Pulsed gas-liquid discharge plasma catalytic degradation of bisphenol A over graphene/CdS: process parameters optimization and O3 activation mechanism analysis
Nan JIANG(姜楠),Xuechuan LI(李學(xué)川),Ju LI(李舉),Jie LI(李杰),2,?,Bing LIAO (廖兵), Bangfa PENG (彭邦發(fā)) and Guo LIU (劉國)
1 School of Electrical Engineering,Dalian University of Technology,Dalian 116024,People’s Republic of China
2 Key Laboratory of Industrial Ecology and Environmental Engineering,University of Technology,Dalian 116024, People’s Republic of China
3State Environmental Protection Key Laboratory of Synergetic Control and Joint Remediation for Soil &Water Pollution, Chengdu University of Technology, Chengdu 610059, People’s Republic of China
Abstract
Keywords: pulsed discharge, gas-liquid discharge, wastewater treatment, parameters optimization, DFT calculation
1.Introduction
Bisphenol A(BPA)is an organic raw material widely used to synthesize chemical products such as epoxy resin,polycarbonate, pesticides, antioxidants and coatings, but it is also an emerging contaminant [1].BPA can be discharged into the environment by means of several different channels,including wastewater produced during industrial papermaking, plastics, as well as from landfill leachates [2].When the human body ingests BPA, it will interfere with hormone activities, causing endocrine disorders, and thus endangering human health.Recently, advanced oxidation processes have been established for contaminant degradation by strong oxidization, including photocatalytic degradation [3-6], electrocatalytic degradation [7], Fenton and photo-Fenton-like oxidation [8], ozone oxidation [9] and non-thermal plasma degradation [10, 11].
Among the above techniques,pulsed discharge plasma is particularly promising on account of its environmentally friendly properties,including generation in the gas phase[12],liquid phase [13] or gas-liquid hybrid phase [14].It is generally believed that a discharge is more easily generated in the gas phase or gas-liquid phase than the liquid phase.During pulsed discharge plasma processes,a mass of reactive oxygen species (ROS) can be generated in situ in liquid and gasliquid environments, such as O3, H2O2, ·OH, ·O, etc, which can uniformly disperse in solution and degrade the organic contaminants.Hydroxyl radical(·OH) is of special concern among the ROS, because the oxidation potential of ·OH is 2.80 V and which is capable of oxidizing a broad range of contaminants non-selectively [15].Simultaneously, physical effects such as ultraviolet light concomitantly produced in a gas-liquid discharge can also improve the efficiency of contaminant degradation.
The amount of O3generated during discharge is greater than that of ·OH, but the oxidizing capacity of O3is much lower than that of ·OH [16].Therefore, it is critical to boost the conversion of O3to ·OH.The most promising way to promote the O3conversion reaction is to introduce a catalyst.Different catalysts have been designed and employed to promote the conversion of O3either in the gas or liquid phase[17, 18].As a kind of n-type semiconductor photocatalyst,CdS has the unique advantage of various crystallite phases to enhance activity, a narrow band gap (2.42 eV) to achieve‘pseudo photocatalysis’ and a suitable band structure for the reduction of O2/O3to·O-2/·O-3[19-23].However,pure CdS has several problems such as a high charge recombination rate,serious photocorrosion,and the photogenerated holes are prone to oxidize sulfide ions in CdS, resulting in poor stabilityand lowphotocatalytic activity [24-26].Therefore, the photocorrosion and photocatalytic activity of CdScan be improved by doping two-dimensional planar monoatomic layer graphene oxide (GO) with a high specific surface area and carrier mobility.In previous work, we have explored the combination of plasma and a series of reduced GO-CdS(rGO(x)/CdS)catalysts to degrade BPA.Similar to the behavior of photocatalysts in producing ROS,the‘pseudo photocatalytic’effect of catalysts can be induced by the discharge and the production of ·OH enhanced [27-29].Generally speaking,ozonation of contaminants can proceed in two ways: (1) the direct reaction of O3molecules and(2) the indirect oxidation of ·OH resulting from the decomposition of O3.However, to the best of our knowledge, there are few literature reports regarding the mechanism of O3activation to ·OH on the catalyst surface.
The present study primarily focused on optimizingthe effects of a series of process parameters on the efficacy of pulsed gas-liquid discharge plasma for BPA degradation,including pulse peak voltage, air flow rate, electrode gap,catalyst dosage, initial BPA concentration, initial solution pH,and solution conductivity.Furthermore, the short-lived active species generated during the pulsed gas-liquid plasma discharge was diagnosed by optical emission spectroscopy(OES).Finally, the activation mechanism of O3on the catalyst surface was analyzed using density functional theory (DFT).
2.Experiment
2.1.Materials
The following chemicals have been used in this work: BPA,GO, cadmium acetate, dimethyl sulfoxide (DMSO), (3-aminopropyl)triethoxysilane,acetone and ethanol.All reagents in this work were of analytical grade and purchased from Dalian Liaodong Chemical Reagent Factory, China.The solutions used in the experiment were all prepared with deionized water.
2.2.Synthesis of rGO/CdS catalysts
The GO/CdS nanoparticles were synthesized by a hydrothermal method.The synthesis procedure is as follows.First,rGO was fabricated by the Hummers method [30], and then different weight ratios of rGO with Cd(AC)2·2(H2O) were dispersed into DMSO solution to obtain respective contents of 0%,1%,5%,9%and 13%rGO nanocomposites,represented as CdS and rGO(x)/CdS (x=1, 5, 9, 13), respectively.The preparation process of the rGO(x)/CdS catalyst is described in detail in the Supplementary Information.As described in our previous work [27], the highest BPA degradation efficiency was achieved over rGO(5)/CdS catalyst.Therefore,the experiments in this work were carried out over rGO(5)/CdS catalyst.
2.3.Experimental setup
The pulsed gas-liquid hybrid discharge plasma (PHDP) for BPA degradation was ignited in a multi-needle to plate electrode,as shown in figure 1.0.10-0.30 g l-1rGO(5)/CdS nanocomposites were added into wastewater for BPA degradation.The initial concentration of BPA varies from 20 to 80 mg l-1and the solution volume for each experiment was 150 ml.The pH values of solution were adjusted to acidic(pH=3, 6), neutral (pH=7.5) and alkaline (pH=9) for the study,and the solution conductivities were 10,50,75 and 100 μS m-1, respectively.The high-voltage electrode consisted of several needles with a 4:1 length to diameter ratio.A steel plate with a 1:3 thickness to diameter ratio served as the ground electrode.The distances between the high-voltage electrode and the ground electrode were 8, 10, and 12 mm.The electric characteristics were measured by a voltage probe,a current monitor, and an oscilloscope.The voltage and current waveforms during the discharge process are demonstrated in figure S1.The gas is pumped into the aeration tank through an air pump and a flowmeter, and then enters the solution tank via the hollow needle electrode to excite the pulsed gas-liquid hybrid discharge plasma.The optical emission spectra (OES) emitted from the pulsed gas-liquid discharge plasma were identified by a spectroscopy (Princeton,Action SP2750).The BPA concentration was determined by high-performance liquid chromatography.The methods for calculation of BPA degradation efficiency and the detection of active species are provided in the Supplementary Information.
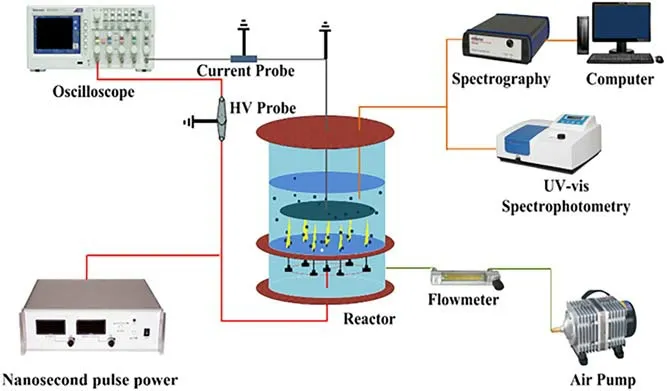
Figure 1.Diagram of the experimental system.
3.Results and discussion
3.1.Optimization of process parameters of the pulsed gasliquid discharge plasma catalysis system
3.1.1.Effect of pulse peak voltage.Contaminant degradation
is affected by the generation rate and quantity of energetic electrons,active species and UV,which are related to the input voltage [31].Generally, a higher peak discharge voltage will produce more high-energy electrons, thereby accelerating the formation of active species.Hence, the effect of the pulse voltage on the catalytic degradation of BPA by the plasma synergistic rGO(5)/CdS catalyst was first investigated(figure 2(a)).The degradation of 20 mg l-1BPA solution was conducted at three pulse peak voltages(15,17 and 19 kV).
Notably, BPA degradation was an increasing function of processing time regardless of the pulse peak voltage applied to the reaction system.It is clear from figure 2(a) that the percentage degradation efficiency of BPA is promoted by increasing the discharge peak voltage, and better degradation performance can be achieved at a higher peak voltage.For target concentrations<1.0 mM, the reaction rate is proportional to the initial concentration based on the Langmuir-Hinshelwood model [32, 33].Hence, the first-order rate constants(r=-dc/dt=kKC=k1C or ln C0/Ct=k1t)were calculated and are displayed in figure 2(b).When the peak voltages were 15,17 and 19 kV,the kinetic constants reached 0.016,0.025 and 0.037 min-1,respectively.That is because the number of active species will increase as the voltage increases.The increase in active species can increase the probability of collision with contaminant molecules, which will resultin higher BPA degradation.Meanwhile, the intensity of the electric field and ultraviolet light generated in the reactor will also increase[34-36].Guo et al[37]studied BPA degradation in aqueous solution by plasma combined with activated carbon under different voltages and came to similar conclusions.Considering various factors comprehensively, 19 kV was selected as the discharge voltage in subsequent experiments.
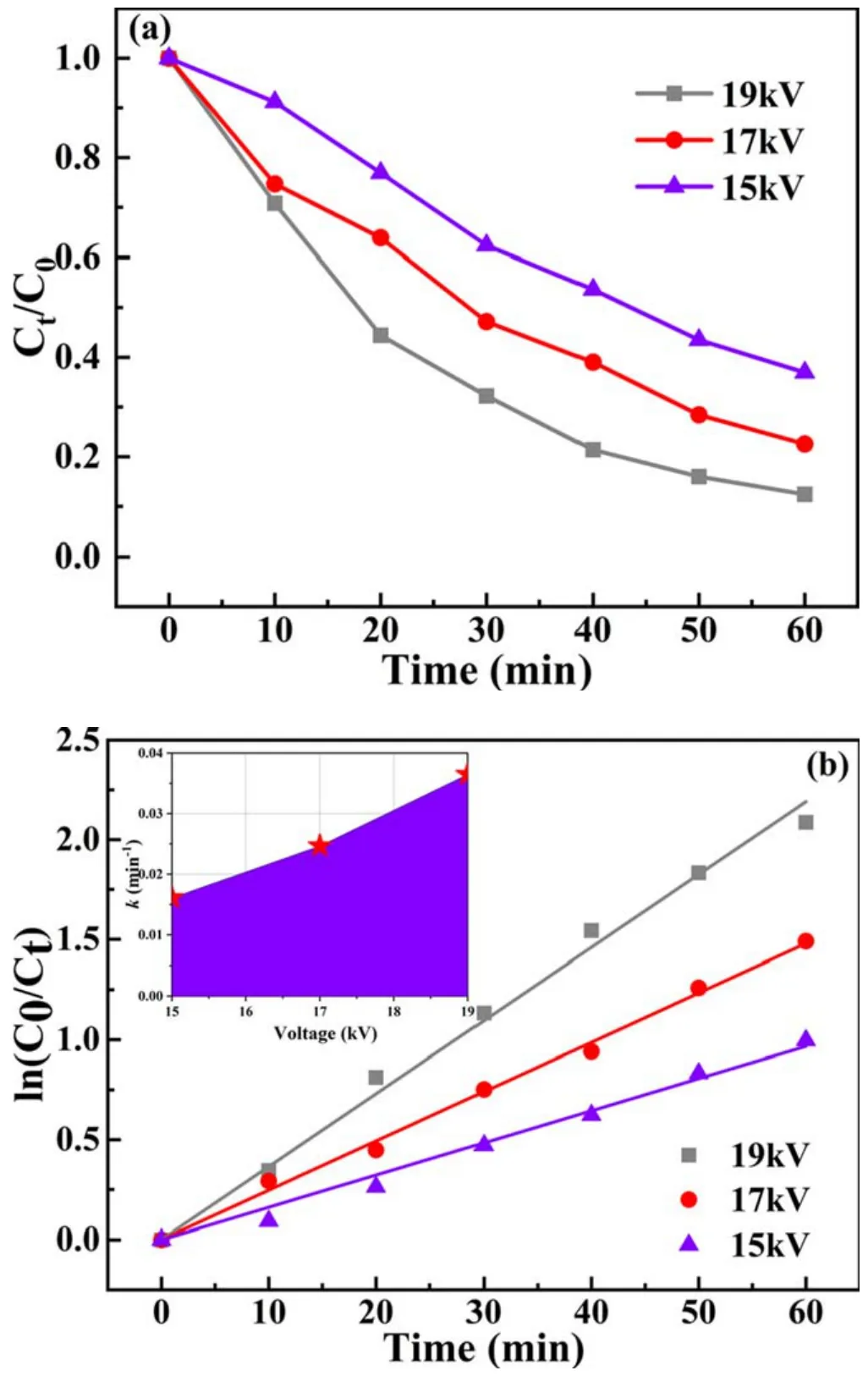
Figure 2.(a)Effect of pulse peak voltage on BPA degradation and(b)kinetic curves of BPA degradation(BPA concentration=20 mg l-1,air flow rate=4 l min-1, electrode gap=10 mm, catalyst dosage=0.2 g l-1, pH value=7.5, solution conductivity=10 mS cm-1).
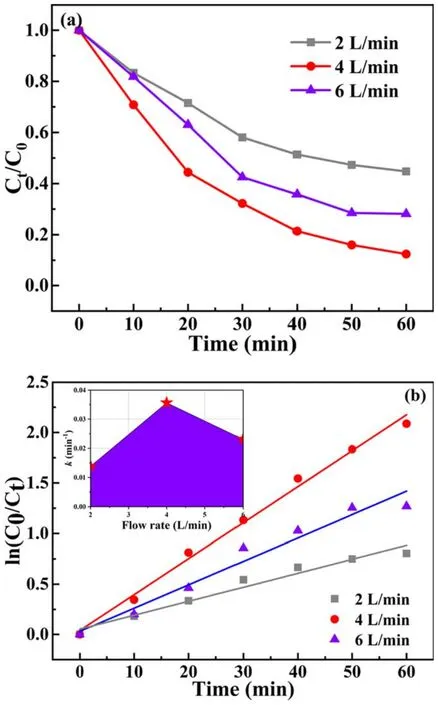
Figure 3.(a) Effect of air flow rate on BPA degradation and (b)kinetic curves of BPA degradation(BPA concentration=20 mg l-1,pulse peak voltage=19 kV, electrode gap=10 mm, catalyst dosage=0.2 g l-1, pH value=7.5, solution conductivity=10 mS cm-1).
3.1.2.Effect of air flow rate.The air flow rate can significantly affect the physical and chemical effects in plasma system,such as the number of active species.When air is pumped into the system during the discharge process, many active species including O·, O3, ·OH, H·, N·, etc can be generated in the reaction system.Figure 3(a)shows the effect of air flow rate on BPA degradation.It can be seen from figure 3(a) that BPA degradation efficiency increases first and then decreases as the air flow rate increases from 2 to 6 l min-1.The highest degradation efficiency was achieved when the air flow rate was 4 l min-1.That is because the number of oxygen molecules increases with the air flow rate, which increases the O3concentration.Zhang et al[38]reported that an increase in air flow rate favored the transfer of O3from the gas phase to the liquid phase.In addition,the residence time of active species in the reactor can also be affected by the air flow rate.As the air flow rate increases, the residence time of active substances in the solution will be shortened, and thus the active particles cannot effectively dissolveinto the solution and participate in the degradation of organic pollutants[39].In contrast,a higher air flow rate will cause a large number of fine bubbles to merge into coarse bubbles,which will destroy the transfer balance and lead to a reduction in the gas-liquid mass transfer of active species [40].In addition, as shown in figure 3(b), it can be concluded that the highest kinetic constant (0.036 min-1) can be obtained when the air flow rate is 4 l min-1.

Figure 4.(a) Effect of electrode gap on BPA degradation and (b)kinetic curves of BPA degradation(BPA concentration=20 mg l-1,pulse peak voltage=19 kV, air flow rate=4 l min-1, catalyst dosage=0.2 g l-1, pH value=7.5, solution conductivity=10 mS cm-1).
3.1.3.Effect of electrode gap.In a discharge plasma system,the discharge mode will alter with the change in the electrode gap,which will affect the production of active species as well as the degradation performance.Figure 4(a) shows the effect of electrode gap on BPA degradation.As shown in figure 4(a), the BPA degradation efficiency increases as the electrode gap increases from 8 to 10 mm.With further increase in the electrode gap to 12 mm,the BPA degradation efficiency decreases.Generally speaking, the electric field is enhanced with a decrease in the electrode gap, which facilitates the formation of reactive species.Nevertheless,when the discharge gap is further reduced to 8 mm, the discharge mode transforms into an unstable spark discharge.Zhan et al [41, 42] also reported that the discharge mode transferred from a corona discharge to a spark discharge when the electrode gap was decreased, which resulted in a rapid decrease in voltage and an increase in current.Furthermore,the kinetic analysis also demonstrates that the maximum kinetic constant can be acquired with an electrode gap of 10 mm (figure 4(b)).Therefore, the optimal electrode gap is 10 mm.
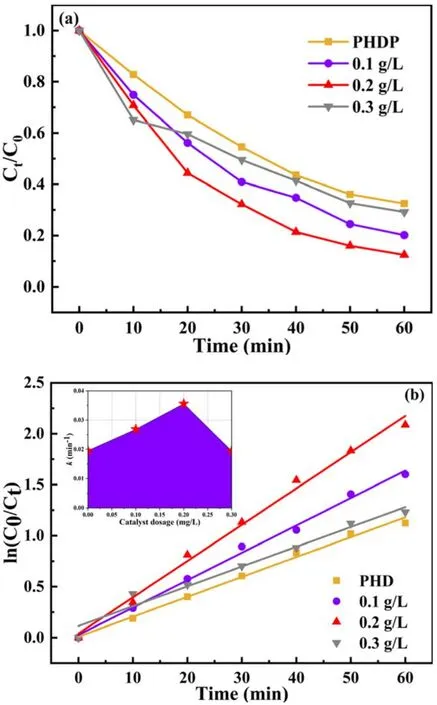
Figure 5.(a) Effect of catalyst dosage on BPA degradation.and (b)pseudo-first-order kinetic curves of BPA degradation (BPA concentration=20 mg l-1, pulse peak voltage=19 kV, air flow rate=4 l min-1,electrode gap=10 mm,pH value=7.5,solution conductivity=10 mS cm-1).
3.1.4.Effect of catalyst dosage.The effect of rGO(5)/CdS catalyst dosage on BPA degradation efficiency was studied.As exhibited in figure 5(a), as the dosage of rGO(5)/CdS catalyst increased from 0.1 to 0.3 g l-1, the degradation efficiency first increased and then decreased.The highest degradation efficiency was achieved at 0.2 g l-1.A significant inhibitory effect appeared whenthe catalyst dosage continued to increase.We speculated that when the dosage of rGO(5)/CdS is increased within a certain range,the plasma can activate ‘pseudo photocatalytic’ behavior of the catalyst and generate more photogenerated e--h+pairs,which is conducive to the production of ·OH, thereby improving the degradation BPA.However,adding too much catalyst not only leads to a higher cost but also reduces the number of reactive active sites due to the aggregation of solid particles, which is not conducive to contaminant degradation [43, 44].Figure 5(b) displays the effect of catalyst dosage on the kinetic constants.It can be seen from figure 5(b) that the pseudo-first-order kinetic curve and the kinetic constants are consistent with those of the catalyst dosage.Therefore, the addition of 0.2 g l-1of catalyst was considered to be the optimal dosage for BPA elimination in this work.
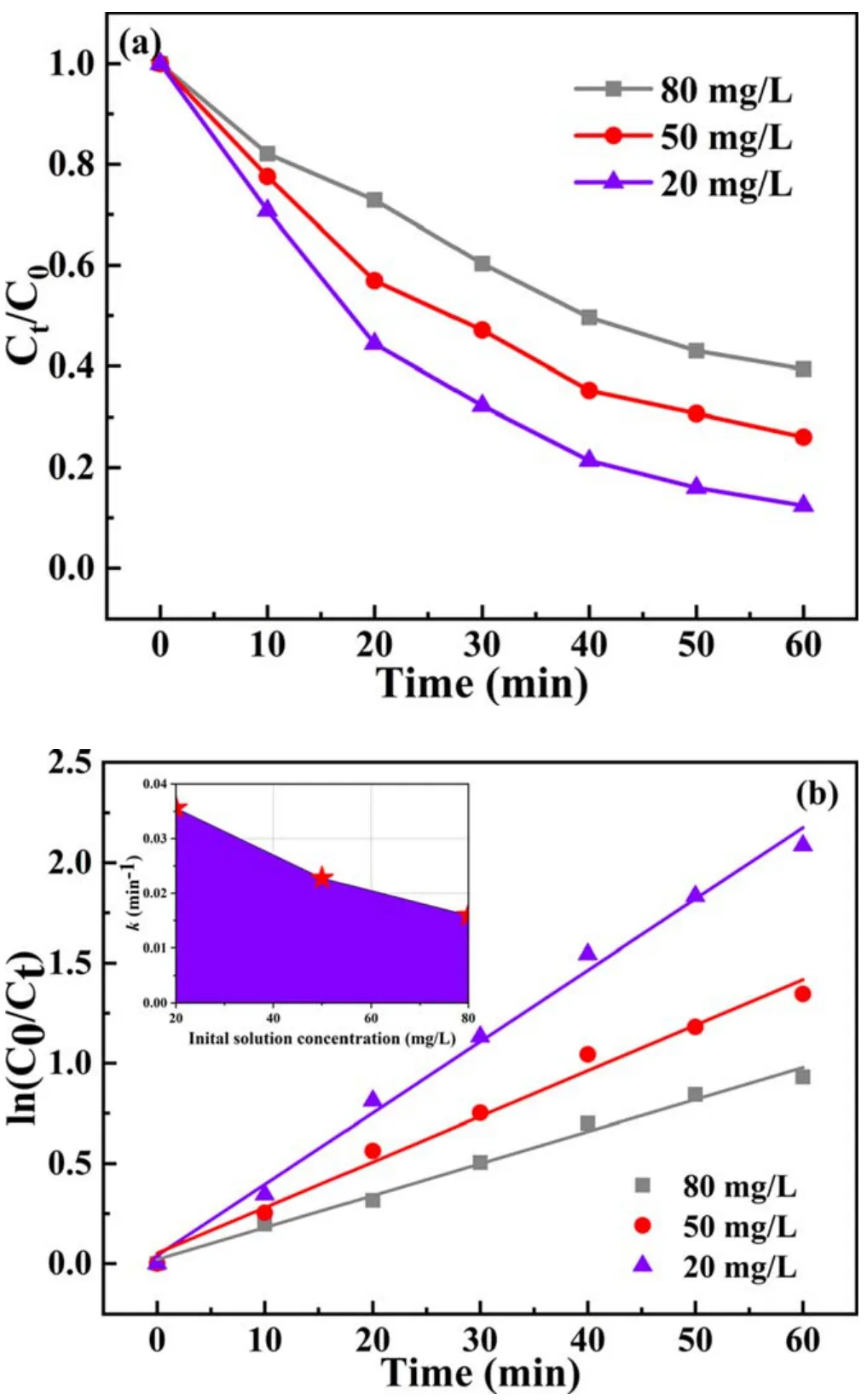
Figure 6.(a)Effect of initial concentration on BPA degradation and(b)kinetic curves of BPA degradation(pulse peak voltage=19 kV,air flow rate=4 l min-1, electrode gap=10 mm, catalyst dosage=0.2 g l-1, pH value=7.5, solution conductivity=10 mS cm-1).
3.1.5.Effect of initial concentration of BPA.Figure 6(a)
exhibits the evolution of BPA degradation efficiency over time at different initial BPA concentrations.It is obvious that BPA degradation efficiency and initial concentration demonstrate a negative correlation.Nezamzadeh-Ejhieh pointed out that increasing the number of molecules per unit volume helps to increase the probability of collision with active particles, which is conducive to improving the degradation efficiency [45, 46].However, we found that the reactive speciesis not enough to react with contaminants,resulting in a lower BPA degradation efficiency at the same time[47].More time was needed for a higher concentration of BPA.Nevertheless, a higher degradation rate does not mean that more contaminants are decomposed.With initial concentrations of 20, 50 and 80 mg l-1, the absolute mass of BPA removed was 2.63 mg, 5.55 mg and 7.27 mg respectively.The absolute mass of BPA removed showed an opposite trend to degradation efficiency.The main reason for this phenomenon was that the collision probability between active species and BPA molecules was greatly increased.The higher initial concentration means that there is intense competition between reactant molecules for reactive species.Correspondingly, a maximum kinetic constant of about 0.036 min-1can be achieved at 20 mg l-1, which is 1.6 and 2.3 times that for the 50 mg l-1and 80 mg l-1solutions(see figure 6(b)).
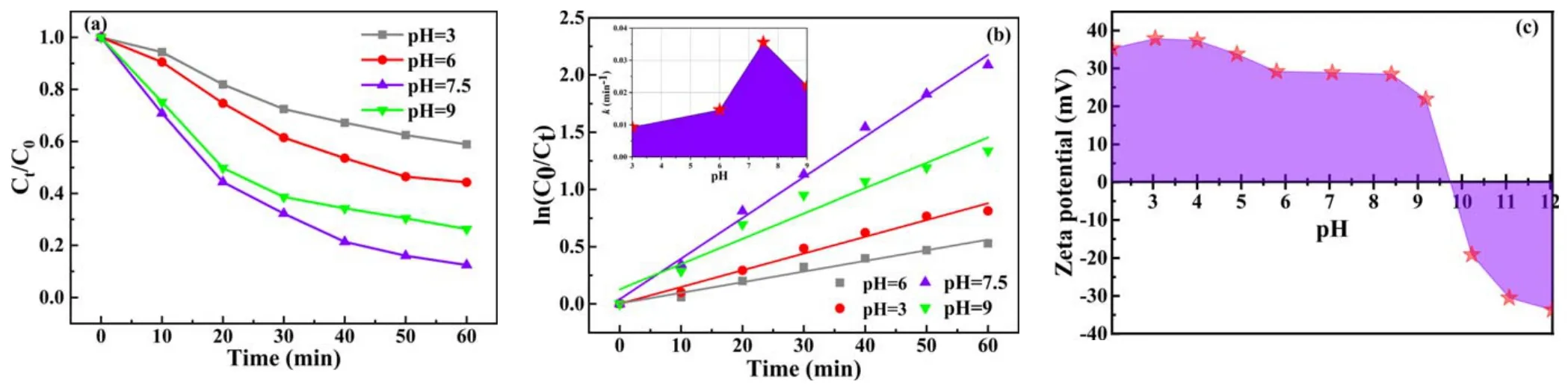
Figure 7.(a)Effect of initial solution pH on BPA degradation,(b)kinetic curves of BPA degradation and(c)effect of pH on zeta potential(BPA concentration=20 mg l-1, pulse peak voltage=19 kV, air flow rate=4 l min-1, electrode gap=10 mm, catalyst dosage=0.2 g l-1,solution conductivity=10 mS cm-1).
3.1.6.Effect of initial solution pH.The initial solution pH can affect the number of active substances and the species of the organic compounds during the discharge plasma process[48].There are two plausible reasons to analyze the effect of pH on the degradation of any organic compound: (1) pH affects the availability of reactive species and (2) pH affects the organic compound, which undergoes structural variations [49].It has been reported that the structure of organic compounds changes due to protonation and deprotonation with variations in solution pH [50-52].Hence, it is vital to evaluate the effect of pH on BPA degradation by changing the solution pH between 3 and 9.
The BPA degradation efficiency and reaction rate constant as a function of initial concentration are presented in figures 7(a) and (b).It can be clearly observed from figure 7(a) that a higher BPA degradation efficiency was acquired under neutral conditions(initial pH 7.5),followed by alkaline and weak acidic conditions.Meanwhile, kinetic constants also follow the same rule.This can be attributed to the fact that BPA is present in molecular form in aqueous solution when the initial solution pH is lower than the BPA dissociation constant (4.9).Conversely, BPA is present as in anion form at pH levels higher than 4.9 [53].It has been reported that pollutants in anion form are react more easily with ROS produced from plasma [53, 54].Secondly, H+in the acidic solution will consume many electrons,which is not conducive to the formation of the active species ·OH [55].
In addition, zeta potentials in different pH solutions were measured, as displayed in figure 7(c).Zeta potential is used to analyze the charge of rGO(5)/CdS catalyst.As shown in figure 7(c),the zeta potential of rGO(5)/CdS decreases with the increase of pH.The zeta potential was located between pH 9-10(~9.7),which is characterized as the point of zero charge(PZC).When the pH value of the solution is less than PZC,the catalyst surface is positively charged, while when the pH value of the solution is greater than PZC, the catalyst surface is negatively charged [56, 57].Hence, there is non-interaction between rGO(5)/CdS and BPA at pH<4.9.As pH begins to increase(4.9
3.1.7.Effect of solution conductivity.Solution conductivity may affect the discharge characteristic as well as the generation of active species.Hence,it is essential to explore the effect of solution conductivity on BPA degradation in the plasma catalysis process.The effect of solution conductivity on BPA degradation efficiency is presented in figure 8(a).It is clear that the greater the solution conductivity, the worse the removal efficiency of BPA solution.That is because higher conductivity will be accompanied by more intensive UV light and higher plasma temperature, which may have a negative effect on reactive species production [50, 60].Correspondingly, the kinetic constant also decreases from 0.035 to 0.014 min-1when increasing the solution conductivity from 10 to 100 μS m-1, as shown in figure 8(b).Therefore, the following experiment was carried out at the initial conductivity of 10 mS cm-1.
3.2.The identification of reactive species and analysis of their contribution

Figure 8.(a) Effect of solution conductivity on BPA degradation and (b) kinetic curves of BPA degradation (BPA concentration=20 mg l-1,pulse peak voltage=19 kV,air flow rate=4 l min-1,electrode gap=10 mm, catalyst dosage=0.2 g l-1,pH value=7.5).
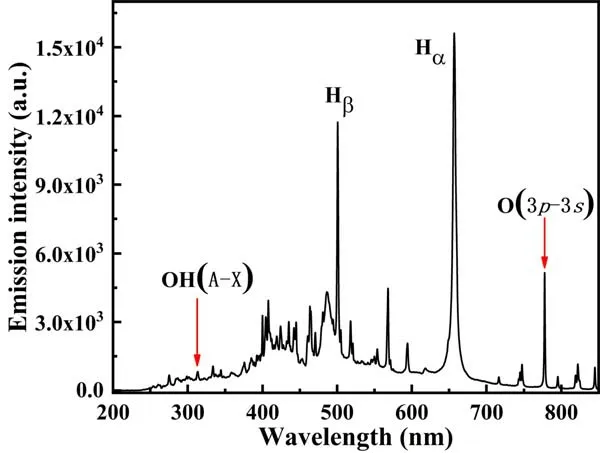
Figure 9.OES of the air gas-liquid hybrid discharge plasma (pulse peak voltage=19 kV, frequency=50 Hz, air flow rate=4 l min-1, electrode gap=10 mm).
3.2.1.Optical emission spectra diagnosis.As is well known,·OH is a vital oxidant that dominates plasma oxidations in water treatment, and ·O plays an important role in the formation of O3.Hence,the OES ranging from 200 to 850 nm were diagnosed to investigate the types of reactive species generated (including ·O and ·OH) in the pulsed gas-liquid plasma system,as shown in figure 9.The results show that the peaks located in the short-wave region belong to the spectrum of OH·emission, which is mainly the energy transition A2Σ+→X2П.The peaks in visible range are the emission line of the hydrogen atom H(n=3,4,5)→H(n=2).The Balmer spectrum of H corresponds to Hα(656.3 nm), Hβ(486.1 nm)and Hγ(434.0 nm),respectively.The peaks in the infrared range belong to the spectrum of the oxygen atom O,corresponding to the energy state transitions of O 3p5P0→3s5S0(777.1 nm) and 3p3P→3s3S0(844.6 nm),respectively.As in the air gas-liquid discharge, the active oxygen atoms are preferentially generated from O2.The formation of active species can be explained by equations (1)-(8) [14, 61]:
3.2.2.The contribution of reactive species to BPA degradation.The contribution of various chemically reactive species to BPA degradation and plasma catalytic processes over the catalyst was investigated by the free radical capture test(figure 10).Benzoquinone (BQ), monopotassium phosphate(MP),iso-propyl alcohol(IPA)and sodium oxalate(SO) were acted as ·O2-(k=2×109M-1s-1), e-(k=1.9×107M-1s-1), ·OH (k=1.9×1010M-1s-1)and h+(k>4.7×107M-1s-1) scavengers, respectively[62-65].The plasma treatment time was 60 min.As presented in figure 10, after adding MP and SO, the degradation efficiency was reduced by about 41.4% and 53.6%,respectively, which was 1.39 and 2.16 times that of the plasma alone.These results indicate that e-and photogenerated h+play an important role in BPA degradation.In our previous work, the ‘pseudo photocatalytic’ mechanism of plasma-induced rGO(5)/CdS catalysts was elucidated.These results show that the adsorption range of visible light is widened and the separation of e--h+pairs is accelerated when rGO is added to CdS [27].
In the case of plasma alone, the presence of BQ had almost no effect on BPA degradation (0.62%).However, the removal rate was decreased by 28.8% after introducing BQ,indicating that the derived reactive species produced byplay an important role in reaction process.In a plasma catalytic system,can also be converted to-to participate in the BPA degradation reaction.Moreover, after the addition of IPA, the BPA degradation efficiency is also reduced to a certain extent, indicating that the presence of·OH in the reaction system would also have an impact on BPA degradation.
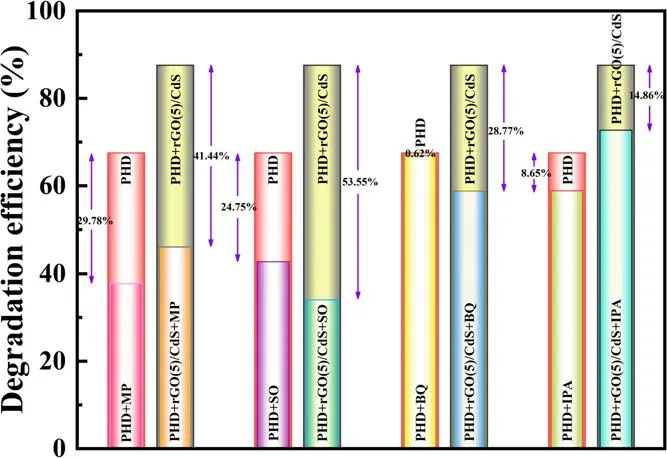
Figure 10.The capture testing of active species in PHDP and PHDP catalytic systems at 60 min.
3.3.O3 activation mechanism analysis
As a long-lived species, O3can diffuse into the liquid phase and react with aqueous contaminants in air or oxygen discharge.At the gas-liquid interface, strongly oxidative compounds such as ·OH radicals and O atoms can be generated and dissolved in water,and organic pollutants can be removed through a series of oxidation reactions [66].Variations in the concentrations of O3and ·OH in PHDP and PHDP catalytic systems at different treatment times (30 min and 60 min) are exhibited in figure S3.As can be seen from that figure, the presence of rGO(5)/CdS catalyst reduces the O3concentration in the PHDP system and promotes the generation of·OH,which facilitates BPA degradation.Some of dominant reactions for O3and ·OH production are presented in equations (9)-(13) [66-68]:
There are few studies on the mechanism of how O3is activated and converted to ·OH on the catalyst surface gasliquid discharge.Therefore, it is also necessary to study the evolution process of active species in the plasma catalytic system,especially the activation of O3on the surface of rGO(5)/CdS nanocomposite catalyst.The activation pathway of O3on the catalyst surface was therefore analyzed in detail by DFT.The method of theoretical simulation is described in text S4.
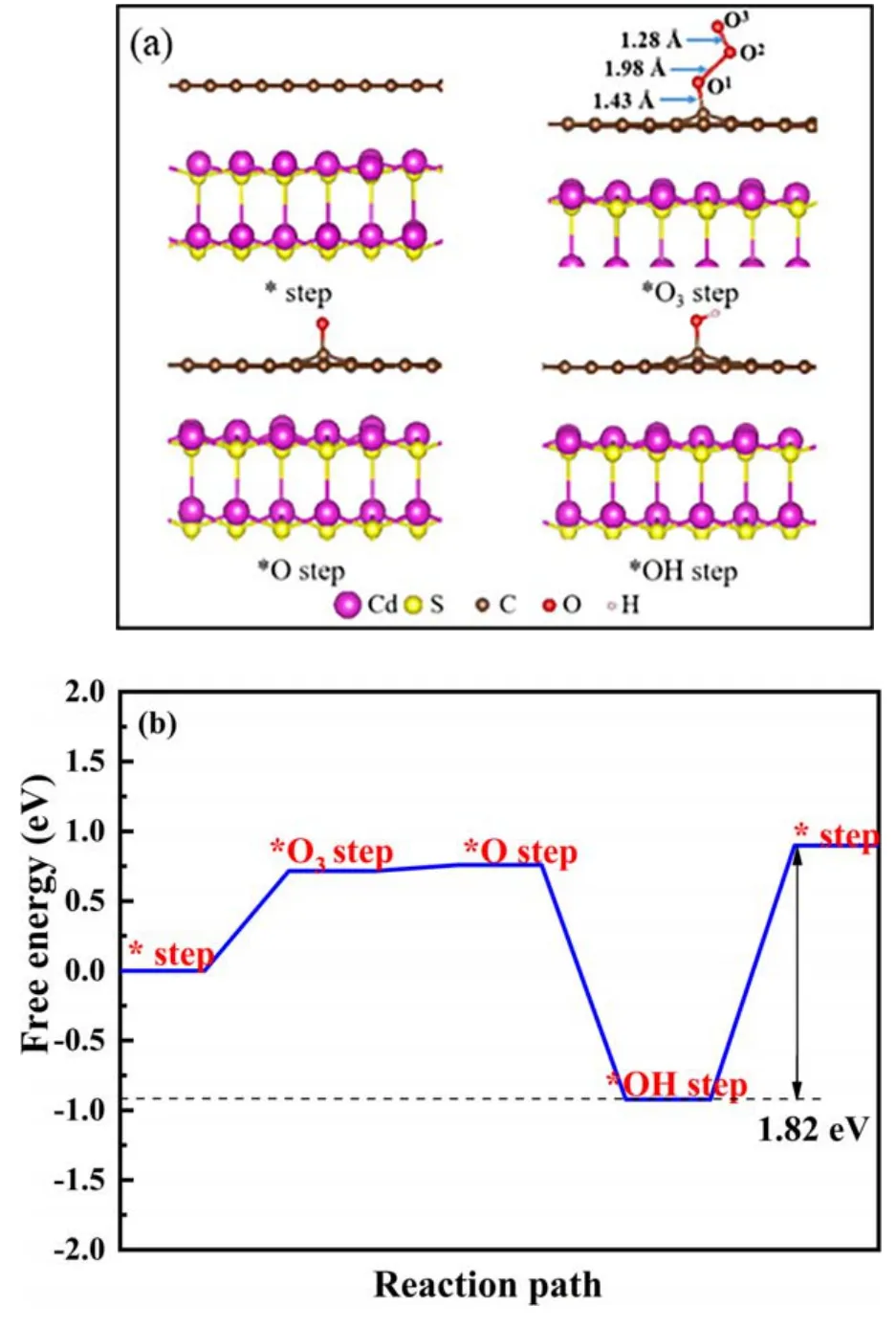
Figure 11.(a) The O3 activation pathway on the rGO(5)/CdS surface and (b)the corresponding energy profile.In free O3 and O2,the O-O bonds have lengths of 1.31 ? and 1.27 ?, respectively.
Based on DFT calculations, the activation path of O3adsorbed on the surface of rGO(5)/CdS is exhibited in figure 11.First,O3binds to the C atom in rGO(figure 11(a)).The adsorption energy of this interaction is 0.72 eV, which effectively weakens the O1-O2bond with a length of 1.98 ?(figure 11(b)), indicating that O3adsorbed on the surface of rGO(5)/CdS is activated.Meanwhile,the O2-O3bond with a length of 1.28 ? is enhanced,which means that O2-O3can be desorbed into free O2with a desorption energy of 0.04 eV(figure 11(b)).Subsequently, an O1atom binds to H2O to form adsorbed ·OH.Finally, ·OH is desorbed, and this step determines the rate of the reaction, for which the energy barrier is 1.82 eV.
The projected density of states(PDOS)of O atoms in O3further confirms the activation mechanism of O3,as shown in figure 12(a).After the adsorption of O3on the rGO(5)/CdS surface,the 2p state of the O1atom is located below the Fermi level, indicating that electrons are clustered in the O1atom.However, some 2p states of O2and O3atoms are above the Fermi level and not occupied by electrons.Therefore, the above results indicate that the electrons in rGO(5)/CdS are mainly concentrated in the O1atom, which is directly bound to the C atom.This is further illustrated by the charge density difference mapping of O3and rGO(5)/CdS (figure 12(b)).The sky blue and yellow isosurfaces represent negative and positive charges,respectively.The charge density isosurfaces in all figures are set as 0.002 e ?-3.The calculated results show that the electrons in rGO(5)/CdS (sky blue region) are mainly located between O1atoms and rGO,which proves that O3is activated.
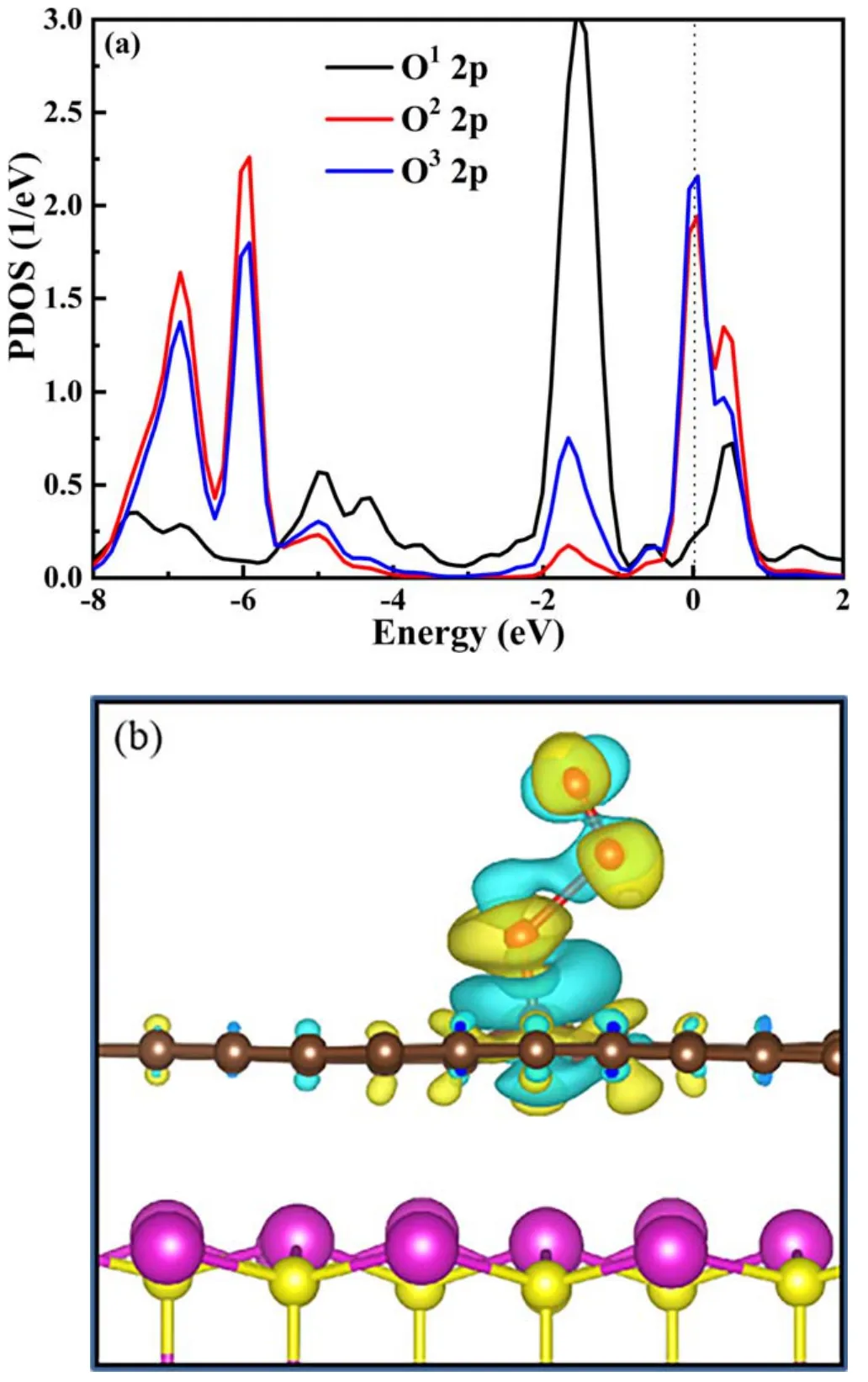
Figure 12.(a)The calculated projected density of state plots of O3 on rGO(5)/CdS and(b)the charge density difference mapping between O3 and rGO(5)/CdS.
4.Conclusions
Evaluation and optimization of the PHDP system over rGO(5)/CdS were performed in terms of BPA degradation performance in aqueous solution.The results indicated that increasing discharge voltage and decreasing initial BPA concentration and solution conductivity were found to be favorable for BPA degradation.With the increase of other process parameters (air flow rate, electrode gap, catalyst dosage and initial solution pH), the BPA degradation rate increased first and then decreased.The short-lived and longlived activespecies generated from pulsed gas-liquid hybrid discharge plasma were detected by optical emission spectroscopy, and their contribution to BPA degradation was evaluated by the free radical capture test.Finally, DFT calculation was used to analyze the activation mechanism of O3on the catalyst surface.The O3adsorbed on the surface of rGO(5)/CdS is activated and desorbed into free O2, and the step of desorption to ·OH is the rate-determining step of the reaction.The projected density of states of O atoms in O3further confirmed the activation mechanism of O3.
Acknowledgments
This work is supported by the Open Fund for State Environmental Protection Key Laboratory of Synergetic Control and Joint Remediation for Soil & Water Pollution (No.GHBK-2020-006) and National Natural Science Foundation of China (No.21876070).
 Plasma Science and Technology2023年10期
Plasma Science and Technology2023年10期
- Plasma Science and Technology的其它文章
- 3D fluid model analysis on the generation of negative hydrogen ions for negative ion source of NBI
- Enhancing surface adhesion of polytetrafluoroethylene induced by two-step in-situ treatment with radiofrequency capacitively coupled Ar/Ar+CH4+NH3 plasma
- Etching characteristics and surface modification of InGaSnO thin films under Cl2/Ar plasma
- A homogeneous atmospheric pressure air plasma in a 10mm gap based on a threeelectrode configuration
- Effect of gas flow on the nanoparticles transport in dusty acetylene plasmas
- Closed corner divertor with B × ?B away from the divertor: a promising divertor scenario for tokamak power exhaust
