p38 MAPK inhibitor SB202190 suppresses ferroptosis in the glutamate-induced retinal excitotoxicity glaucoma model
Lemeng Feng ,Chao Wang ,Cheng ZhangWulong ZhangWeiming ZhuYe HeZhaohua XiaWeitao Song
Abstract Glutamate excitotoxicity has been shown to play an important role in glaucoma,and glutamate can induce ferroptosis.The p38 mitogenactivated protein kinase (MAPK) pathway inhibitor SB202190 has a potential ability to suppress ferroptosis,and its downstream targets,such as p53,have been shown to be associated with ferroptosis.However,whether ferroptosis also occurs in retinal ganglion cells in response to glutamate excitotoxicity and whether inhibition of ferroptosis reduces the loss of retinal ganglion cells induced by glutamate excitotoxicity remain unclear.This study investigated ferroptosis in a glutamate-induced glaucoma rat model and explored the effects and molecular mechanisms of SB202190 on retinal ganglion cells.A glutamate-induced excitotoxicity model in R28 cells and an N-methyl-D-aspartate-induced glaucoma model in rats were used.In vitro experiments showed that glutamate induced the accumulation of iron and lipid peroxide and morphological changes of mitochondria in R28 cells,and SB202190 inhibited these changes.Glutamate induced the levels of p-p38 MAPK/p38 MAPK and SAT1 and decreased the expression levels of ferritin light chain,SLC7A11,and GPX4.SB202190 inhibited the expression of iron death-related proteins induced by glutamate.In vivo experiments showed that SB202190 attenuated N-methyl-D-aspartate-induced damage to rat retinal ganglion cells and improved visual function.These results suggest that SB202190 can inhibit ferroptosis and protect retinal ganglion cells by regulating ferritin light chain,SAT1,and SLC7A11/Gpx4 pathways and may represent a potential retina protectant.
Key Words:ferroptosis;glaucoma;glutamate excitotoxicity;p38 MAPK;retinal ganglion cell;SB202190
Introduction
Glaucoma is a group of optic neuropathies characterized by progressive degeneration of retinal ganglion cells (RGCs)and the leading cause of irreversible blindness worldwide(Weinreb et al.,2014).An estimated 118 million people will be diagnosed with glaucoma worldwide by 2040 (Kang and Tanna,2021).While elevated intraocular pressure (IOP) has been traditionally associated with RGC loss,other factors also contribute to disease progression (Schuster et al.,2020;Susanna et al.,2015;Mohan et al.,2022).Glutamate excitotoxicity,first recognized in 1957,has garnered significant attention in glaucoma research (Lucas and Newhouse,1957).Glutamatergic excitotoxicity is involved in several neurodegenerative diseases,including glaucoma(Wang et al.,2019;Verma et al.,2022).Glutamate is the major excitatory neurotransmitter in the central nervous system(Thoreson and Witkovsky,1999;Kemp and McKernan,2002).Previous studies demonstrated that intraocular injection of glutamatergic substances is toxic to RGCs (Azuma et al.,1989;Siliprandi et al.,1992).Moreover,clinical studies revealed that vitreous glutamate concentrations were significantly elevated in primary open angle glaucoma (POAG) and primary angle closure glaucoma (PACG) groups compared with the cataract control group (Dreyer et al.,1996;Dreyer and Grosskreutz,1997;Vorwerk et al.,1999).N-methyl-D-aspartate (NMDA)receptors are abundantly distributed in the ganglion cell layer (GCL) of the retina and play an important role in visual pathway signal transduction (Rosini et al.,2019).Pathologically elevated glutamate overactivates NMDA receptors.NMDA receptor overactivation is related to calcium overload (Hartveit et al.,1994;Cull-Candy et al.,2001;Bai et al.,2013;Rosini et al.,2019).The consequent calcium influx activates several cell injury pathways including calpain activation,oxidative stress,and endoplasmic reticulum stress in the retina,and can eventually cause RGC damage (Awai et al.,2006;Maekawa et al.,2017).Thus,inhibition of glutamatergic excitotoxicity is one of the potential targets for combating glaucoma.
RGC loss is the common outcome in all types of glaucoma(Weinreb et al.,2014;Chen et al.,2022;Mueller-Buehl and Reinehr,2023;Shen et al.,2023).Thus,strategies to delay or halt RGC loss have been suggested as potentially beneficial to preserve eyesight in glaucoma (Almasieh et al.,2012).Several types of cell death,such as apoptosis,necroptosis,autophagy and pyroptosis,were shown to be involved in RGC loss in glaucoma (Chen et al.,2020;Li et al.,2022;Qin et al.,2022).Various potential drugs were proposed from these studies;however,they failed to effectively block RGC loss in the clinic.Currently there are no clinically effective drugs that target RGCs for protection.
Ferroptosis is a novel cell death modality that is morphologically,biologically,and genetically distinct from other types of cell death and characterized by irondependent lipid peroxidation (Dixon et al.,2012;Li et al.,2020;Yang et al.,2023).Many diseases have been shown to be associated with ferroptosis,such as stroke,Alzheimer’s disease and hepatocellular injury (Wang et al.,2017;Zille et al.,2017;Masaldan et al.,2019;Shan et al.,2020).In the acute ocular hypertension glaucoma model,retinal ischemiareperfusion induces ferroptosis in glaucomatous RGCs (Qin et al.,2022;Yao et al.,2022).Glutamate has been confirmed to be a classical inducer of ferroptosis (Li and Huang,2022).Proteomic analysis showed that ferroptosis also plays an important role in RGC loss in the NMDA glaucoma model(Suo et al.,2022).These studies suggest that ferroptosis is a potential therapeutic target for glaucoma.
p38 mitogen activated protein kinases (MAPKs) are a family of evolutionarily conserved serine/threonine protein kinases that respond to extracellular signals to regulate various cellular processes,including redox homeostasis (Ono and Han,2000;Kheiri et al.,2018).SB202190,a widely used p38 MAPK pathway inhibitor(English and Cobb,2002),was shown to exert anti-tumor effects and rescue memory deficits(Nemoto et al.,1998;Davies et al.,2000).Studies showed that the downstream targets of SB202190,such as p53,were associated with ferroptosis,which suggested that SB202190 may be a potential inhibitor of ferroptosis (Jiang et al.,2015;Li and Huang,2022).
In this study,we examined whether ferroptosis plays a role in a glutamate-induced glaucoma cell models and explored the potential effect and mechanism of SB202190 in protecting RGCs.
Methods
Experimental design In vivo experiments
SD male rats (250–260 g) were obtained from Hunan SJA Laboratory Animal Co.,Ltd.(Changsha,Hunan,China) (license No.SYXK (Xiang) 2020-0019).Before experiments,the animals were adaptively fed for one week under specific pathogenfree (SPF) feeding conditions of a 12-hour light/dark cycle at 21 ± 1°C.Food and water were available ad libitum.All the experiments were performed in accordance with the Association for Research in Vision and Ophthalmology (ARVO)Statement for the Use of Animals in Ophthalmic and Vision Research.All experimental procedures were approved by the Animal Ethics Committee of Xiangya Hospital of Central South University,China (approval No.202209019,approval date:September 9,2022).
The rats were randomized into three groups (10 rats per group): NMDA group (20 mM),NMDA+SB202190 group (20 mM NMDA [M3262,Sigma-Aldrich,St.Louis,MO,USA]+1 mM SB202190 [T2301,Topscience,Shanghai,China]) and control group (4 μL of sterile saline).Rats were anesthetized with pentobarbital (1%,80 mg/kg,intraperitoneal injection).Oxybuprocaine hydrochloride (Shentian Pharmaceutical Co.,Ltd.,Shanghai,China) and tropicamide phenylephrine(Shentian Pharmaceutical Co.,Ltd.) eye drops were used for ocular surface anesthesia and pupil dilation,respectively.A 34G needle was inserted into the vitreous cavity at the limbus.Drug treatments (4 μL) were injected into the vitreous cavity through the incision using a microsyringe (Hamilton,Reno,NV,USA).NMDA and SB202190 were given at the same time in the NMDA+SB202190 group.The control group received intravitreal injection with the same volume of saline.The entire operation was performed under a stereomicroscope.Animals were sacrificed 24 hours after intravitreal injection for subsequent analyses (retinal immunofluorescence staining,n=5;hematoxylin-eosin (H&E) staining,n=4,1 animal died from anesthetic overdose;in each group;Figure 1).
Figure 1 | Schematic of the experimental timelines of in vivo (top) and in vitro(bottom) modeling.
In vitro experiments
Rat retinal precursor (R28) cells (Cat# CVCL_5I35) were provided by Central South University (Changsha,China).R28 cells were maintained in T25 culture flasks with Dulbecco’s modified Eagle’s medium (DMEM) (Procell,Wuhan,China)containing 1 g/L glucose and supplemented with 10%FBS (Gibco,Grand Island,NY,USA) at 37°C with 5% CO2.Passaging was performed when the cell density reached 80%.Glutamate was obtained from Gibco (Grand Island,NY,USA),SB202190 was purchased from Topscience (Shanghai,China,T2301),and ferrostatin-1 was obtained from Ape Bio (Houston,TX,USA).Glutamate was dissolved in cell culture medium to a concentration of 10 mM.Ferrostatin-1 and SB202190 were dissolved in DMSO (MP Biomedicals,Shanghai,China) and diluted in culture medium containing 10 mM glutamate (Figure 1).
Cell viability
Cell viability was detected using a CCK-8 kit (Topscience,Shanghai,China).R28 cells were seeded in into 96-well culture plates (5 × 103cells/well).Cells were cultured with in DMEM(Procell) and 10% FBS containing glutamate or SB202190 for 24 hours.The culture medium was removed and 10% CCK-8 solution was added.Cells were then incubated at 37°C for 3 hours and analyzed at 450 nm with a Synergy LX multidetection microplate reader (BioTek,Covina,CA,USA).Cell viability was calculated using the following formula: relative cell viability (%)=(absorbance at 450 nm of treated group–absorbance at 450 nm of blank)/(absorbance at 450 nm of control group– absorbance at 450 nm of blank) × 100.
Propidium iodide/Hoechst 33342 staining
R28 cells were seeded into 12-well culture plates (1 × 105cells/well) and divided into four groups: the control group,glutamate group (10 mM glutamate),SB202190 group (10 mM glutamate+25 μM SB202190) and ferrostatin-1 group(glutamate 10 mM+1 μM ferrostatin-1 [Ape Bio]).Glutamate was dissolved in cell culture medium to a concentration of 10 mM.Ferrostatin-1 and SB202190 were dissolved in DMSO (MP Biomedicals) and diluted in culture medium containing 10 mM glutamate.Cells were treated with the respective treatments for 24 hours.
For propidium iodide (PI)/Hoechst 33342 staining,cells were incubated with cell staining buffer,Hoechst staining solution,and PI staining solution at 4°C in the dark for 30 minutes following the manufacturer’s instructions (Beyotime,Shanghai,China).Cells were washed with PBS and observed using a fluorescence microscope (Nikon,Tokyo,Japan).Propidium iodide (PI) indicated dead cells (red fluorescence)and Hoechst 33342 stained all cells (blue fluorescence).The brightness parameters were consistent between the groups during image capture.The photos were analyzed using ImageJ software (Fiji;National Institutes of Health,Bethesda,MD,USA;Schneider et al.,2012).The percentage of cell death was calculated using the formula: cell death rate%=PI-positive cells/Hoechst-positive cells × 100.
Transmission electron microscopy
Cells (2 × 105cells/well) seeded in a 6-well plate were cultured in DMEM and 10% FBS containing 10 mM glutamate and 25 μM SB202190 for 24 hours in a 37°C,5% CO2incubator.The cells were fixed with 2.5%glutaraldehyde fixative (Servicebio,Wuhan,China) at room temperature in the dark for 5 minutes.The cells were scraped off with a cell scraper and centrifuged (100 ×g,5 minutes).The fixative was discarded and fresh electron microscope fixative was added (Servicebio,Wuhan,China).The cells were fixed at room temperature for 30 minutes in the dark.Next,1% osmium tetroxide (Servicebio) was used for post-fixing at room temperature (20°C) for 2 hours.After dehydration with gradient concentrations of alcohol (50%,70%,80%,90%,and 100%) and acetone,cells were embedded in epoxy resin (Servicebio).The samples were cut into 60–80 nm ultra-thin sections for uranium-lead (Servicebio) double staining and then examined under a transmission electron microscope (Hitachi,Tokyo,Japan).
Intracellular ferrous ion detection
The detection of ferrous iron ions was performed using the FerroOrange assay kit (Dojindo Laboratories,Tokyo,Japan).FerroOrange undergoes an irreversible reaction with intracellular Fe2+in live cells,resulting in fluorescence emission (Ex: 543 nm and Em: 580 nm).R28 cells were seeded in a 48-well plate at a density of 2 × 104cells/well.After 24 hours of adaptive growth on 9 mm cell slides (Biosharp,Anhui,China),0 mM glutamate (control group),10 mM glutamate or 10 mM glutamate+25 μM SB202190 were added and cells were incubated for 24 hours in a 37°C,5% CO2incubator.The drug-containing culture medium (containing glutamate[Gibco],SB202190 [Topscience,T2301],DMEM [Procell]) was removed and cells were washed with HBSS.Cells were then incubated with 1 μM FerroOrange (Dojindo Laboratories,M374) working solution for 30 minutes and then observed under a fluorescence microscope.The brightness parameters were consistent between the groups during image capture.
Intracellular lipid peroxide detection
R28 cells were seeded in a 48-well plate (2 × 104cells/well).After 24 hours of adaptive growth on 9 mm cell slides(Biosharp).The cells were divided into several groups and treated for 24 hours: control group,10 mM glutamate group and 10 mM glutamate+25 μM SB202190 group.The medium was removed and cells were washed twice with 200 μL HBSS.Cells were then incubated in 200 μL of 1 μM Liperfluo HBSS solution (Dojindo Laboratories) at 37°C in a 5% CO2incubator for 30 minutes;the solution was removed and cells were washed twice with HBSS.Liperfluo is selectively oxidized by lipid peroxides,and the oxidized form of Liperfluo exhibits strong fluorescence at the cell membrane (Ex: 524 nm and Em:535 nm).Cells were examined with a fluorescence microscope(Nikon).The brightness parameters were consistent between the groups during image capture.
Detection of intracellular reactive oxygen species
The intracellular reactive oxygen species were detected using a kit (Abcam,Cambridge,UK,Cat# ab113851).The experimental group and drug treatment followed the same protocol as intracellular lipid peroxidation detection.After 24 hours of drug treatment,cells were collected by 0.25% Trypsin-EDTA (NCM Biotech,Wuhan,China) and then centrifuged (room temperature,1000 r/min,5 minutes).Cells were incubated with 20 μM 2′,7′-dichlorofluorescein diacetate (DCF-DA) (Abcam) at 37°C for 30 minutes.Cells were washed with 1× wash buffer three times and immediately analyzed on a flow cytometer (BD Biosciences,San Jose,CA,USA).DCF-DA was excited by a 488 nm laser and the fluorescence intensity was detected at 535 nm (typically fluorescent channel 1).The mean fluorescence intensity (MFI) of reactive oxygen species (ROS) was calculated from the flow cytometry data using FlowJo software (version 10,BD Life Sciences,Franklin Lakes,NJ,USA).
Malondialdehyde detection
Malondialdehyde (MDA) content was detected with a MDA content detection kit (AKFA013M,Boxbio,Beijing,China).Cell grouping and drug treatment were performed in the same way as the intracellular lipid peroxidation detection.Cells were collected by trypsinization and MDA extract was added.Cells were then centrifuged (8000 ×g) for 10 minutes at 4°C.The supernatant was collected and placed on ice;the protein concentration was detected using BCA Protein Assay Kit (Thermo Fisher Scientific,Waltham,MA,USA).The MDA detection working solution was added and the sample was placed in a boiling water bath for 60 minutes.After cooling to room temperature in an ice bath,the sample was centrifuged at 10,000 ×gfor 10 minutes at room temperature.The supernatant (200 μL) was pipetted into a 96-well plate and the absorbance was measured at 450 nm,532 nm and 600 nm with a Synergy LX multi-detection microplate reader (BioTek).
Intracellular glutathione detection
A micro-intracellular glutathione (GSH) assay kit (Solarbio,BC1175) was used to detect the content of reduced glutathione.Cell grouping and drug treatment were performed in the same way as the intracellular lipid peroxidation detection.After 24 hours of drug treatment,5000 cells were collected using 0.25% trypsin-EDTA (NCM Biotech,Wuhan,China).Cells were washed PBS twice.After adding GSH extract,samples underwent two freeze-thaw cycles in liquid nitrogen and a 37°C water bath.The sample was centrifuged (8000 ×g) at 4°C for 10 minutes and the supernatant was collected.GSH content was detected following the manufacturer’s instructions.
Western blotting assay
R28 cells were seeded in 60 mm cell culture dishes (8 × 105cells/dish) and the plates were divided into three groups:control group,10 mM glutamate group and 10 mM glutamate+25 μM SB202190.After 24 hours of drug treatment,cells were lysed in RIPA lysis buffer (Beyotime) and protein concentrations were quantified using a BCA Protein Assay Kit(Thermo Fisher Scientific).Samples were separated by 12%SDS-PAGE and then transferred to PVDF membranes (Millipore,Burlington,MA,USA).The membrane were incubated with blocking buffer (5% nonfat milk in TBST) for 90 minutes at room temperature,followed by incubation with primary antibodies overnight at 4°C: beta-actin monoclonal antibody(1:1000,Proteintech,Rosemont,IL,USA,Cat# 66009-1-Ig,RRID: AB_2687938),p38 MAPK polyclonal antibody (1:1000,Proteintech,Cat# 14064-1-AP,RRID: AB_2878007),ferritin light chain (FTL) polyclonal antibody (1:1000,Proteintech,Cat# 10727-1-AP,RRID: AB_2278673),and SAT1 polyclonal antibody (1:1000,Proteintech,Cat# 10708-1-AP,RRID:AB_2877739);phospho-p38 MAPK (1:1000,Thr180/Tyr182 Rabbit mAb,Cell Signaling Technology,Danvers,MA,USA,Cat# 4511);recombinant anti-glutathione peroxidase 4(GPx4) antibody (1:1000,Abcam,Cat# ab125066,RRID:AB_10973901);and SLC7A11 polyclonal antibody (1:1000,Thermo Fisher Scientific,PA1-16893).The membranes were washed and incubated with the goat anti-rabbit IgG H&L (HRP;Zen Bio,Chengdu,China,511203) for 90 minutes at room temperature.The band signals were detected using enhanced chemiluminescence reagent (Bio-Rad,Hercules,CA,USA).The grayscale intensities were quantified with ImageJ and protein expression was normalized to β-actin.
Flash visual evoked potential analysis
The rats (4 rats per group) were anesthetized with pentobarbital 3 days after drug intravitreal injection.After 15 minutes of dark adaptation,we inserted electrodes under the skin of the rat back (ground electrode),anterior bregma(cathode),and the occipital bone (anode).The contralateral eye was covered with thick gauze and foil,and the visual function was recorded and evaluate with a multifocal electroretinography recorder (Gotec,Chongqing,China,GT-2008V–VI) and the Ganzfeld system (Gotec,Chongqing,China).
Hematoxylin and eosin staining
Rats (4 rats per group) were sacrificed 3 days after intravitreal injection.Eyeballs were fixed in 4% formalin,and the samples were dehydrated in a graded series of ethanol (80%,95%,and 100%) and embedded in paraffin.Samples were cut into 3 μm vertical sections using freezing microtome (CM1860 UV,Leica,Germany).The slices were stained with hematoxylin and eosin (H&E),visualized using a light microscope (Nikon)and analyzed using CaseViewer software (3DHISTEC;Sysmex,Budapest,Hungary).The thickness of the retinal ganglion cell body complex (GCC) was measured at 500,1000,1500 and 2000 μm from the optic nerve center.
Immunofluorescence staining of retinal whole-mounts
Rats (5 rats per group) were sacrificed 3 days after intravitreal injection.The eyeballs were fixed in 4% paraformaldehyde for 30 minutes,and the retinas were dissected under a stereomicroscope.Samples were blocked in 2% bovine serum albumin (BSA) (Servicebio) in PBS containing 0.5%Triton-X100 (Servicebio) for 1 hour at room temperature,followed by incubation with anti-Brn3a antibody (a marker for RGCs;1:100,Abcam,Cat# ab245230,RRID: AB_2916038)overnight at 4°C.Samples were washed with 0.5% Triton-X10 three times,fixed in 4% paraformaldehyde for 10 minutes and washed.The sample was incubated with goat anti-rabbit IgG H&L,Alexa Fluor 488 (1:200,Abcam,Cat# ab150077,RRID:AB_2630356) for 1.5 hours at room temperature and washed with PBS.Primary and secondary antibodies for p-p38 MAPK,FTL,SAT1,GPx4 and SLC7A11 are listed above in section 2.10.The samples were examined and photographed using a fluorescence microscope.The immunoreactivity and RGC numbers were analyzed by ImageJ software.
Immunofluorescence staining of retinal sections
Paraffin sections of eyeballs (3 μm thick) were deparaffinized with xylene and alcohol series.After washing the slices,endogenous peroxidase activity was blocked with 3%hydrogen peroxide (Servicebio) for 5 minutes.Sections were immersed in 0.01 M citrate antigen retrieval solution (pH 6.0) for antigen retrieval and heated in a microwave oven,followed by cooling for 40 minutes.Samples were then incubated with primary antibody overnight at 4°C,followed by incubation with secondary antibody (goat anti-rabbit IgG H&L,Alexa Fluor 488,1:200,Abcam,Cat# ab150077,RRID:AB_2630356) for 1 hour at room temperature.The following primary antibodies were used for staining: p38 MAPK rabbit polyclonal antibody (1:200,Proteintech,Cat# 14064-1-AP,RRID: AB_2878007),FTL rabbit polyclonal antibody (1:200,Proteintech,Cat# 10727-1-AP,RRID: AB_2278673) and SAT1 rabbit polyclonal antibody (1:200,Proteintech,Cat# 10708-1-AP,RRID: AB_2877739);phospho-p38 MAPK Thr180/Tyr182 rabbit mAb (1:200,Cell Signaling Technology,Cat#4511);rabbit recombinant anti-GPx4 antibody (1:200,Abcam,Cat# ab125066,RRID: AB_10973901);and SLC7A11 rabbit polyclonal antibody (1:200,Thermo Fisher Scientific,Cat#PA1-16893).DAPI (Servicebio,Wuhan,China,G1012) staining was performed 5 minutes.Sections were photographed using a fluorescence microscope (Nikon).The brightness parameters were consistent between the groups during image capture.
Quantitative real-time polymerase chain reaction
Total RNA was extracted from cells using TRIzol reagent(Invitrogen,Waltham,MA,USA),and cDNA was synthesized with the Hifair III 1stStrand cDNA Synthesis Kit (Yeasen Biotechnology,Shanghai,China).Quantitative real-time polymerase chain reaction (RT-PCR) was performed using HieffqPCR SYBR Green Master Mix (Low Rox,Yeasen Biotechnology,Shanghai,China) with a sequence detection system (Prism 7500,Applied Biosystems,Waltham,MA,USA) following the manufacturer’s instructions.Primers were designed by Sangon Biotech: SLC7A11 (forward 5′-GAC AGT GTG TGC ATC CCC TT-3′ and reverse 5′-GCA TGC ATT TCT TGC ACA GTT C-3′),Gpx-4(forward 5′-GAT GAA AGT CCA GCC CAA GG-3′ and reverse 5′-TAG AGA TAG CAC GGC AGG TC-3′),FTL (forward 5′-CCG CAC AGA CCC TCA CCT C-3′ and reverse 5′-AGA GAT ACT CGC CCA GAG ATG C-3′),SAT1 (forward 5′-TCC CAG ATT AGC CAA AGA GCC-3′ and reverse 5′-GTG TGG CTG TGT TGT TGA G-3′),p38-MAPK (forward 5′-GCA CAC TGA TGA CGA AAT GAC C-3′ and reverse 5′-CCC ACG GAC CAA ATA TCC ACT -3′),and β-actin(forward 5′-CTA CAA TGA GCT GCG TGT GGC-3′ and reverse 5′-CAG GTC CAG ACG CAG GAT GGC-3′).The relative expression of each gene was normalized to β-actin mRNA using the 2–ΔΔCtmethod.Each sample was measured in triplicate wells,and the experiments were repeated three times.
Statistical analysis
Data are expressed as means ± SD,and at least three independent experiments were performed.GraphPad Prism(version 9.1.1;GraphPad,San Diego,CA,USA,www.graphpad.com) was used for statistical analyses.Two-tailed Student’st-test was used to compare differences between two groups.Multiple data were analyzed using one-way analysis of variance (ANOVA),followed by Tukey’s multiple comparison test.P<0.05 indicated statistical significance.
Results
SB202190 protects R28 cells from glutamate-induced cell death
The R28 cell line has been widely used to explore the neuroprotection and pathological mechanism of RGCsin vitroin several studies (Dai et al.,2022;Liu et al.,2022;Yao et al.,2023).To explore the effects of SB202190 on glutamatergic toxicity,we used R28 cells as anin vitromodel.We cultured R28 cells for 24 hours with various concentrations of glutamine and found that cell viability was approximately 50%in the presence of 10 mM glutamate (Figure 2A).Therefore,we chose 10 mM for subsequent experiments.
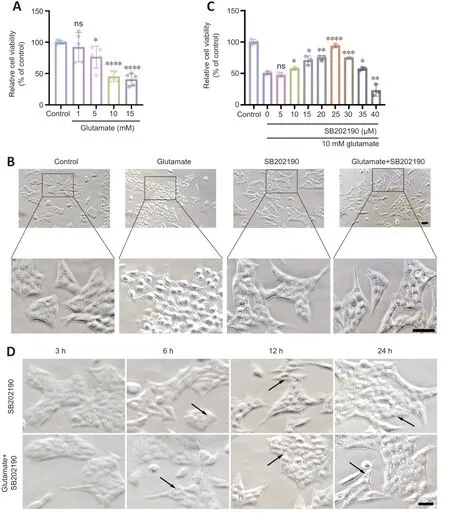
Figure 2 | p38 MAPK inhibitor SB202190 protects R28 cells from glutamate-induced cell death.
Light microscopy showed that untreated R28 cells exhibited a spindle-shaped morphology with tight intercellular connections (Figure 2B).After 24 hours of glutamate treatment,most cells underwent morphological changes,appearing rounded,and the intercellular connections gradually disappeared.SB202190 exhibited protective effects on R28 cells by inhibiting p38 MAPK.CCK-8 assay showed that SB202190 had a protective effect on R28 cells treated with glutamate (Figure 2C).While glutamate reduced cell viability(48.98%),co-treatment with 10 μM SB202190 increased viability to 57.73% (P<0.05).At 25 μM SB202190,cell viability reached 93.79% (P<0.0001) and then began to decrease with increasing drug concentration (P<0.05).Excessively high concentrations of SB202190 may have caused toxicity.We therefore chose 25 μM for subsequent experiments.
We examined cell morphology after drug treatment under a light microscope and detected vesicle-like structures with SB202190 treatment (Figure 2D).These were detected at 12 hours and were more obvious at 24 hours.
SB202190 protects R28 cells from glutamate-induced ferroptosis
We next examined whether the glutamate-induced death of R28 cells involved ferroptosis and explored whether the rescue effect of SB202190 was associated with reversal of ferroptosis.Ferrostatin-1 is a classical inhibitor of ferroptosis(Miotto et al.,2020) and was used as a positive control.Hoechst/PI staining showed that both SB202190 and ferrostatin-1 significantly reduced glutamate-induced cell death (Figure 3AandB).Transmission electron microscopy revealed that the glutamate-treated group showed morphological changes indicative of ferroptosis (Dixon et al.,2012),such as mitochondrial shrinkage,reduction of mitochondrial cristae and mitochondrial outer membrane rupture.Notably,the mitochondrial damage induced by glutamate was prevented by SB202190 treatment (Figure 3C).Iron and lipid peroxide accumulation are important biochemical features of ferroptosis (Chen et al.,2021).The intracellular iron content was detected using Mito-FerrOrange.The fluorescence intensity in the glutamate group increased,indicating that intracellular iron was elevated by glutamate treatment (Figure 3DandE).Upon co-treatment with SB202190,the fluorescence intensity decreased (P<0.001).We next used Liperfluor to detect intracellular lipid peroxides.The fluorescence intensity was significantly increased in the glutamate group compared with the control group (P<0.001)and decreased upon SB202190 treatment (Figure 3FandG).MDA and GSH assays were also used to detect changes in intracellular lipid peroxidation levels.MDA levels were significantly increased in the glutamate group compared with the control group (P<0.0001),while it was significantly decreased with SB202190 co-treatment (P<0.01;Figure 3H).While GSH was significantly decreased in the glutamate group compared with the control group (P<0.001),co-treatment with SB202190 did not significantly increase levels (Figure 3I).We suggest that this may be because scavenging excess reactive oxygen species (ROS) depletes intracellular GSH.At the time point of observation,though the alived R28 in SB202190 group could continuely synthesized GSH,the part of synthesized GSH cannot compensate the part of depleted.
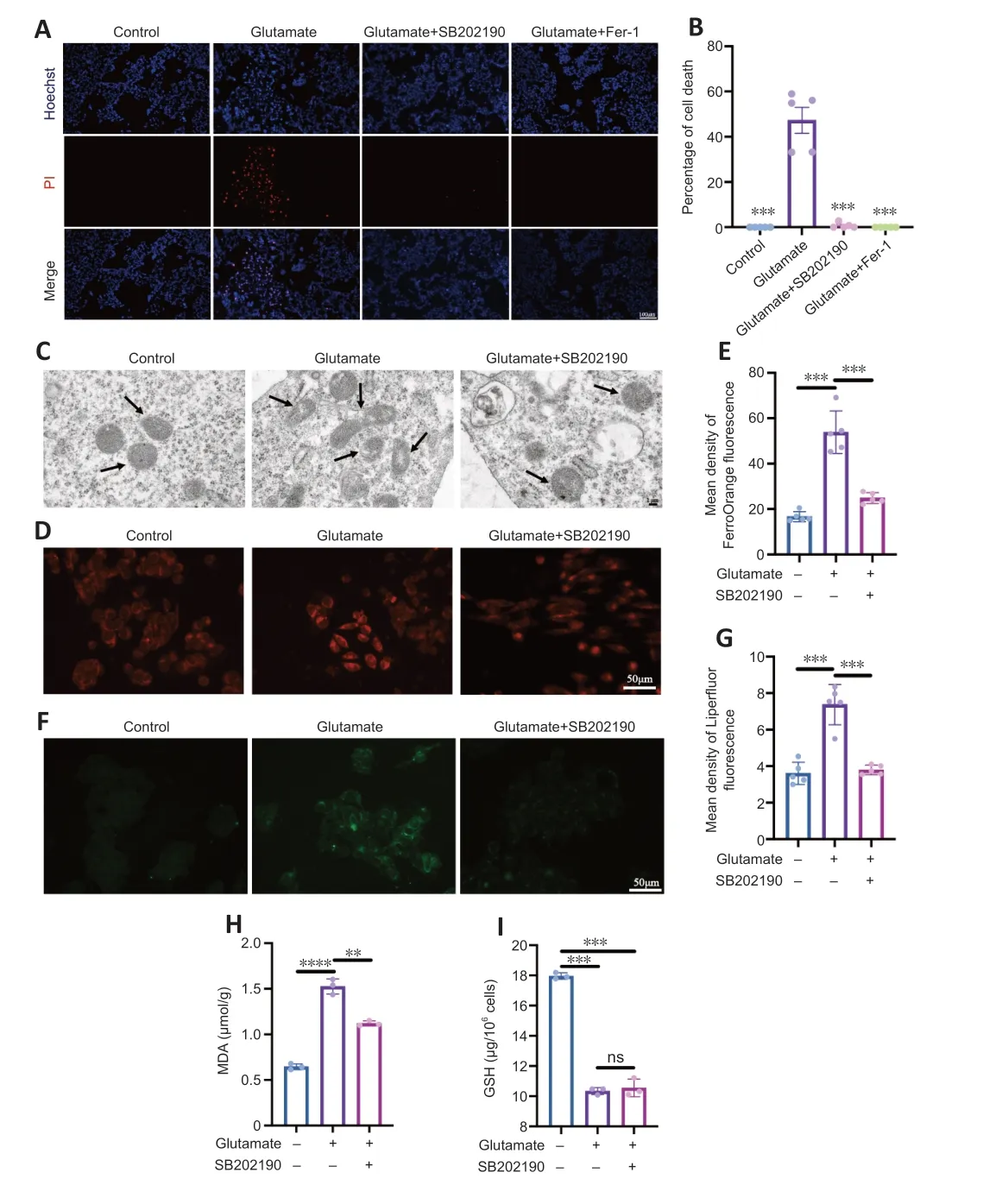
Figure 3 | p38 MAPK inhibitor SB202190 protects R28 cells from glutamate-induced ferroptosis.
SB202190 regulates glutamate-induced ferroptosis in R28 cells by acting on FTL and SAT1
p38 MAPK is activated upon phosphorylation (English and Cobb,2002).We detected the expressions of p38 and p-p38 in R28 cells after drug treatment by western blotting (Figure 4AandB).We found that p-p38 MAPK/p38 MAPK increased in the glutamate group compared with control group (P<0.001)and SB202190 co-treatment decreased the level (P<0.05).This was also supported by the results of immunofluorescence staining of paraffin sections from animal models.The fluorescence intensity of p-p38 MAPK labeling in RGCs of NMDA group was higher than that in the other two groups(Figure 4EandF).
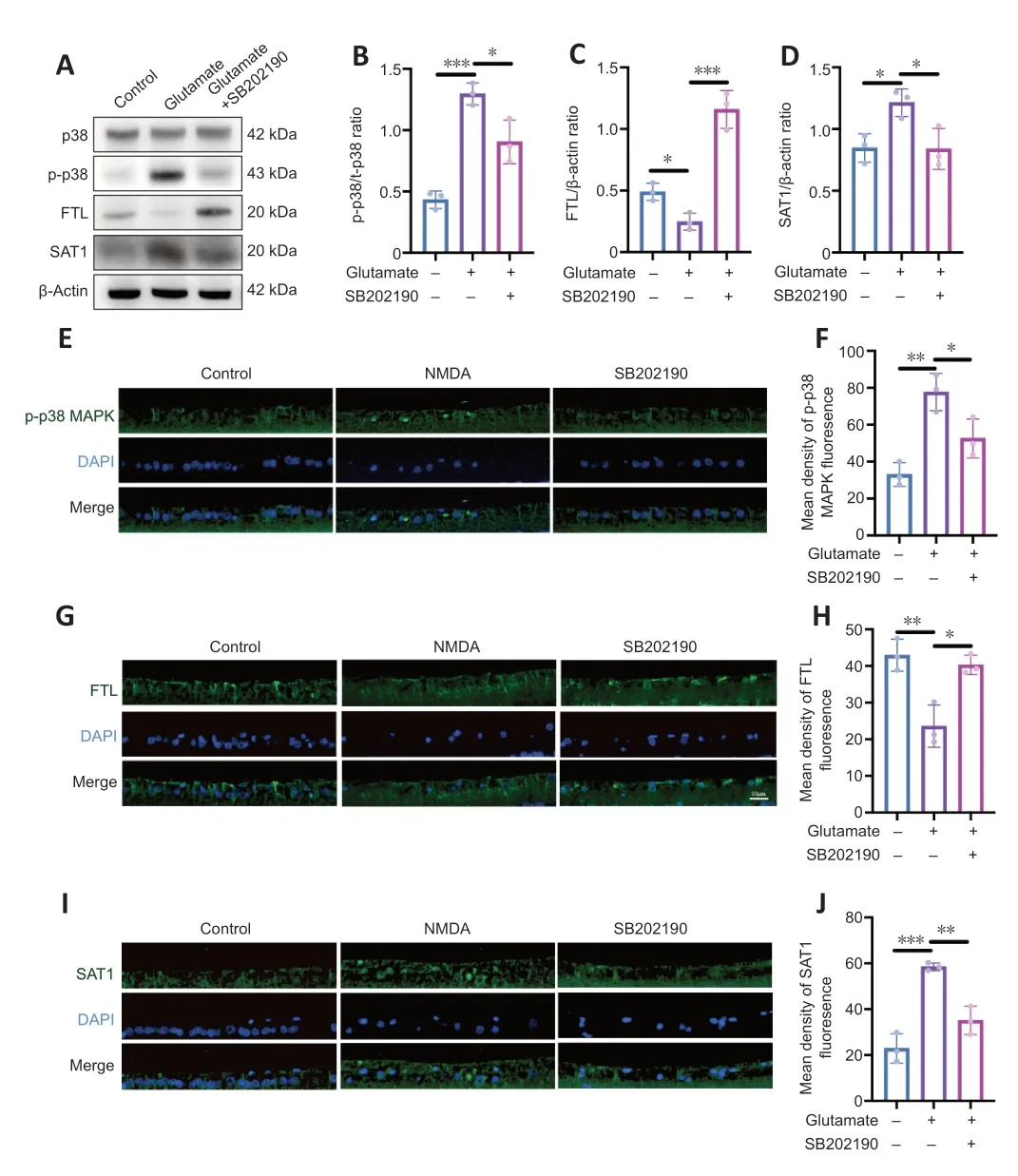
Figure 4 | p38 MAPK inhibitor SB202190 regulates ferroptosis in a glutamate R28 cell model by regulating FTL and SAT1.
We also used qRT-PCR to examine the mRNA levels of p38 MAPK (Additional Figure 1).We found no significant difference in the mRNA levels of p38 MAPK between the control group and the glutamate group (P>0.05).However,p38 MAPK mRNA level increased in the SB202190 group compared with the glutamate group (P<0.01).However,this is not contradictory to the results obtained from western blot,as an increase in mRNA levels may not necessarily reflect the level of protein phosphorylation.
To further explore the mechanism of SB202190 on glutamateinduced ferroptosis,we examined the expression of ferroptosis-related proteins.Western blot showed that FTL expression decreased and SAT1 expression increased in glutamate-treated R28 cells compared with controls (P<0.05;Figure 4A,C,andD).SB202190 co-treatment reversed the effects of glutamate on the expression of these two proteins(P<0.05).We also performed qRT-PCR to measure the mRNA levels of FTL and SAT1 (Additional Figure 1).While the mRNA level of FTL was unchanged by glutamate treatment,FTL mRNA increased with SB202190 co-treatment (P<0.05);the mRNA level of SAT1 increased with glutamate treatment and decreased with SB202190 co-treatment (P<0.05).Fluorescence microscopy of paraffin sections from animal models revealed that the trend of FTL and SAT1 fluorescence intensity changes was compatible with the results of cell experiments (Figure 4G–J).FTL expression was decreased and SAT1 expression increased in the NMDA group compared with the control group.Addition of SB202190 prevented the changes induced by NMDA.
SB202190 alleviates lipid peroxidation by regulating the SLC7A11/GSH/GPX-4 pathway
We examined intracellular ROS content after drug treatment of R28 cells (Figure 5AandB).Glutamate increased ROS relative to the control group (P<0.0001),and the mean fluorescence intensity (MFI) value of ROS in glutamate group was approximately 5.13-fold of that in control group.However,the MFI significantly decreased with co-treatment of SB202190 compared with glutamate alone (P<0.0001).The SLC7A11/GSH/GPX-4 axis is the main intracellular antioxidant system that protects against ferroptosis and regulates lipid reactive oxygen species production (Jiang et al.,2021).Our results showed SB202190 co-treatment did not impact glutamate-induced GSH changes in R28 cells (Figure 3I).We also examined SLC7A11 and GPX-4 protein expression in R28 cells (Figure 5C–E).SLC7A11 protein showed no significant changes between the glutamate and control groups (P>0.05).However,SLC7A11 increased after SB202190 co-treatment compared with glutamate alone (P<0.05).GPX-4 expression was lower in the glutamate group than in the control group(P<0.05) and it increased with co-treatment of SB202190(P<0.05).We used qRT-PCR to detect the mRNA levels of SLC7A11 and GPX-4 (Additional Figure 1).Both SLC7A11 and GPX-4 mRNA levels showed a significant increase upon SB202190 co-treatment compared with glutamate alone (P<0.05).Immunofluorescence analysis of paraffin sections of animal models showed that the trend of SLC7A11 and GPX-4 expression changes were compatible with the results in R28 cells (Figure 5F–I).
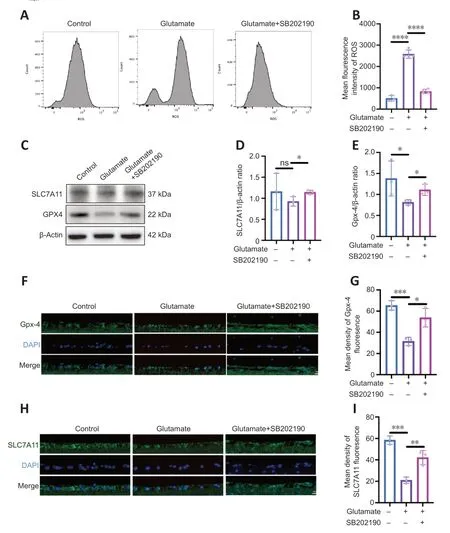
Figure 5 | p38 MAPK inhibitor SB202190 alleviates lipid peroxidation by regulating the SLC7A11/GSH/GPx4 pathway.
SB202190 prevents NMDA induced damage in rat retina
NMDA binds to NMDA receptors on RGCs to induce excitatory retinal damage (Doozandeh and Yazdani,2016).We established an glutamate excitotoxicity glaucoma animal model by intravitreal injection of NMDA in rats.To assess the effects of SB202190 on retinas from NMDA model animals,we performed retinal tiling and immunofluorescence staining with Brn3a antibody,which is used to identify and quantify RGCs in the retina (Nadal-Nicolás et al.,2009;Figure 6AandB).After 3 days of intravitreal injection,the density of RGCs in the retina in the NMDA group was significantly reduced to approximately 38.16% relative to the control group (P<0.0001).After treatment with SB202190,the damage of RGCs induced by NMDA was reduced and the density of RGCs was restored to 57.57% of the control group (P<0.0001).We further performed H&E staining to observe the retinal morphology (Figure 6C).At 3 days after intravitreal injection,the number of nuclei in the GCL per 100 μm was reduced to approximately 29.67% of that in the control group and increased to 46.88% in the SB202190-treated group (P<0.01;Figure 6D).We further determined the thickness of the retinal ganglion cell body complex (GCC) at 500,1000,1500,and 2000 μm from the optic center of optic disc.The GCC was thinner at all measurement points in the NMDA group compared with the control group (P<0.05).The damage induced by NMDA was prevented in the SB202190-treated group (Figure 6EandF).Müller cells are neuroglial cells that play an important regulatory role in retinal metabolism,which are essential for extracellular glutamate clearance and the synthesis of intraretinal glutamate and GABA (Pfeiffer et al.,2020;Xu et al.,2022).Glutamine synthetase (GS) is the major neuroglial enzyme involved in extracellular glutamate clearance by Müller cells (Villar et al.,2023).We found that intravitreal injection of NMDA increased the expression of GS in Müller cells in the rat retina to some extent (Additional Figure 2).
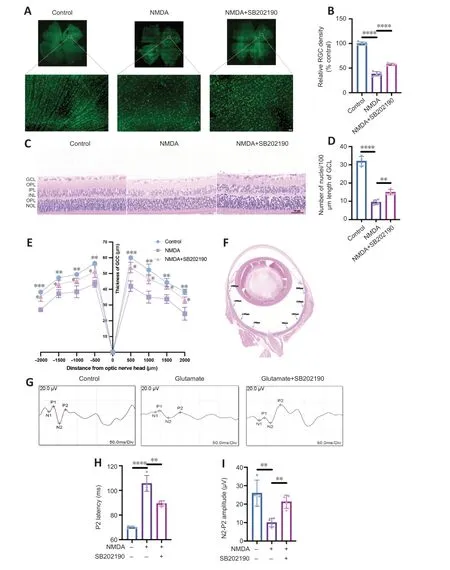
Figure 6 | p38 MAPK inhibitor SB202190 attenuates N-methyl-D-aspartate (NMDA)-induced damage in the rat retina.
f-VEP is a common examination for the clinical assessment of visual conduction function.The f-VEP amplitude is considered as an indicator of the receptive function of the intact synaptic contacts number.The f-VEP latency is considered as an indicator of nerve conduction and axon myelin sheath integrity (Li et al.,2018) We evaluated the effects of SB202190 on the latency and amplitude of P2 wave in the NMDA model by detecting flash visual evoked potential (Figure 6G).At 3 days after NMDA treatment,the latency of P2 wave was prolonged and the amplitude was reduced compared with observations in the control group (Figure 6HandI),indicating that intravitreal NMDA injection caused retinal dysfunction in rats.SB202190 treatment partially restored the P2 wave.
Discussion
In the present study,we observed a protective effect of SB202190 on RGCs in the NMDA rat model.In cellular assays,we found that SB202190 protected R28 cells from glutamateinduced ferroptosis by regulating FTL,SAT1 and SLC7A11/GSH/GPx4 pathways (Figure 7).These proteins showed the same trends of change in the NMDA model.These findings suggest that SB202190 may be a potential therapeutic agent to achieve protection of RGCs in glaucoma.

Figure 7 |A schematic model for the mechanism of p38 MAPK inhibitor SB202190 in alleviating ferroptosis in the glutamate-induced retinal excitotoxic glaucoma model.
The specific molecular mechanisms underlying ferroptosis in glutamatergic models of glaucoma are unknown.Imbalances in iron metabolic homeostasis,reduced cellular antioxidant capacity,and abnormal mitochondrial structure are the three characteristic alterations of ferroptosis that trigger lipid peroxidation (Mou et al.,2019;Qiu et al.,2020;Jiang et al.,2021;Mao et al.,2021).In the present study,we found that iron ion accumulation,lipid peroxide accumulation and decreased GSH occurred in R28 cells after glutamate treatment.Electron microscopy revealed an increased density of decreased mitochondrial cristae and outer mitochondrial membrane rupture in response to glutamate treatment.These results all suggested that glutamate induced ferroptotic death in R28 cells.
Ferroptosis is regulated by multiple signaling pathways,and the upstream molecular mechanisms are very complex.The SLC7A11/GSH/GPx4 pathway is the major antioxidant system against ferroptosis in cells.SLC7A11,also known as XCT,is a cystine/glutamate transporter (Koppula et al.,2021) that transports cystine intracellularly and oxidizes it to cysteine to catalyze the synthesis of GSH.GSH is an essential factor for GPx4 to scavenge lipid reactive oxygen species (Ursini and Maiorino,2020;Zhang et al.,2021).SLC7A11 reduction can induce ferroptosis by affecting GPx4 activity.Our study demonstrated that SB202190 inhibited ferroptosis by reducing lipid ROS accumulation in R28 cells via modulating the SLC7A11/GSH/GPx4 signaling pathway.Previous studies have shown that FTL is an intracellular iron storage protein that stores iron in a state that is soluble and nontoxic (Bu et al.,2012;Honarmand Ebrahimi et al.,2015).FTL downregulation can lead to an imbalance in intracellular iron homeostasis and accelerated onset of ferroptosis (Bu et al.,2012).Previous studies showed that partial p38 MAPK inhibitors induce vacuole formation in a variety of cell lines (Hassan et al.,2004;Menon et al.,2011).SB 202190 has been mentioned in glaucoma related research.Nassar et al.(2015) found that SB 202190 can inhibit the proliferation and migration of human Tenon fibroblasts,and it is a potential drug to inhibit scar formation after glaucoma filtration surgery.However,no more reports were found to explore the effect of SB 202190 on RGCs.SB202190 is a classical p38 MAPK inhibitor.In previous studies,it was found that melatonin can promote the survival of RGCs in the glaucoma NDMA model.This effect may be achieved by inhibiting pro-inflammatory cytokines derived from microglial cells,thereby suppressing the activation of p38 MAPK (Zou et al.,2023).Another study revealed that Interleukin-17A regulates retinal inflammation by modulating microglial cell activation through the inhibition of the p38 MAPK pathway (Chen et al.,2023).These studies suggest that inhibiting the activation of p38 MAPK is a potential target for protecting RGCs.In our study,in bothin vivoandin vitroexperiments,we found that glutamate excitotoxicity can induce the activation of p38 MAPK.SB202190 induced the formation of vesicle-like structures in R28 cells.Our results showed that SB202190 upregulated the expression of FTL,decreased intracellular free divalent iron ions,and inhibited ferroptosis to some extent.SAT1 (spermidine/spermine N1 acetyltransferase 1) is an important regulator of polyamine metabolism,and its activation can induce lipid peroxidation,triggering ferroptosis by ROS (Kang et al.,2019;Mou et al.,2022).We found that SB202190 inhibited the high SAT1 expression caused by glutamate.This further suggested that SB202190 plays a role in the intervention of ferroptosis by regulating lipid peroxidation.
In this study,we found intravitreal injection of NMDA increased the expression of GS in Müller cells in the rat retina to some extent.This finding is consistent with previous research (Shen et al.,2004).We speculate that the activation of Müller cells may have a beneficial effect on the survival of RGCs by promoting NMDA clearance,but the underlying relationship requires further investigation.
This study has several limitations.Our study was only focused on a glutamate excitotoxicity model.While several studies on the protection of RGCs in glaucoma have been conducted using this model (Honda et al.,2019;Liu et al.,2022;Duan et al.,2023),the mechanisms of RGC damage in glaucoma are complex,and a single model is insufficient to fully simulate the pathological and physiological processes of RGC loss in clinical glaucoma patients (Casson et al.,2012;Pang and Clark,2020).To comprehensively analyze ferroptosis in RGCs and the therapeutic effects of p38 MAPK inhibitor,future studies should be performed with more glaucoma animal models,such as acute high-pressure models,optic nerve crush models,or intracameral injection microbead models.Additionally,thein vitroexperiments in this study were performed in R28 cells and not primary RGCs,and these should be investigated in future research.
In conclusion,our study indicated that ferroptosis plays an important role in the glutamate-induced excitotoxicity glaucoma model.SB202190 prevented RGC loss caused by glutamate toxicity by regulating ferroptosis.The p38 MAPK inhibitor SB202190 inhibits ferroptosis and protects RGCs by regulating FTL,SAT1 and SLC7A11/Gpx4 pathways,indicating SB202190 represents a potential retina protectant.
Author contributions:Conceptualization,investigation,visualization,and writing– original draft:LF and CW;data curation:LF;funding acquisition:WS and CW;methodology:ZX,LF,CW,CZ,WuZ and WeiZ;resources:LF,CW;project administration,validation,and writing– review &editing:WS;supervision:YH and WS.All authors approved the final version of the manuscript.
Conflicts of interest:The authors declare that they have no competing interests.
Data availability statement:All data relevant to the study are included in the article or uploaded as Additional files.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional files:
Additional Figure 1:mRNA expression of ferroptosis related proteins induced by glutamate in R28 cells.
Additional Figure 2:Morphological changes of glutamine synthetase-positive cells after p38 MAPK inhibitor SB202190 treatment in rat with NMDA-induced retinal injury.
- 中國神經(jīng)再生研究(英文版)的其它文章
- Corrigendum: Sorl1 knockout inhibits expression of brain-derived neurotrophic factor:involvement in the development of late-onset Alzheimer’s disease
- Global trends in diabetic eye disease research from 2012 to 2021
- Lycium barbarum glycopeptide (wolfberry extract)slows N-methyl-N-nitrosourea-induced degradation of photoreceptors
- Magnesium-L-threonate treats Alzheimer’s disease by modulating the microbiota-gut-brain axis
- 3′-Deoxyadenosin alleviates methamphetamineinduced aberrant synaptic plasticity and seeking behavior by inhibiting the NLRP3 inflammasome
- Small extracellular vesicles from hypoxiapreconditioned bone marrow mesenchymal stem cells attenuate spinal cord injury via miR-146a-5p-mediated regulation of macrophage polarization

