Small extracellular vesicles from hypoxiapreconditioned bone marrow mesenchymal stem cells attenuate spinal cord injury via miR-146a-5p-mediated regulation of macrophage polarization
Zeyan Liang ,Zhelun Yang ,Haishu Xie,Jian Rao,Xiongjie XuYike LinChunhua Wang,Chunmei Chen
Abstract Spinal cord injury is a disabling condition with limited treatment options.Multiple studies have provided evidence suggesting that small extracellular vesicles (SEVs) secreted by bone marrow mesenchymal stem cells (MSCs) help mediate the beneficial effects conferred by MSC transplantation following spinal cord injury.Strikingly,hypoxia-preconditioned bone marrow mesenchymal stem cell-derived SEVs(HSEVs) exhibit increased therapeutic potency.We thus explored the role of HSEVs in macrophage immune regulation after spinal cord injury in rats and their significance in spinal cord repair.SEVs or HSEVs were isolated from bone marrow MSC supernatants by density gradient ultracentrifugation.HSEV administration to rats via tail vein injection after spinal cord injury reduced the lesion area and attenuated spinal cord inflammation.HSEVs regulate macrophage polarization towards the M2 phenotype in vivo and in vitro.MicroRNA sequencing and bioinformatics analyses of SEVs and HSEVs revealed that miR-146a-5p is a potent mediator of macrophage polarization that targets interleukin-1 receptor-associated kinase 1.Reducing miR-146a-5p expression in HSEVs partially attenuated macrophage polarization.Our data suggest that HSEVs attenuate spinal cord inflammation and injury in rats by transporting miR-146a-5p,which alters macrophage polarization.This study provides new insights into the application of HSEVs as a therapeutic tool for spinal cord injury.
Key Words:bone marrow mesenchymal stem cells;hypoxia preconditioning;interleukin-1 receptor-associated kinase 1;macrophages;mesenchymal stem cells;small extracellular vesicles;spinal cord injury
Introduction
Spinal cord injury (SCI) has devastating consequences on patients’ physical,economic,and psychosocial health (Ahuja et al.,2017;Anjum et al.,2020).Secondary SCI after SCI involves complex pathophysiological processes,such as ischemia,oxidative stress,neuronal death,blood-brain barrier dysfunction,inflammatory events,apoptosis,and necrosis,ultimately causing irreparable damage to the patient’s sensory and motor functions (Hutson and Di Giovanni,2019;Schmidt and Quintá,2023).Currently,available treatments are limited and provide only supportive care to patients with lifelong disabilities (Khorasanizadeh et al.,2019).Existing evidence suggests that central nervous system-resident microglia and macrophages from the peripheral circulation are key effectors of secondary injury after SCI (Porta et al.,2015;Milich et al.,2019).After acute SCI,macrophages from the peripheral circulation migrate into the spinal cord and initiate a classically activated M1-type transition,producing pro-inflammatory cytokines (TNF-α and IL-1),reactive oxygen species,and nitric oxide,which in turn promote tissue inflammation and damage(Wang et al.,2015;Van Broeckhoven et al.,2021).Therefore,inhibiting or reversing classical activation of macrophages is a promising strategy for alleviating secondary injury to the spinal cord.
Bone marrow mesenchymal stem cell (BMSC)-derived small extracellular vesicles (SEVs) are often rich in nucleic acids that play a crucial role in altering the phenotype and function of recipient cells (Carlson et al.,1998;Zhang et al.,2015).According to the literature,BMSC-SEVs can induce changes in macrophage phenotype by delivering microRNAs (miRNAs),thereby reducing inflammation (Zhao et al.,2019;Liang et al.,2022).Additionally,oxygen concentration is important for mesenchymal stem cell (MSC) proliferation,differentiation,and self-renewal (Hu et al.,2014;Zhu et al.,2018).Liu et al.(2020) demonstrated that SEVs produced by hypoxiapreconditioned MSCs (HSEVs) can further promote functional recovery after SCI.
Using high-throughput sequencing,we identified a significant disparity in miR-146a-5p content between SEVs and HSEVs.Prior studies have highlighted the role of miR-146a-5p in orchestrating macrophage polarization,leading to the amelioration of sepsis and atherosclerosis (Li et al.,2015;Song et al.,2017).However,the intricate mechanisms underpinning how miR-146a-5p encapsulated within BMSC-HSEVs governs macrophage polarization within the context of SCI remain unknown.We hypothesize that HSEVs may have superior therapeutic efficacy in rats subjected to SCI,compared with SEVs.Furthermore,we conjecture that the different outcomes in animals treated with HSEVs or SEVs may stem from the distinct miRNA cargos that they harbor.Finally,we suggest that miR-146a-5p is a key regulator within HSEVs that is largely responsible for modulating macrophage polarization.
Methods
Animals
Sprague-Dawley (SD) rats (150 rats) were purchased from Sprague (Beijing) Biotechnology Co.,Ltd.(license No.SCXK(Jing) 2019-0010).All rats were male,aged 6–8 weeks,and weighing 200–260 g at the time of surgery.All animals were maintained under specific pathogen-free conditions at the Animal Facility of Fujian Medical University (Fuzhou,China) (temperature: 20–24°C;humidity: 40–60%;standard photoperiod: 12 hours light (day)/12 hours dark (night)).Each SD rat was housed individually to avoid mutual interference and influence.During the feeding period,each rat was free to eat and drink at any time to meet its physiological needs.All experimental procedures were approved by the Institutional Laboratory Animal Care and Use Committee of Fujian Medical University on November 29,2021 (FJMU IACUC 2021-0501) and were designed and reported according to the Animal Research: Reporting ofIn VivoExperiments (ARRIVE)guidelines (Percie du Sert et al.,2020).The number of animals used in the study was limited to the minimum required for sufficient statistical power.
Cell isolation and culture
BMSCs were isolated from 25 SD rats (aged 6 weeks,weighing 150–180 g).Briefly,rats were anesthetized with isoflurane(induction concentration 3–4%,Lunan Better Pharmaceutical Co.,Ltd.,Shandong,China),sacrificed,and soaked in 75%alcohol for disinfection.Rat femurs and tibias were harvested,after which the bone marrow was flushed out using highglucose Dulbecco’s modified eagle medium (DMEM,Hyclone,Logan,UT,USA,Cat# SH30243.01) containing 10% fetal bovine serum (FBS,Gibco,Waltham,MA,USA,Cat# 10270-106) and 1% penicillin/streptomycin (P/S,10,000 μg/mL,Sigma,St.Louis,MO,USA,P0781) and disaggregated into a single-cell suspension.Cells were centrifuged at 300 ×gfor 5 minutes,and pellets were resuspended in DMEM highglucose medium containing 10% FBS,1% P/S and inoculated into a 75-cm2culture flask (Corning,Steuben County,NY,USA)that was then incubated at 37°C,in an atmosphere containing 5% CO2and 21% O2.The growth medium was changed every two days.Passages four transferred into the fourth passage were used for subsequent experiments.Bone marrow-derived macrophages (BMDMs) were obtained from rat femur and tibia bone marrow using the method described above.Cells were centrifuged at 300 ×gfor 5 minutes,then the pellet was resuspended in red blood cell lysis buffer (Servicebio,G2015-500ML,Wuhan,China) and lysed at 4°C for 3 minutes.Cells were then centrifuged again and resuspended in high-glucose DMEM containing 10% FBS and 1% P/S and cultured in a 25-cm2culture flask (Corning) for 6 hours to separate adherent cells.Cells were then cultured in DMEM containing 10% FBS,1%P/S,and recombinant rat macrophage colony stimulating factor(20 ng/mL,Peprotech,Rocky Hill,NJ,USA,Cat# 400-28) for 7 days.The medium was changed every 2 days (the first medium change was a half-volume change).To induce M1 polarization,we stimulated BMDMs with 500 ng/mL lipopolysaccharide(LPS,MedChemExpress,Monmouth Junction,NJ,USA,Cat#HY-D1056) for 24 hours.
Identification of BMSCs
Passage four BMSC surface proteins were labeled with PE Armenian hamster anti-CD29 antibody (1:200,eBioscience,Waltham,Massachusetts,USA,Cat# 12-0291-82,RRID:AB_763478),APC mouse anti-CD90 antibody (1:200,eBioscience,Cat# 17-0900-82,RRID: AB_469420),FITC rat anti-CD34 antibody (1:200,eBioscience,Cat# 11-0341-82,RRID: AB_465021),and V450 mouse anti-CD45 antibody(1:200,BD Bioscience,Franklin Lakes,NJ,USA,Cat# 561587,RRID: AB_10926193),and cells were sorted by flow cytometry to determine their purity.The three-lineage differentiation ability of the BMSCs was also assessed.For osteogenic differentiation,when BMSCs reached 70% confluency,the medium was replaced with osteogenic differentiation medium (Cyagen,Suzhou,China,Cat# RAXMX-90021) to induce differentiation until day 21.Osteogenic differentiation was detected by Alizarin Red S staining (Cyagen,Cat# ALIR-10001) for 30 minutes.When BMSCs reached 80% confluency,they were trypsinized,centrifuged to prepare BMSC pellets,cultured in chondrogenic differentiation medium (Cyagen,Cat# RAXMX-90041) until day 21,and stained with Alcian blue (Cyagen,Cat# ALCB-10001) for 30 minutes to detect chondrogenic differentiation.When BMSCs reached 100%confluency,they were cultured in adipogenic differentiation medium (Cyagen,Cat# RAXMX-90031) until day 21 and stained with Oil Red O (Cyagen,Cat# OILR-10001) for 30 minutes to detect adipogenic differentiation.
BMSC-SEV isolation and characterization
To extract SEVs,we replaced the medium with complete medium containing 10% exosome-free FBS (ViVaCell,Shanghai,China,Cat# C3801005) when BMSCs reached 80% confluency.After 48 hours of culture under hypoxic (37°C,5% CO2and 1% O2) or normoxic (37°C,5% CO2and 21% O2) conditions,we collected the culture supernatant and centrifuged at 4°C for 10 minutes to remove dead cells,then centrifuged at 2000 ×g,4°C for 10 minutes to remove cell debris,and finally centrifuged at 10,000 ×g,4°C for 30 minutes.After the initial centrifugation,the supernatant was slowly added to an ultracentrifuge tube (Beckman,US) containing 4 mL of a 30% sucrose/D2O cushion and spun in an ultracentrifuge(Optima XE-100,Beckman,US) at 110,000 ×gat 4°C for 70 minutes,after which the supernatant was discarded.Phosphate-buffered saline (PBS,Hyclone,SH30256.02,USA)was added to dilute the sucrose layer,and after thorough mixing the solution was centrifuged again at 110,000 ×gat 4°C for 70 minutes.The supernatant was removed,and the BMSC-SEV pellet was resuspended in 1 mL PBS to obtain a BMSC-SEV suspension.The morphology of the obtained SEVs was observed using transmission electron microscopy (Hitachi,Japan,Cat# HT-7700).The distribution of the SEV and HSEV diameters was analyzed using a nanoparticle tracking analysis(NTA) particle size analyzer (NanoFCM,Xiamen,China,Cat#N30E).SEV and HSEV protein concentrations were determined using a BCA protein concentration assay kit (Beyotime,Cat#P0012S).Specific SEV surface markers,such as CD9,CD63,and TSG101,were quantitated by western blotting.The following reagents were used: hypersensitive ECL reagent(MedChemExpress,Cat# HY-K1005),mouse anti-TSG101 antibody (1:1000,Invitrogen,Waltham,MA,USA,Cat# MA1-23296,RRID: AB_2208088),rabbit anti-CD9 antibody (1:1000,Sigma,Cat# SAB4503606,RRID: AB_10752277),rabbit anti-CD63 antibody (1:1000,Invitrogen,Cat# PA5-92370,RRID:AB_2806456),HRP-conjugated goat anti-mouse secondary antibody (1:10000,Invitrogen,Cat# 31430,RRID: AB_228307),and HRP-conjugated goat anti-rabbit antibody (1:10,000,Proteintech,Rockford,IL,USA,Cat# SA00001-2,RRID:AB_2722564).
Rat SCI modeling and grouping
We established an SCI model using adult male SD rats (6–8 weeks old,200–260 g).Briefly,rats were anesthetized with isoflurane (induction concentration 3–4%,maintenance concentration 1.5–2%),and laminectomy was performed at the T9–T11 level,exposing the dura at the T10 level at the back of the dural sac.We then used a spinal impactor (RWD,Shenzhen,China,Cat# 68099) to strike the dorsal side of the dural sac with a metal rod (with a striking head 3 mm in diameter) at a speed of 0.5 m/s,to a depth of 1 mm,with a duration of 1 ms.After the injury,muscle and skin were sutured layer-by-layer.The SCI rats were placed in a room with constant temperature and humidity (ambient humidity 55–60%,temperature 22–24°C),subcutaneously injected with 2 mL of normal saline,and intramuscularly injected with 0.1 mL of penicillin sodium (for 3 consecutive days).Each animal’s bladder was manually voided twice a day until bladder function recovered.The criteria for successful establishment of the SCI model were as follows: the dura mater was intact,purple-red,and swollen after the impact,the lower limbs fluttered in a retracted position,after rats awakened from anesthesia their hind limbs exhibited flaccid paralysis,and the Basso-Beattie-Bresnahan (BBB) score was 0.
Rats were divided into four groups (n=20/group) using the random number table method.One group was a sham operation group that underwent simple laminectomy without treatment.Rats in the other three groups were given PBS (500μL),100 μg BMSC-derived SEVs (500 μL),or 100 μg HSEVs (500μL) via tail vein injection after SCI for 3 consecutive days.
BMDM uptake of SEVs
BMSCs were fluorescently labeled using a kit according to the manufacturer’s instructions.Briefly,BMSCs in the logarithmic growth phase (adjusted to a cell density of 1 × 106/mL) were treated with carboxyfluorescein succinimidyl ester (CSFE,MedChemExpress,Cat# HY-D0938) at a final concentration of 5 μM and incubated at 37°C for 10 minutes.After fully washing cells with PBS,complete DMEM was added.Reactions were terminated by incubating at 37°C for 10 minutes.Culture flasks were then re-inoculated with 10% exosome-free FBS DMEM complete medium and cultured for 48 hours under hypoxic (37°C,5% CO2and 1% O2) or normoxic (37°C,5% CO2and 21% O2) conditions.These supernatants were collected to obtain CFSE-labeled SEVs or HSEVs.These CFSE-labeled SEVs or HSEVs were then co-cultured with BMDMs for 24 hours,after which the cells were washed with PBS and fixed in 4% paraformaldehyde.BMDMs were labeled with Alexa 647 rabbit anti-Iba1 antibody (1:400,Cell Signaling Technology,Danvers,MA,USA,Cat# 78060S,RRID: AB_2943374),and nuclei were stained with antifade mounting medium with DAPI (Beyotime,Shanghai,China,Cat# P0131).The uptake of CFSE-labeled SEVs or HSEVs by BMDM was observed using an inverted fluorescence microscope (DMI8,Leica Microsystems,Wetzlar,Germany).
In vitro BMDM grouping
To compare the efficacy of SEVs and HSEVs in stimulating macrophage polarization,BMDMs were divided into four groups.One group was the conventional control group (NC),in which medium was replaced with fresh medium alone,while the other groups were exposed to SEVs or HSEVs.After LPS(500 ng/mL,24 hours) stimulation,cells were treated with PBS(200 μL,24 hours,group: LPS+PBS),SEVs (200 μL,24 hours,group: LPS+SEVs),or HSEVs (200 μL,24 hours,group: LPS +HSEVs).After 24 hours,cells and cell supernatants from the four groups were collected for subsequent experiments.
To verify whether HSEVs induce phenotypic changes in BMDMs by delivering miR-146a-5p,the BMDMs were divided into four groups (n=3/group).Each group was stimulated with 500 ng/mL LPS for 24 hours to induce polarization to the M1 phenotype.LPS+miR-146a mimic (GenePharma,Shanghai,China) group: after co-incubating 5 μL of miR-146a mimic at a concentration of 20 μM and 5 μL of Hieff Trans liposomal transfection reagent (Yeasen,Shanghai,China,Cat#40802ES02) at room temperature for 20 minutes,the nucleic acid/liposome solution was mixed with 100 μL of 0.2 μg/μL of fresh HSEV medium and incubated with BMDMs for 24 hours.LPS+NC mimic (GenePharma) group: after co-incubating 5 μL of 20 μM NC mimic (negative control mimic of miR-146a) and 5 μL Hieff Trans liposome nucleic acid transfection reagent at room temperature for 20 minutes,the nucleic acid/liposome solution was mixed with 100 μL fresh HSEV medium at a concentration of 0.2 μg/μL and incubated with cells for 24 hours.LPS+miR-146a inhibitor group: after coincubating 5 μL of 20 μM miR-146a inhibitor (GenePharma)with 5 μL of Hieff Trans liposome nucleic acid transfection reagent at room temperature for 20 minutes,the nucleic acid/liposome solution was mixed with 100 μL of 0.2 μg/μL of fresh HSEV medium.Cells were incubated with fresh HSEV culture medium for 24 hours.LPS+NC inhibitor (GenePharma)group: after co-incubating 5 μL of 20 μM NC inhibitor (negative control for miR-146a inhibitor) and 5 μL Hieff Trans liposome nucleic acid transfection reagent at room temperature for 20 minutes,the nucleic acid/liposome solution was mixed with 100 μL of fresh 0.2 μg/μL HSEV medium and co-incubated with cells for 24 hours.Cells and supernatants from each group were collected for subsequent experiments.
Motor function assessment BBB motor function scores
BBB scores (Basso et al.,1995) were used to evaluate recovery of hind limb motor function.Rats were placed on a 3-m2platform,and researchers who were blinded to the group assignments observed and recorded hind limb walking and physical activity scores.Scores ranged from 0 points (complete paraplegia) to 21 points (normal function).Hind limb joint mobility,hind limb coordination,and fine paw movements were assessed.Each group was scored 1 day before surgery and on days 1,3,7,14,21,and 28 post-SCI.
Catwalk-assisted gait analysis
Gait analysis was performed for each group of rats using a CatWalk XT automated quantitative gait analysis system(Noldus,Wageningen,Netherlands) 28 days after SCI.This experiment mainly assessed the influence of the following three gait parameters on relevant behavioral changes after SCI: base of support,measured as the width of the area between the left and right hind paws;stride length;and regularity index.Parameter results were automatically calculated using the analysis system’s software.
Electrophysiology
The motor-evoked potentials of rats on day 28 after SCI were tested using a neuroelectrophysiological monitor (Medtronic,Minneapolis,MN,USA) to evaluate functional recovery.Rats were anesthetized with isoflurane (induction concentration 3–4%,maintenance concentration 1.5–2%).The stimulating electrode was then applied to the rostral end of the operated spinal cord,the recording electrode was placed at the flexor tendon of the biceps femoris,the reference electrode was inserted into the distal tendon of the hind limb muscles,and the ground electrode was placed subcutaneously.Single square-wave stimuli (duration,75 seconds;stimulus intensity,50 V;filter,200 Hz) were applied.Area under the curve and peak amplitude were used to detect nerve conduction function of rat hind limbs.For all motor function assessments,each rat was evaluated by two independent examiners blinded to the treatment regimen.
Hematoxylin-eosin staining and lesion measurement
On day 28,rats were anesthetized with isoflurane (induction concentration 3–4%) and sequentially perfused with 0.9%normal saline and 10% formaldehyde through the heart.Lesioned spinal cord tissue (2 cm in length,centered on the injury site) was harvested and fixed overnight in 10%formaldehyde.After stepwise dehydration in a graded ethanol series,the samples were cleared,and embedded in paraffin,and sliced into 3-μm continuous coronal sections using a HistoCore AUTOCUT (Leica Camera AG,Germany).Staining was performed using a hematoxylin-eosin (H&E) staining kit (Solarbio,Beijing,China,Cat# G1120) according to the manufacturer’s instructions.The lesion size in the H&E-stained images was analyzed using ImageJ software (version 1.53k,Media Cybernetics,Silver Spring,MD,USA).
Immunofluorescence
Tissue immunofluorescence staining
Spinal cord sections were prepared as described above.For tissue immunofluorescence staining,sections were blocked with blocking buffer (1× PBS/5% BSA/0.3% TritonTMX-100) for 1 hour at room temperature.Sections were incubated with mouse anti-Iba1 antibody (1:100,Abcam,Cambridge,UK,Cat# ab283319,RRID: AB_2924797) and rabbit anti-inducible nitric oxide synthase (iNOS) antibody (1:50,Invitrogen,Cat#PA1-036,RRID: AB_325773) or mouse anti-Iba1 antibody and rabbit anti-Arginase 1 (Arg1) antibody (1:200,Invitrogen,Cat# PA5-85267,RRID: AB_2792410) overnight at 4°C,then Alexa Fluor 488 goat anti-rabbit antibody (1:200,Invitrogen,Cat# A-11034,RRID: AB_2576217) and Alexa Fluor 568 goat anti-mouse antibody (1:200,Invitrogen,Cat# A-11004,RRID:AB_2534072) were added at room temperature,and thetissue sections were incubated in a dark box.After incubation for 60 min,the slides were mounted with antifade reagent containing DAPI.An OlyVIA VS200 microscope was used to view and image the sections.The proportion of different types of cells was determined using ImageJ software.
BMDM immunofluorescent staining
BMDM cultured for 7 days were inoculated into a sixwell plate (a sterile glass slide was placed in each well in advance).Cells were stimulated with LPS,then treated with PBS,SEVs,or HSEVs,as above.Cells were then fixed in 4%paraformaldehyde and stored at room temperature.After blocking with blocking buffer (1 × PBS/5% BSA/0.3% TritonTMX-100) for 1 hour,mouse anti-Iba1 antibody and rabbit anti-iNOS antibody or mouse anti-Iba1 antibody and rabbit anti-Arg1 antibody were added to the six-well plate and incubated at 4°C overnight.Alexa Fluor 488 goat anti-rabbit antibody and Alexa Fluor 568 goat anti-mouse antibody were added,and the cells were incubated for 60 min at room temperature in a dark box.The slides were then removed and sealed with an anti-fluorescence quencher containing DAPI.A Leica confocal laser-scanning microscope (Leica,TCA SP8) was used to capture images.The proportion of different types of cells was determined using ImageJ software.
Flow cytometry analysis
BMDMs were collected after LPS stimulation and treatment with PBS,SEVs,or HSEVs,as described above.Then the cells were permeabilized and fixed using a fixation/permeabilization kit (BD Bioscience,Cat# 554714) according to the manufacturer’s protocol.The cells were then incubated with rabbit anti-Arg1 antibody at 4°C for 30 minutes,washed with PBS,then incubated with PE donkey anti-rabbit antibody(1:200,eBioscience,Cat# 12-4739-81,RRID: AB_1210761)at 4°C for 30 minutes.After washing with PBS,the cells were incubated with CoraLite? plus 488 rabbit anti-iNOS antibody(1:500,Proteintech,Cat# CL488-18985,RRID: AB_2919161)and Alexa 647 anti-Iba1 antibody at 4°C for 30 minutes.Flow cytometry was performed using a C6 Plus flow cytometer (BD Biosciences),and data were analyzed using FlowJo software(TreeStar,Ashland,OR,USA).
Western blotting
Proteins were extracted from SEVs,HSEVs,cells,and tissues using radio immunoprecipitation assay (RIPA) lysis buffer(Meilunbio,Dalian,China,Cat# MA0151) containing a protease inhibitor (Meilunbio,Cat# MB2678) and a protein phosphatase inhibitor (Solarbio,Cat# P1260).Protein concentration was determined using a BCA protein assay kit (Beyotime,Cat# P0012S).Twenty micrograms of each sample was loaded into a FuturePAGE protein precast gel(ebio-ace,Nanjing,China,Cat# ET12420LGel) and subjected to electrophoresis for 1 hour to separate equal amounts of protein.The wet transfer method was used to transfer protein to nitrocellulose membranes (Pall,Beijing,China,66485).Membranes were incubated with 5% nonfat dry milk for 3 hours on a shaker at room temperature,then incubated with primary antibody for 12 hours at 4°C.β-Actin was used as a loading control.Membranes were incubated with appropriate peroxidase-conjugated secondary antibodies with shaking at room temperature for 1 hour.Protein bands were visualized using ultrasensitive ECL reagents and a chemiluminescent imager (e-BLOT,Shanghai,China).Protein band intensity was semi-quantified using ImageJ.The following antibody were used: mouse anti-β-actin antibody (1:10,000,Invitrogen,Cat#MA1-140,RRID: AB_2536844),rabbit anti-CD206 antibody(1:2000,Abcam,Cat# ab64693,RRID: AB_1523910),rabbit anti-IRAK1 antibody (1:2000,ABclonal,Wuhan,China,Cat#A12624,RRID: AB_2759468),rabbit anti-iNOS antibody(1:2000,Invitrogen,Cat# PA1-036,RRID: AB_325773),rabbit anti-TNF receptor-associated factor 6 (TRAF6) antibody(1:2000,Abcam,Cat# ab33915,RRID: AB_778572),rabbit anti-NF-κB antibody (1:2000,Abcam,Cat# ab76302,RRID: AB_1524028),HRP-conjugated goat anti-mouse secondary antibody (1:10,000,Invitrogen,Cat# 31430,RRID:AB_228307),and HRP-conjugated goat anti-rabbit antibody(1:10,000,Proteintech,Cat# SA00001-2,RRID: AB_2722564).
Enzyme-linked immunosorbent assay
As described above,rat spinal cord tissue was obtained on day 7 after surgery and cut into small pieces (1 mm3),added to pre-cooled RIPA lysate containing protease inhibitor,ground in liquid nitrogen to make a homogenate,ultrasonicated to shear the nucleic acids,lysed at 4°C for 20 minutes,and centrifuged at 12,000 ×gfor 5 minutes.Supernatants were analyzed to detect cytokine expression levels using an enzyme-linked immunosorbent assay kit (TNF-α [Beyotime,Cat# PT516],IL-1β [Beyotime,Cat# PI303],TGF-β [Beyotime,Cat# PT878],IL-10 [Beyotime,Cat# PI525]).Optical densities were measured using a microplate reader (Multiskan MK3,Thermo Fisher Scientific,Waltham,MA,USA),and the corresponding cytokine concentrations were extrapolated from a standard curve.
Quantitative reverse transcription-polymerase chain reaction
Total RNA was extracted from cells using TRIzol reagent(Invitrogen,15596026),according to the manufacturer’s instructions.RNA was reverse transcribed into cDNA using an miRNA fluorescence quantitative reverse transcriptionpolymerase chain reaction (qRT-PCR) kit (GenePharma).A real-time fluorescent quantitative PCR instrument (ABI 7500,Thermo Fisher Scientific) and SYBR Green PCR mix(GenePharma) were used to amplify cDNA.U6 was used as a reference.The 2–ΔΔCTmethod was used to calculate and analyze miRNA expression levels.Primers for miR-146a-5p and U6 were designed and synthesized by Shanghai Gemma Pharmaceutical Technology Co.,Ltd.,China.Primer sequences are listed inTable 1.

Table 1 |Primer sequences used for quantitative reverse transcription-polymerase chain reaction
Detection of the miRNA content of SEVs and HSEVs and differential expression analysis
MiRNA detection in SEVs and HSEVs was performed by extracting RNA extraction from SEVs and HSEVs,followed by miRNA-seq library construction and high-throughput sequencing using an Illumina HiSeq (Illumina,San Diego,CA,USA),which can obtain millions of miRNA sequences simultaneously.Finally,quantitative statistical analysis was performed to assess differences in miRNA levels.Differential expression analysis was performed by Nanjing Jiangbei New District Biomedical Public Service Platform Co.,Ltd.using DESeq2 (http://www.bioconductor.org/packages/release/bioc/html/DESeq2.html).
Statistical analysis
No statistical methods were used to predetermine sample sizes;however,our sample sizes are similar to those reported in a previous publication (Liu et al.,2020).GraphPad Prism 8.0(GraphPad Software,Inc.,San Diego,CA,USA,www.graphpad.com) was used to process and analyze data and images.Data are shown as mean ± standard deviation.We calculatedPvalues using Student’st-test for two-group comparisons and one-way analysis of variance for more than two-group comparisons followed by Tukey’spost hoctest.All tests were two-sided,and values ofP<0.05 were considered statistically significant.
Results
Identification of BMSCs,primary macrophages,and BMSCderived exosomes
BMSCs grew adherently and were fusiform or irregularly triangular in shape at 80–90% confluency (Additional Figure 1A).Adipogenic (Additional Figure 1B),osteogenic (Additional Figure 1C),and chondrogenic differentiation (Additional Figure 1D) of BMSCs was confirmed using appropriate kits (described in the Methods).Flow cytometry analysis confirmed that BMSCs were positive for CD90 and CD29,but negative for CD34 and CD45 (Additional Figure 1E).BMDMs were isolated from rat bone marrow as described in the Methods and cultured until day 7 (Additional Figure 1F),when their identity was confirmed by flow cytometry and their purity was confirmed by Iba1 staining (Additional Figure 1G).SEVs and HSEVs were isolated from BMSC culture supernatants by density gradient ultracentrifugation and analyzed by electron microscopy,NTA,and western blotting.A typical exosome structure was observed by transmission electron microscopy (Figure 1A).The NTA results for SEVs(average particle size: 84.82 nm,range: 52.25–149.25 nm)and HSEVs (average particle size: 84.04 nm,range: 50.25–148.75 nm) showed similar particle size distributions (Figure 1B),and the concentration of particulates was higher under anoxic conditions than under normoxic conditions (Figure 1C).Western blotting was used to detect the cellular markers CD63,CD9,and TSG101 in the SEVs (Figure 1D).

Figure 1 | Characterization of BMSC-derived SEVs and HSEVs.
HSEVs attenuate spinal cord injury in rats more effectively than SEVs
To determine whether exosomes from MSCs exposed to hypoxic conditions improve motor function after SCI,we first assessed functional recovery in SCI rats treated with PBS,SEVs,or HSEVs using BBB scoring.At 14,21,and 28 days postinjury,rats in the SEV group exhibited superior functional improvement compared with those in the PBS group (P<0.05).Notably,at the 28-day mark post-injury,the BBB scores of the HSEV group significantly exceeded those of the SEV group (P<0.05;Figure 2A).Electrophysiological testing of rat hind limbs supported the conclusions derived from the BBB scores (Figure 2B).On day 28 after injury,the area under the curve (Figure 2C) and amplitude (Figure 2D) of motor-evoked potentials in the HSEV group were significantly higher than those in the SEV group (P<0.05),indicating that the hind limbs showed better neurological status after HSEV administration.Gait analysis indicated that hind limb gait recovery was significantly better in the SEV (P<0.05) and HSEV (P<0.05) groups than in the PBS group (Figure 2E–H).H&E staining of the spinal cord showed that the areas of the spinal cord lesions in the SEV (P<0.05) and HSEV groups (P<0.05) were significantly smaller than those in the PBS group,and the lesion areas in the HSEV group were significantly smaller than those in the SEV group(P<0.05;Figure 2IandJ).These results indicate that BMSCderived SEVs or HSEVs reduced the severity of SCI in rats,and that HSEVs were more effective than SEVs.
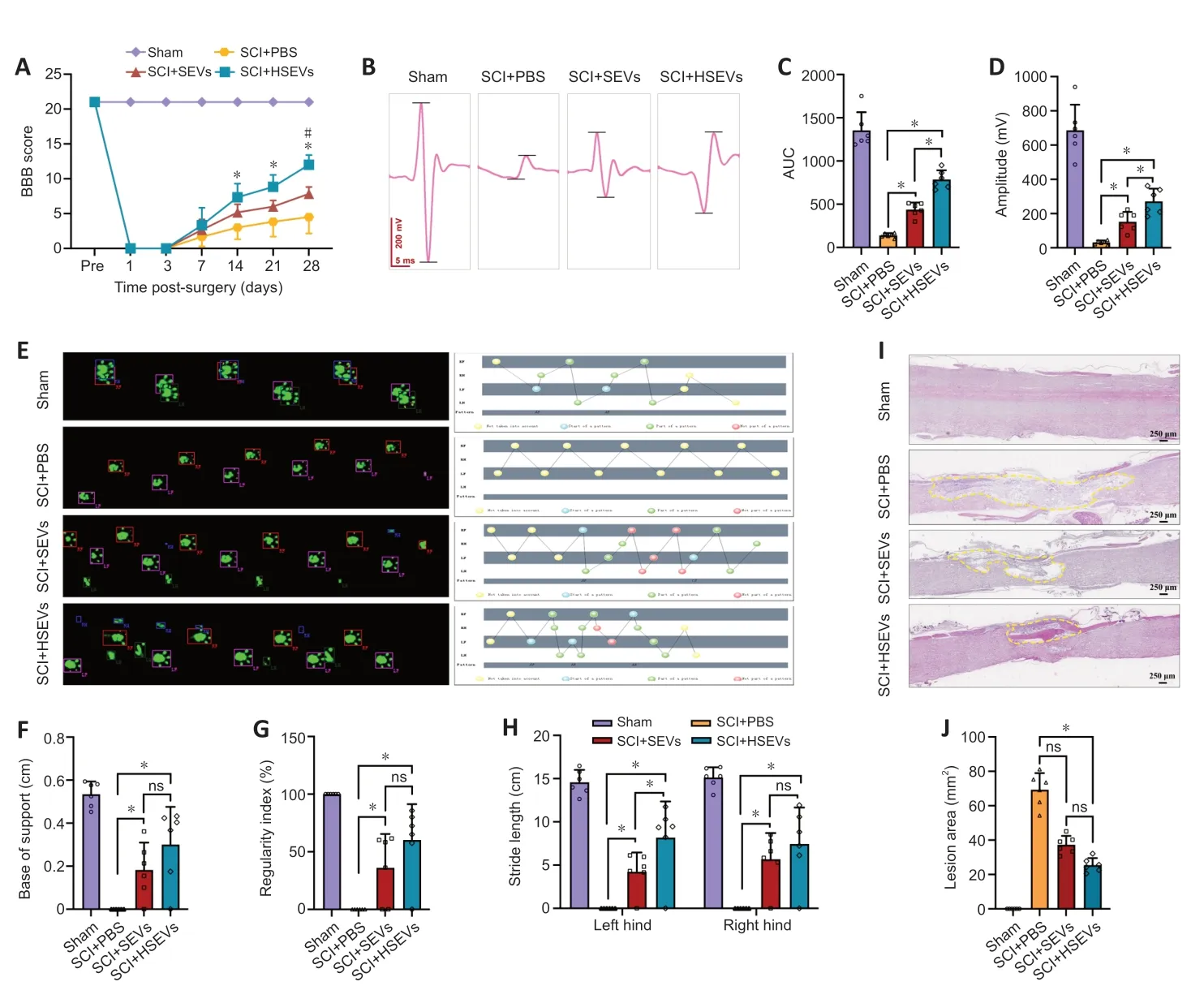
Figure 2 |BMSC-derived HSEVs promote motor function recovery in SCI rats.
HSEVs alter local macrophage M1/M2 phenotypic polarization in injured spinal cords
During SCI,macrophages may regulate the progression of inflammation by altering their polarization state.In this study,we found that BMSC-derived SEVs and HSEVs improved motor function after SCI.To determine whether this phenomenon was associated with SEV-or HSEV-mediated modulation of macrophage polarization,macrophage M1/M2 subsets were detected by immunofluorescence in spinal cord sections from rats 7 days post-injury.The results demonstrated a significant decrease in the proportion of Iba1+iNOS+cells (M1 subtype) within both the SEV group (P<0.05) and the HSEV group (P<0.05) compared with that in the PBS group (Figure 3A).Additionally,a notable increase was observed in the proportion of Iba1+Arg1+cells (M2 subtype) in the SEV (P<0.05) and HSEV groups (P<0.05) compared with that in the PBS group (Figure 3B).Comparing with the SEV group,the proportion of Iba1+iNOS+cells was significantly decreased (P<0.05),and the proportion of Iba1+Arg+cells was significantly increased,in the HSEV group (P<0.05).Next,total protein was extracted from rat spinal cord tissue 7 days after injury and subjected to western blot analysis.The results showed that,compared with the findings in the SEV group,the reduction in iNOS expression (P<0.05) and the increase in CD206 expression (P<0.05) were more pronounced in the HSEV group (Figure 3C).Seven days after SCI,we measured concentrations of the pro-inflammatory cytokines TNF-α and IL-1β and the anti-inflammatory cytokines TGF-β and IL-10 in the rat spinal cord by enzyme-linked immunosorbent assay.The administration of either SEVs (P<0.05) or HSEVs(P<0.05) significantly decreased the concentrations of proinflammatory cytokines compared with the findings in the PBS control group (Figure 3D).The inhibition of pro-inflammatory cytokine secretion was more pronounced in the HSEVs group compared with that in the SEVs group (P<0.05).Additionally,the administration of either SEVs (P<0.05) or HSEVs (P<0.05) significantly increased the concentrations of antiinflammatory cytokines compared with the findings in the PBS control group (Figure 3E).The promotion of anti-inflammatory cytokine secretion was more pronounced in the HSEV group compared with that in the SEV group (P<0.05).These results indicate that BMSC-derived SEVs or HSEVs effectively altered the balance of the M1/M2 subpopulations,and that HSEVs were more effective than SEVs at promoting M2 polarization.
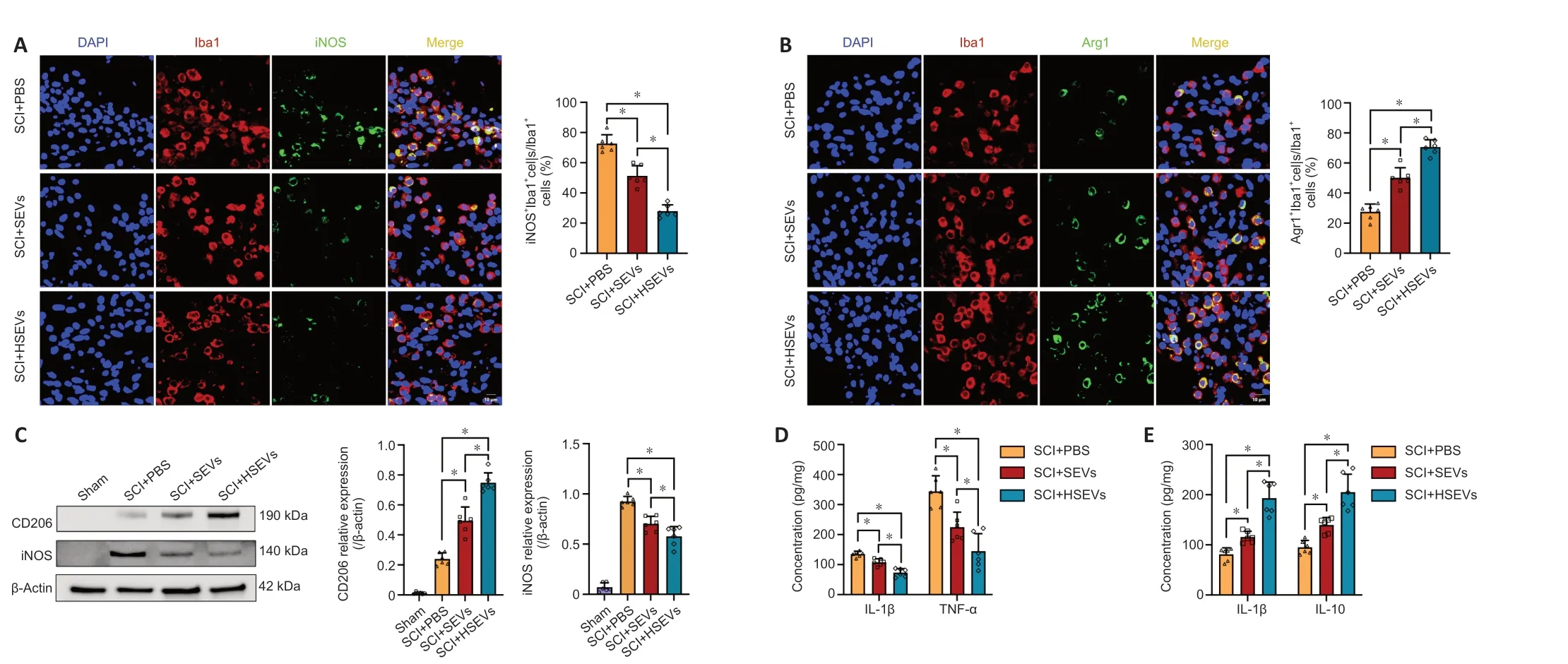
Figure 3 | Effect of BMSC-derived HSEVs on local M1/M2 subpopulation ratio in the injured rat spinal cord.
HSEVs trigger macrophage polarization from an M1 to an M2 phenotype in vitro
We demonstrated that SEVs/HSEVs alter local M1/M2 subpopulations in the injured spinal cord.Next,we asked whether SEVs or HSEVs exert similar therapeutic effectsin vitroas those observedin vivo.To test this,mononuclear cells were isolated from rat bone marrow and cultured under macrophage colony stimulating factor stimulation for 7 days to obtain BMDMs.When CFSE-labeled SEVs or HSEVs were added to the BMDM culture medium,they were taken up by the BMDMs and were visible in the cytoplasm within 12 hours (Figure 4A).LPS (50 ng/mL) was added to the culture medium,and BMDMs were incubated for 12 hours to induce an inflammatory microenvironment before the being incubated with PBS,SEVs,or HSEVs for 24 hours.Western blot analysis showed downregulation of iNOS and increased expression of CD206 in the SEV (P<0.05) and HSEV groups (P<0.05) compared with the findings in the PBS group (Figure 4B),similar to thein vivoresults.Cell immunofluorescence analysis revealed downregulation of iNOS and increased Arg-1 expression in the SEV (P<0.05) and HSEV groups (P<0.05;Figure 4CandD) compared with the findings in the PBS group,with more pronounced effect in the HSEV group than in the SEV group (P<0.05).Flow cytometric analysis of the polarization state of M1/M2 BMDMs showed that HSEVs exerted more beneficial effects than SEVs (Figure 4E).These results suggest that HSEVs plays a more pronounced role than SEVs in regulating BMDM polarization toward an M2 phenotypein vitro,further confirming thein vivoresults.
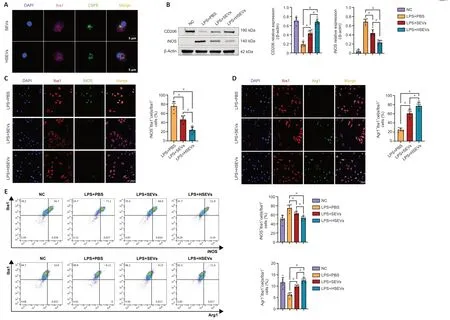
Figure 4 |BMSC-derived HSEVs trigger polarization of primary macrophages (BMDMs) from an M1 to an M2 phenotype in vitro.
HSEVs regulate the Irak1/Traf6/NF-κB signaling pathway through miR-146a-5p
Ourin vivoandin vitroassays demonstrate that HSEVs are more effective than SEVs at promoting macrophage polarization to the M2 phenotype and attenuating the effects of SCI.Previous studies have shown that SEVs can regulate gene expression in target cells via miRNAs (Liang et al.,2022).To understand how HSEVs regulate macrophage polarization,we performed high-throughput sequencing and differential analysis of the miRNAs carried by BMSC-derived SEVs and HSEVs.We found that three miRNAs (miR-146a-5p,miR-21-5p,and miR-125a-5p) were significantly upregulated in HSEVs compared with the findings in SEVs (Figure 5AandB).Comparison to the relevant literature and the fact that miR-146a-5p was the most highly expressed of the three miRNAs in HSEVs led us to select miR-146a-5p as the key cargo by which HSEVs regulate macrophage polarization.
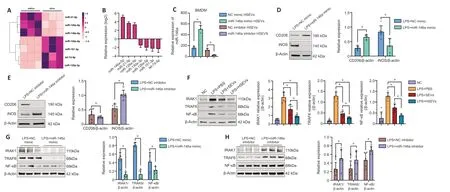
Figure 5 | BMSC-HSEV-transported miR-146a-5p regulates macrophage polarization by targeting IRAK1.
To confirm the role of miR-146a-5p in BMDM polarization,we performed miR-146a-5p overexpression (miR-146a mimic) and knockdown (miR-146a inhibitor) assays using a lipofection-based approach incorporating BMSCs and corresponding negative controls (NC and NC inhibitors).Transfection efficiency was confirmed by qRT-PCR (Figure 5C).We then treated LPS-stimulated BMDMs with NC mimic HSEVs or miR-146a mimic HSEVs for 24 hours and extracted total protein for western blot analysis.The miR-146a mimic HSEVs induced significantly downregulation of iNOS expression and upregulation of CD206 expression,compared with NC mimic HSEVs (P<0.05;Figure 5D).We also treated BMDMs with NC inhibitor HSEVs or miR-146a inhibitor HSEVs and performed western blot analysis.These results showed that HSEVs containing the miR-146a inhibitor upregulated iNOS expression (P<0.05,compared with NC inhibitor HSEVs)and downregulated CD206 expression (P<0.05,compared with NC inhibitor HSEVs;Figure 5E).These results indicate that miR-146a-5p is a key component of HSEV-mediated M2 macrophage polarization.To better understand how miR-146a-5p regulates macrophage phenotype,we consulted the miRNA target database (miRBase) and related literature and found that interleukin-1 receptor-associated kinase 1 (IRAK1)may be involved in the attenuation of potential targets (Zhang et al.,2018,2021;Li et al.,2020).Previous studies have shown that,during inflammation,IRAK1 upregulates TRAF6 and NFκB expression,thereby promoting inflammation (Zhang et al.,2021;Xiong et al.,2022).Therefore,we next collected PBS-,SEV-,and HSEV-treated BMDMs for western blot analysis.Compared with those in the PBS group,the expression levels of IRAK1,TRAF6,and NF-κB in the SEV group (P<0.05) and the HSEV group (P<0.05) were clearly elevated,and this effect was more pronounced in the HSEV group than in the SEV group (Figure 5F).To confirm that the IRAK1/TRAF6/NFκB signaling pathway is targeted by miR-146a in macrophages,we measured IRAK1 expression in macrophages treated with HSEVs containing the miR-146a mimic.IRAK1,TRAF6,and NF-κB expression levels were significantly decreased in the miR-146a mimic group (P<0.05) compared with those in the group NC mimic (Figure 5G).Furthermore,the miR-146a inhibitor group showed higher expression levels of IRAK1,TRAF6,and NF-κB than the NC inhibitor group (P<0.05;Figure 5H).Taken together,these results suggest that HSEVs promotes M2 macrophage polarization via miR-146a-5pmediated downregulation of the IRAK1/TRAF6/NF-κB pathway.
Discussion
SCI is a serious central nervous system injury with poor prognosis,often involving the destruction of axons and neurons,activation of inflammatory cells,apoptosis,reactive glial scar formation,and other pathological processes (Hutson and Di Giovanni,2019;Locke et al.,2022;Myatich et al.,2023;Shen et al.,2023).In this study,a rat SCI model was constructed and used to verifyin vivothat SEVs and HSEVs alleviate SCI and promote M2 polarization of macrophages.Furtherin vitroexperiments verified that HSEV exposure promotes macrophage M2 polarization and relieves spinal cord inflammation.We also demonstrated that miR-146a-5p is enriched in HSEVs and regulates macrophage polarization through the IRAK1/TRAF6/NF-κB pathway.
Macrophages are effector cells that participate in the occurrence and resolution of spinal cord inflammation in SCI.M1-type macrophages are typically rapidly induced and maintained in the injured spinal cord,thereby promoting the progression of spinal cord inflammation (Gensel and Zhang,2015;Kong and Gao,2017).The importance of macrophages in SCI models has been reviewed previously (David and Kroner,2011;Ding et al.,2021).Previous studies have demonstrated that MSC-derived SEVs potently modulate macrophage polarization towards the M2 phenotypein vitroand in SCI rats.SEV-induced macrophage polarization attenuates the pro-inflammatory cascade and enhances subsequent repair activities,thereby limiting the area of SCI (Sun et al.,2018a;Lai et al.,2022).These previous findings are consistent with the conclusions from the current study.Furthermore,HSEVs,which were generated by exposing BMSCs to hypoxia,enhanced the restriction of secondary inflammatory responses due to SCI.
SEVs are thought to play important roles in various diseases(He et al.,2018;Kalluri and LeBleu,2020;Li et al.,2021).MiRNAs are considered essential components of SEVs and largely determine the impact of SEVs on target cells (Zhang et al.,2015;Sun et al.,2018b).We believe that the difference in potency between SEVs and HSEVs is related to the different compositions of the miRNA cargoes that they carry.Thus,we performed miRNA sequencing of SEV and HSEV miRNA contents.By comparing our own sequencing data with publicly available exosomal miRNA profiles and miRNA target prediction databases,we identified miR-146a-5p as the best candidate.To date,few studies have explored the relationship between miR-146a-5p and SCI,and the role of miR-146a-5p in the immunomodulatory effects of HSEVs remains unclear.Our results show that inhibiting miR-146a-5p in HSEVs attenuates the immunomodulatory effects of HSEVs on macrophages.
Previous studies have shown that miR-146a-5p specifically targets and downregulates the IRAK1/TRAF6/NF-κB pathway(Gao et al.,2015;Zhang et al.,2018).Here,we explored whether miR-146a-5p and the IRAK1/TRAF6/NF-κB pathway are involved in HSEV-dependent macrophage polarization.Ourin vitroexperimental data confirmed that IRAK1,TRAF6,and NF-κB expression levels were significantly downregulated in the HSEV group compared with those in the PBS and SEV groups.Overexpression of the miR-146a-5p mimic in the HSEV group inhibited the IRAK1/TRAF6/NF-κB pathway,and inhibition of the IRAK1/TRAF6/NF-κB pathway was partially reversed by decreasing the amount of miR-146a-5p carried by the HSEVs.These data strongly suggest that miR-146a-5p in HSEVs mediates macrophage polarization by inhibiting the IRAK1/TRAF6/NF-κB signaling cascade.Zhang et al.(2019)reported that miR-146-5p drives macrophage differentiation toward the M2 phenotype by targeting STAT1 to exacerbate viral transmission in thrombocytopenia.This potential mechanism of crosstalk between the IRAK1/TRAF6/NF-κB and STAT1 signaling pathways merits further exploration.
Our results highlight the important role that miR-146a-5p plays in mediating the anti-inflammatory effects of HSEVs and the mechanism by which HSEVs alleviate neuroinflammation in the injury microenvironment after SCI.HSEVs are well known to transmit biologically active substances such as proteins and various RNAs and DNAs to recipient cells,thereby altering gene expression in the recipient cells (He et al.,2018).The anti-inflammatory effect of HSEVs is thus not determined by a single substance;rather,it is regulated by multiple factors.We found miR-146a-5p to be a key miRNA in this process,and verified that it positively regulates the anti-inflammatory effects of HSEVsin vitro.While this only partially accounts for the anti-inflammatory potency of HSEVs,it does provide a potential target for therapeutic intervention.Further research is required to determine the specific mechanisms by which HSEV cargoes activated or inhibit downstream targets in recipient cells,and whether this network could be therapeutically modulated to enhance neuroprotection.
This study has several limitations that should be acknowledged.Firstly,even though our results demonstrated improved motor function in SCI rats following 28 days of treatment with HSEVs,we have yet to explore the mechanism by which HSEVs traverse the circulatory system to reach the injury site,and whether there are differences in the targeting properties of SEVs and HSEVs.Secondly,our high-throughput sequencing analysis highlighted an elevated content of miR-21-5p and miR-125a-5p in HSEVs compared with the findings in SEVs,and these miRNAs may also influence macrophage polarization in the context of SCI.Thus,the observed impact of miR-146a-5p on SCI could be affected by these additional factors,thereby leading to either an overestimation or an underestimation of its effects.Furthermore,while our study yielded meaningful insights within the 28-day observation period,extending the duration of the observation period would be important for capturing potential longer-term dynamics and outcomes.
In conclusion,our study demonstrated that HSEVs derived from BMSCs exposed to hypoxic conditions can suppress spinal cord inflammation in SCI rats via miR-146a-5p and alleviate neurological impairment.High levels of miR-146a-5p in HSEVs promote macrophage polarization towards the M2 antiinflammatory phenotype by inhibiting the IRAK1/TRAF6/NFκB signaling pathway,highlighting their therapeutic potential.Furthermore,the diverse roles of SEVs and their plentiful cargo cannot be detailed but should not be overlooked.
Author contributions:CC and CW designed and supervised this study.ZL,ZY,HX,and JR conducted the majority of the experiments and completed the manuscript.XX and YL analyzed the data.HSX and CW produced the spinal cord injury model.All authors approved the final version of the manuscript.
Conflicts of interest:The authors declare that they have no competing interests.
Data availability statement:Most of the datasets supporting the conclusions of this article are included within this article and its additional files.The datasets used or analyzed during the current study are available on reasonable request.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional file:
Additional Figure 1:Identification of BMSCs and BMDMs.
- 中國神經(jīng)再生研究(英文版)的其它文章
- Corrigendum: Sorl1 knockout inhibits expression of brain-derived neurotrophic factor:involvement in the development of late-onset Alzheimer’s disease
- Global trends in diabetic eye disease research from 2012 to 2021
- p38 MAPK inhibitor SB202190 suppresses ferroptosis in the glutamate-induced retinal excitotoxicity glaucoma model
- Lycium barbarum glycopeptide (wolfberry extract)slows N-methyl-N-nitrosourea-induced degradation of photoreceptors
- Magnesium-L-threonate treats Alzheimer’s disease by modulating the microbiota-gut-brain axis
- 3′-Deoxyadenosin alleviates methamphetamineinduced aberrant synaptic plasticity and seeking behavior by inhibiting the NLRP3 inflammasome

