately chose a specific time frame for analysis to focus our investigation.While this approach allowed us to examine a relevant and significant period,it inherently limited the breadth of our scope.Consequently,our findings may not provide a fully exhaustive representation of all DED research.The emphasis on highly cited papers could potentially lead to misunderstandings regarding influential research,overlooking emerging studies with promising insights that have yet to accumulate substantial citations.When discussing new drug development,our focus on traditional small-molecule drugs might not sufficiently encompass the contemporary pharmaceutical landscape,which is increasingly influenced by biologics and gene therapy approaches.Moreover,in the era of globalization,international collaboration has become pivotal for countries to foster scientific and technological advancements.While an increasing number of influential papers rely on successful international collaborations,it cannot be assumed that such collaborative papers are of higher research caliber than those originating from a single country.To thoroughly investigate this,it is imperative to conduct independent analyses observing the impact of papers resulting from collaborations between various nations.
Future studies could use enhanced data collection and analysis methods to encompass a broader range of sources and regions,thereby ensuring a more comprehensive representation of the global research landscape.Recognizing the intricate interplay between data,collaboration,funding,and research methodologies is essential to advancing our understanding of this field.
In conclusion,although the number of patients with diabetes and DED is rapidly increasing worldwide,the basic and translational research related to DED over the past decade has been somewhat lacking,with limited treatment methods explored and only a few new drugs approved.Because of the growing demand for DED research,the global scientific community needs to make a substantial commitment to basic and clinical research into DED,particularly DC and DG,through active academic investigation,global collaboration,and novel drug development.
Author contributions:ST and JX designed the study.YY,SJ,and JX collected all required data.YY,SJ,YS,ZC,LX,analyzed the data.YY,ST,and JX wrote and revised the manuscript.All authors approved the final version.ST and JX are the guarantors of this work and,as such,had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflicts of interest:No potential conflicts of interest relevant to this study were reported.
Data availability statement:All relevant data are within the paper and its Additional files.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional file:
Additional Table 1:Papers from the top 5 countries with the highest average citation frequencies among the top 10 countries for each diabetic eye condition from 2012 to 2021.
- 中國神經(jīng)再生研究(英文版)的其它文章
- Corrigendum: Sorl1 knockout inhibits expression of brain-derived neurotrophic factor:involvement in the development of late-onset Alzheimer’s disease
- p38 MAPK inhibitor SB202190 suppresses ferroptosis in the glutamate-induced retinal excitotoxicity glaucoma model
- Lycium barbarum glycopeptide (wolfberry extract)slows N-methyl-N-nitrosourea-induced degradation of photoreceptors
- Magnesium-L-threonate treats Alzheimer’s disease by modulating the microbiota-gut-brain axis
- 3′-Deoxyadenosin alleviates methamphetamineinduced aberrant synaptic plasticity and seeking behavior by inhibiting the NLRP3 inflammasome
- Small extracellular vesicles from hypoxiapreconditioned bone marrow mesenchymal stem cells attenuate spinal cord injury via miR-146a-5p-mediated regulation of macrophage polarization

