Establishment and Characterization of a Fin Cell Line Derived from the Atlantic Salmon Salmo salar and Its Application to Fish Virology Study
JIA Peng, LIN Lirong, XU La, YI Meisheng, and JIA Kuntong,
Establishment and Characterization of a Fin Cell Line Derived from the Atlantic Salmonand Its Application to Fish Virology Study
JIA Peng1), 2), 3), LIN Lirong1), XU La4), YI Meisheng1), 2), 3), and JIA Kuntong1), 2), 3),*
1),,510000,2),519082,3),,510000,4);,266104,
Atlantic salmon () is an important economic fish that is seriously threatened by various viruses. A cell line designated as ASF derived from the caudal fin tissue of Atlantic salmon was established and characterized in this study. ASF cells grew well in Dulbecco’s modified Eagle’s medium (DMEM) containing 20% fetal bovine serum at 20℃. DNA sequencing and comparative analysis of thegene verified that the ASF cell line originated from Atlantic salmon. Chromosome analysis indicated that the modal chromosome number of ASF cells was 58. Viral susceptibility test showed that ASF cells were susceptive to two important fish viruses, viral hemorrhagic septicemia virus (VHSV) and red-spotted grouper nervous necrosis virus (RGNNV). Viral replication in ASF cells was further confirmed by qRT-PCR and transmission electron microscopy. Moreover, VHSV and RGNNV infectionscould induce the cellular responsesin ASF cells, as indicated by the differential expression of cellular antiviral response-related genes,and. In conclusion, the newly established ASF cell line can be applied as antool in fish virology and immunity studies.
;cell line; viral hemorrhagic septicemia virus; nervous necrosis virus; immune response
1 Introduction
Atlantic salmon () is a popular aquatic pro- duct because of its delicious taste and rich nutrition (Lo- zano-Mu?oz., 2020). With the decline of wild fish populations due to environmental pollution and habitat de- struction, artificial farming has become the main way for people to obtain Atlantic salmon (Jin., 2020). Atlan- tic salmon is a significant salmonid species in terms of va- lue and production scale in global aquaculture (Brudeseth., 2013). However, more and more viruses infect At- lantic salmon (Munro., 2015; Eriksson-Kallio., 2020;Gjessing., 2020;Jenberie., 2020; Pham., 2020; Samsing., 2021). Viral hemorrhagic septicemia virus (VHSV) is a massively destructive virus with a high mortality rate of up to 100% in fry, causing great losses to Atlantic salmon aquaculture (Emmenegger., 2013; Ito., 2016; Zhang., 2019). Nervous necrosis virus (NNV), infecting more than 120 marine and freshwater teleost species, is also considered a potential threat to sal- mon farming (Li., 2019; Jia., 2020; Zhang., 2020). Although no report stated that NNV infects salmon under natural conditions, Korsnes. (2005) found that viral nervous necrosis broke out in Atlantic salmon after intraperitoneal challenge with nodavirus from Atlantic ha- libut (). Therefore, the patho- genic mechanisms of NNV and VHSV in Atlantic salmon must be elucidated to develop an effective therapy.
Fish cell lines are essential tools for culturing viruses, studying the mechanism of host-virus interactions, and de- veloping vaccines (Collet., 2018; Pham., 2020). For instance, the FtGF cell line, derived from the fin of fan- tail goldfish, was used forthepropagation of Cy- prinid herpes virus-2 (Dharmaratnam., 2020). Tran- scriptome analysis was performed in hirame natural em- bryonic cells with or without NNV infection to determine the innate immune response-related genes against the vi- rus (Kim., 2020). A formalin-inactivated red sea breamiridovirus vaccine was also developed using the culture supernatant of afin cell line persistently in- fected with IVS-1 strain (Kwon., 2020). Although morethan 880 fish cell lines have been established, more cell lines from various species are still needed to meet the di- verse needs of virology and immunology studies, consi- dering the specificity of host-virus interaction (Robin., 2020). Several cell lines have been established from dif- ferent tissues of Atlantic salmon (Martin., 2007; Ro- driguez Saint-Jean., 2014; Pham., 2017b;Gjes- sing., 2018). Some of these cell lines are susceptible to various viruses, including infectious hematopoietic ne- crosis virus, VHSV, infectious pancreatic necrosis virus, At- lantic salmon reovirus, and Pacific salmon paramyxovirus, and the cell lines have been used to develop antiviral strate- gies (Gjessing., 2018; Rodriguez Saint-Jean., 2014). However, none of them are susceptible to NNV. In the present study, an Atlantic salmon caudal fin-derived cell line that is susceptible to NNV and VHSV was estab- lished and characterized. This study can be a basis to elu- cidate the infection mechanisms of NNV and VHSV in At- lantic salmon and contribute to the AS invitrome, a collec- tion of different cell lines derived from this species (Bols., 2017).
2 Materials and Methods
2.1 Fish and Viruses
Healthy juvenile Atlantic salmon (approximately 50g in weight) for the development of primary cells were ob- tained from a local fish farm in Shangdong Province, Chi- na, and were maintained in seawater at 15℃. All proce- dures carried out with Atlantic salmon were approved by the Ethics Committee of Sun Yat-sen University. The fish were completely anesthetized using MS222 before eutha- nasia (Sigma, St. Louis, MO).
Red-spotted grouper nervous necrosis virus (RGNNV) (strain SBN147) was isolated from sicked sea perch and propagated inbrain cells (Le., 2017). VHSV IVa (strain VHSVLB2018) was isolated from largemouth bass and propagated infin cells (Zhang., 2019).
2.2 Primary Cell Culture and Subculture
The work was carried out on a Clean Bench, and sterile techniques were employed in all cell culture procedures. First, the surface of Atlantic salmon was sterilized by 75% ethanol before dissection. Then, caudal fin tissues were de- tached and transferred to a sterile Petri dish supplemented with 5mL of phosphate-buffered saline (PBS) containing antibiotics (penicillin, 100UmL?1; streptomycin, 100μgmL?1) for washing three times. Then, the fin samples were minced into pieces and transferred into a 25cm2culture flask containing 1mL of Dulbecco’s modified Eagle’s me- dium (DMEM) supplemented with 20% fetal bovine serum (FBS) and 4.76gL?1HEPES with a final pH of 7.4, and then were cultured at 20℃. Subsequently, 3mL of DMEM was added after the explants adhered to the bottom of the flask. The medium was exchanged every 4–5 days. When cell monolayers had developed, they were detached with DMEM containing 20% FBS using the standard trypsini- zation method, and subcultured at 1:2 split. The cell culture was denoted as ‘ASF cells’.
2.3 Species Authentication
A partial sequence of the Atlantic salmon() gene was analyzed as previously described with some modifications to identify the origin of ASF cells (Li., 2019). Total genomic DNAs were extracted from At- lantic salmon caudal fin tissues and ASF cells (at passage 10) by using the Tissue DNA Kit (Omega, Norcross, GA), respectively. A 514bp fragment of thegene was am- plified using primers-F andR (Supplemented in Table 1). The PCR products were then sequenced and analyzed.

Table 1 Primers used for cloning and expression analysis
2.4 Chromosome Analysis
Chromosomes of ASF cells were analyzed at passage 20. Cells were seeded in 25cm2culture flasks and treated with colchicine (Sigma) (10μgmL?1) at 70%–80% con- fluence for 24h. The cells were harvested and centrifugedat 1000rmin?1for 5min, resuspended in 1mL of 0.075molL?1KCl, and then incubated at 37℃ for 45min. Then, the cells were fixed with freshly mixed methanol and acetic acid (3:1, V/V) for 10min and collected by centrifuging at 1000rmin?1for 5min. After one more fixation, cells were dropped onto pre-cooled microslides and stained with 10%Giemsa (Sigma). Chromosomes were photographed andcounted.
2.5 Growth Curves
For growth characteristic studies, ASF cells (25th pas- sage) were seeded in 24-well plates. Cells were cultured at 10℃, 15℃, 20℃, and 28℃ to determine the effect of temperature on cell growth. Cells were cultured in DMEMcontaining different concentrations of FBS (10%, 15%, and 20%) to investigate the influence of FBS concentration.Cells were harvested and counted using a hemacytometer after 4, 8, 12, and 14 days, respectively. Experiments were conducted in triplicate.
2.6 Cell Migratory and Proliferative Abilities
Migratory and proliferative abilities of ASF cells (pas-sage 16) were determined as previously described with somemodifications (Gjessing., 2018). ASF cells were seed- ed in 6-well plates and cultured at 20℃until confluent mo- nolayers developed. A scratch was made down through the cell monolayer by a sterile 1mL pipette tip. The medium was changed, and the scratch was photographed daily for 7 days. The width of the scratch was measured in cm on enhanced snapshots.
2.7 Viral Susceptibility and Replication
RGNNV and VHSV were applied to evaluate the viral susceptibility of ASF cells. Cells were seeded into 6-well plates and grown to 80% confluence for infection. Then, the cells were infected with RGNNV or VHSV (multipli- city of infection=1) at 28℃ and 20℃. After adsorbing for 4h, the medium was exchanged. ASF cells incubated with PBS served as controls. Cytopathic effects (CPEs) were assessed daily, and the cells were collected at 24 and 48h post infection (hpi) for RT-PCR and qPCR assays to de- tect RGNNV and VHSV replication as previously described (Li., 2019).
Viral replication was confirmedtransmission elec- tron microscopy. ASF cells were seeded into a 75cm2cul- ture flask and infected with RGNNV or VHSV as men- tioned above. The infected cells were harvested and fixed with 2.5% glutaraldehyde at 4℃ for 24h and then fixed with 2.0% osmium tetroxide for 1h. The samples were dehydrated by graded ethanol (30%, 50%, 70%, 80%, 95% and 100%), embedded in epoxy resin for sectioning, and then stained with uranyl acetate/lead citrate. The viral par-ticles were observed under a Philips CM10 electron micro- scope.
2.8 Expression Analyses of IFN-1 and Mx-1Genes in ASF Cells After RGNNV and VHSV Infections
ASF cells were infected by RGNNV or VHSV as de- scribed in ‘Viral susceptibility and viral replication’. Cells were harvested for RNA extraction at 24 and 48hpi, and then cDNAs were synthesized using PrimeScript Reverse Transcriptase in accordance with the manufacturer’s ins- tructions. Transcription levels of() andwere determined by qRT-PCR in a LightCycler 480 II (Roche) as previously described (Zhang., 2018). The Atlantic salmon geneserved as the reference gene. Primers for qRT-PCR are listed in Table 1. All samples were performed in triplicate, and the 2?ΔΔCtmethod was used to analyze gene expression levels.
2.9 Statistical Analysis
Data represented the average value of three replicates and were expressed as mean±SD (standard deviation). SPSS version 20 was used for statistical analysis. Statis- tical differences between different groups were determin- ed using one-way ANOVA. Statistical significance was con- sidered at0.05.
3 Results
3.1 Primary Culture and Subculture of ASF Cells
The ASF cell line was derived from the caudal fin of healthy juvenile Atlantic salmon. Three days after seeding, epithelial-like cells began to migrate from the explants.The cells grew to full confluence 15 days later (Fig.1A). Then, the cells were subcultured at a split ratio of 1:2 every 7– 10 days in DMEM (15% FBS) at 20℃. Until now, ASF cells have been subcultured more than 50 times (Fig.1B).

Fig.1 Morphology of cells derived from the caudal fin of Atlantic salmon. A, Primary cultures on day 6; B, ASF cells at the 50th passage. Bar=100μm.
3.2 Species Authentication and Karyotyping
Partial amplification of the Cgene was performed using ASF cells and caudal fin tissue of Atlantic salmon to evaluate the potential origin of ASF cells, and an ex- pected fragment of 514bp was amplified (Fig.2A). Sub- sequent sequencing showed that the sequenced fragments from the ASF cells and caudal fin tissue of Atlantic sal- mon were identical, and revealed 99.6% similarities with the knownsequence of Atlantic salmon (GenBank accession no. JQ390056.1). These results verify that the ASF cell line was derived from Atlantic salmon.
One hundred metaphases plates were analyzed to de- termine the chromosome numbers of ASF cells at passage 20. As shown in Figs.2B and 2C, the modal chromosome number of ASF cells was 58.

Fig.2 Species authentication and karyotyping. A, PCR amplification of partial Cyt B gene sequences in ASF cells and Atlantic salmon fin tissue. M, DNA marker (2000bp); Lane 1, ASF cells; Lane 2, fin tissue; Lane 3, negative control. B, Fre-quency distribution of chromosomes in ASF cells counted at the 20th passage. C, Giemsa-stained metaphase chromosomes.
3.3 Cell Growth Characteristics
The effects of temperature and FBS concentration onASF cell growth at passage 25 were tested. ASF cells grew best in DMEM with 20% FBS, and slowed growth rate was observed with decreasing concentration of FBS at 20℃ (Fig.3A). For temperature, ASF cells cultured in DMEM with 20% FBS exhibited a maximum growth rate at 20℃ (Fig.3B).
3.4 Migratory and Proliferative Abilities
Cell migration assay showed a gradually healing scratch of ASF cells after 7 days (Figs.4A–4F). The width varia- tion of the scratch is shown in Fig.4G.
3.5 Viral Susceptibility and Viral Replication
Compared with the control cells (Figs.5C and 5F), RGNNV-or VHSV-infected ASF cells exhibited signifi- cant CPEs. After challenging with RGNNV, ASF cells show-ed shrinking characteristics and appeared spherical at 24hpi, and approximately 90% of cells detached from the bot- tom of the plate at 48hpi (Figs.5A and 5D). A few ASF cells infected with VHSV started to show morphological changes, such as cell rounding and detachment, at 24hpi (Fig.5B). Then, the monolayers were damaged gradually and disintegrated at 48hpi (Fig.5E). Subsequently, infect- ed ASF cells were confirmed by RT-PCR. Partial fragments of the RGNNVgene and the VHSVgene were am- plified from RGNNV- and VHSV-infected ASF cells, res- pectively (Figs.5G, 5H).
The proliferation of RGNNV and VHSV in ASF cells was further confirmed. The expression of RGNNVand VHSVincreased significantly from 24 to 48hpi in RGNNV- or VHSV-infected ASF cells, respectively (Figs.6Aand 6D). Moreover, transmission electron microscopy re- vealed the presence of several virus particles in the cy- to- plasm of RGNNV- or VHSV-infected ASF cells (Figs.6B, 6C and Figs.6E, 6F).
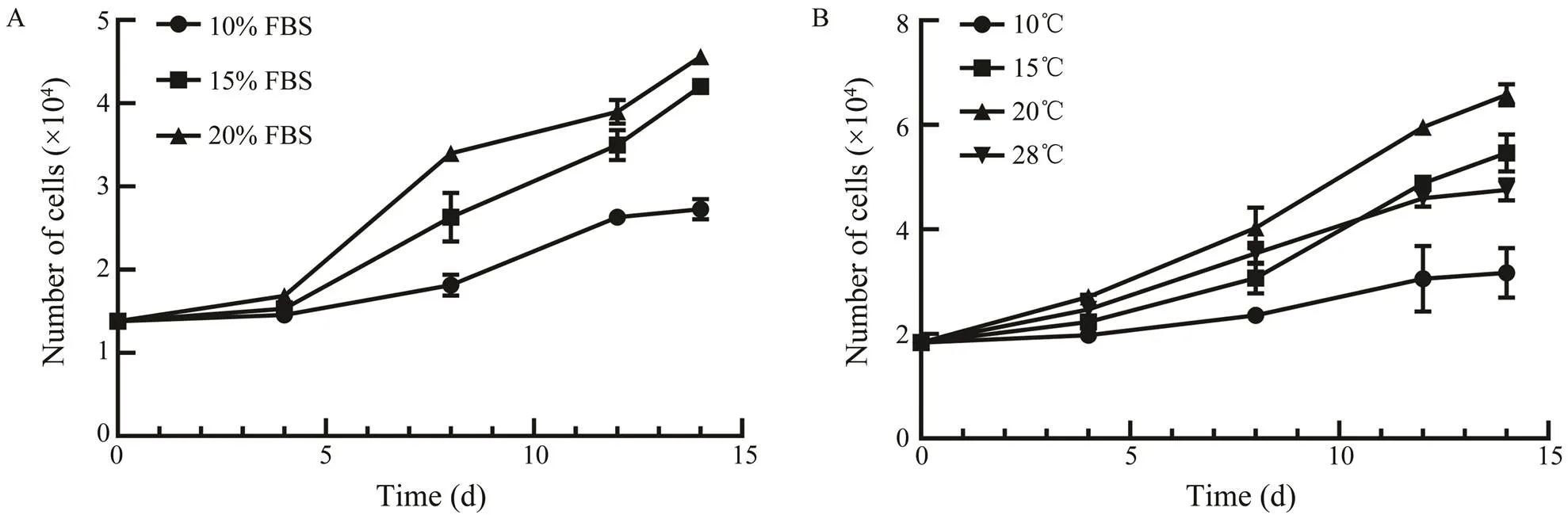
Fig.3 Effects of FBS concentrations and temperatures on the proliferation of ASF cells.A, ASF cells were incubated with DMEM containing 10%, 15%, or 20% FBS at 20℃; B, ASF cells were incubated with DMEM containing 20% FBS at 10℃, 15℃, 20℃, or 28℃.
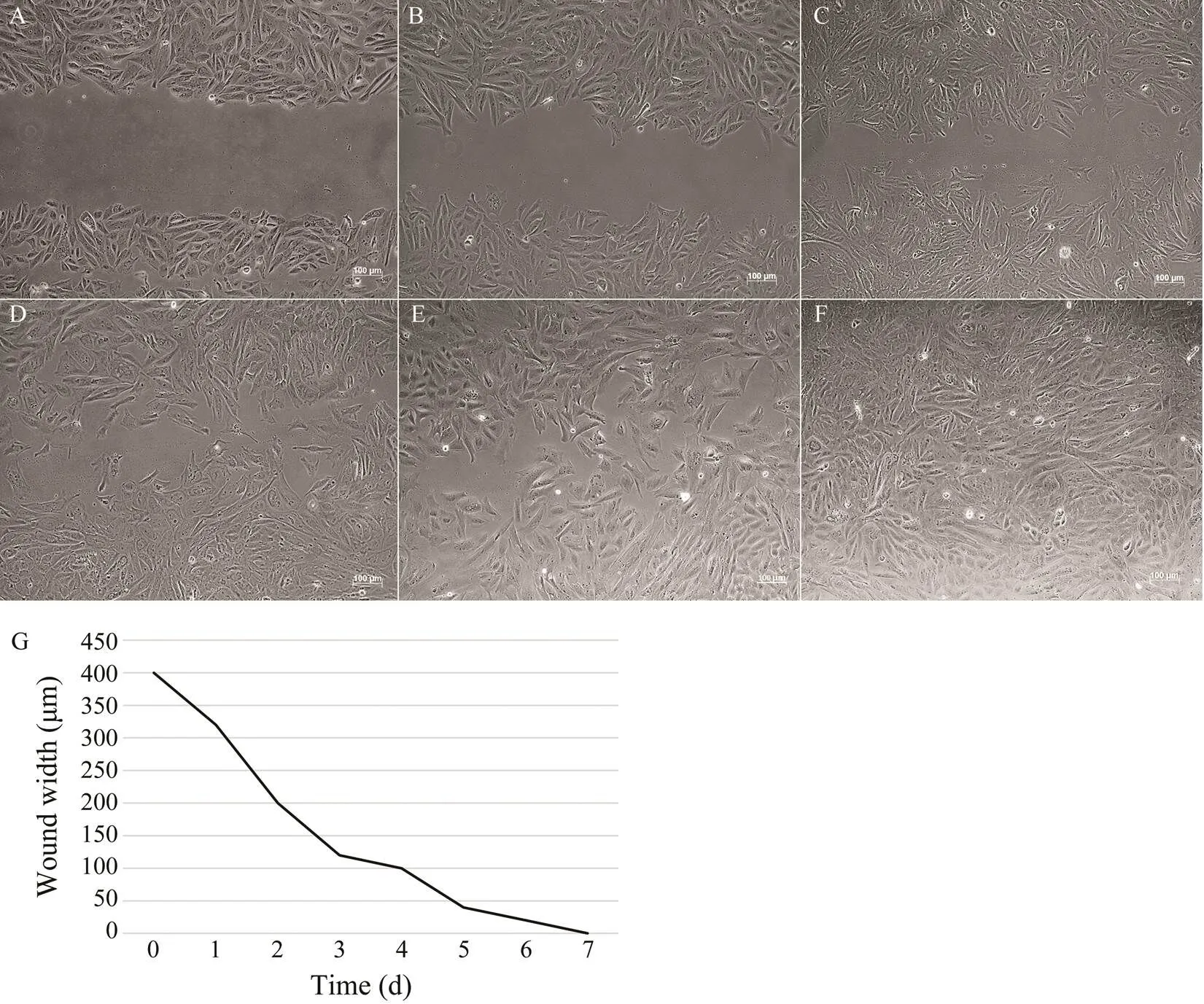
Fig.4 Wound healing. A scratch was made through the ASF cell monolayer by a sterile 1mL pipette tip. Cells were cultured continuously at 20℃ and photographed every day. Representative pictures of ASF at day 0 (A), day 1 (B), day 2 (C), day 3 (D), day 4 (E), and day 7 (F) are shown. Bar=100μm. G, measurement of scratch width.

Fig.5 Susceptibility of ASF cells to RGNNV and VHSV.(A), (D) showed CPEs of ASF cells infected with RGNNV at 24hpi and 48hpi, respectively. (B), (E) showed CPEs of ASF cells infected with VHSV at 24hpi and 48hpi, respectively. (C), (F) showed Mock-infected ASF cells at 24h and 48h , respectively. Bar=100μm. (G) showed agarose gel electrophoresis of PCR products from RGNNV-infected ASF cells using specific primer for the RGNNV CP gene. M, DNA marker (2000bp); Lane 1, ASF cells without RGNNV infection;Lane 2, blank control; Lane 3, ASF cells infected with RGNNV for 48h; Lane 4, RGNNV positive control. (H) showed agarose gel electrophoresis of PCR products from VHSV-infected ASF cells using specific primers for the VHSV N gene. M, DNA marker (2000bp); Lane 1, ASF cells without VHSV infection;Lane 2, blank control; Lane 3, ASF cells infected with VHSV for 48h; Lane 4, VHSV positive control.
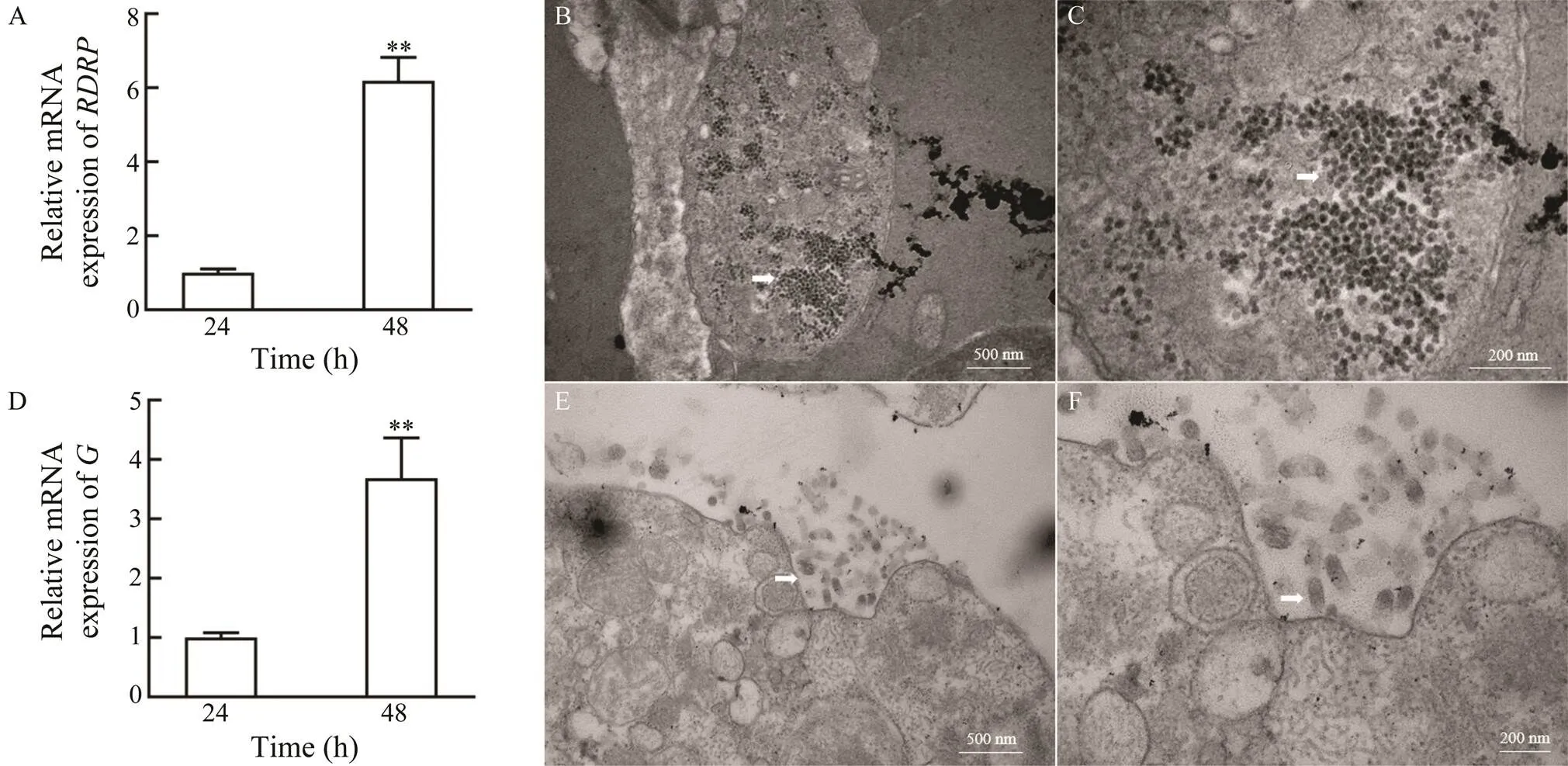
Fig.6 Replication of RGNNV and VHSV in ASF cells. Expression analysis of the RGNNV RDRP gene (A) and the VHSV G (D) gene in ASF cells at 24h and 48h after RGNNV infection (**P<0.01) were showed. B–C, Virus particles in RGNNV-infected ASF cells under low magnification (×40000) (B) and high magnification (×100000) (C). E–F, Virus particles in VHSV-infected ASF cells under low magnification (×40000) (E) and high magnification (×80000) (F).
3.6 Expression of Antiviral Genes in ASF Cells After RGNNV and VHSV Infections
The inductions ofandby RGNNV or VHSV infection were analyzed to investigate the antiviral response of ASF cells upon viral infection. As shown in Figs.7A–7B, RGNNV infection suppressed the expression ofandat 24 and 48hpi. By contrast, VHSV significant- ly induced the expression ofandat 24 and 48hpi (Figs.7C–7D). These data indicate that ASF cells can be utilized as a potentialtool to explore the cellu- lar antiviral response of Atlantic salmon to fish pathogens.

Fig.7 Transcription levels of IFN-1 and Mx-1 genesin ASF cells after RGNNV at (A) 24h and 48h (B), and VHSV infection at 24h (C) and 48h (D). EF-1α was used as an internal control. Results were expressed as mean±SD from three independent experiments performed in triplicates. Asterisks indicate significant differences between groups (**P<0.01).
4 Discussion
Fin tissues are the major entry points in fish of several viruses, such as infectious hematopoietic necrosis virus,VHSV, and infectious salmon anemia virus (Harmache., 2006; Quillet., 2007; Aamelfot., 2015). Although several cell lines from various tissues of Atlantic salmon have been established, few studies have been conducted onfin-derived cell lines in this species. In the present study, a cell line designated as ASF was successfully established and characterized from the caudal fin of Atlantic salmon. Previous studies showed that several cell lines from At- lantic salmon were usually cultured in L-15 medium (Martin., 2007; Rodriguez Saint-Jean., 2014; Pham., 2017b; Gjessing., 2018). Our study found that DMEM was also suitable for ASF cell culture. The ASF cell line showed stable growth in DMEM with 20% FBS and had been cultured for more than 50 passages so far. The opti- mal concentration of FBS for ASF cells was estimated to be 20%, which is consistent with ASHe and BAASf, two other cell lines from Atlantic salmon (Pham., 2017a, 2017b). Previous studies cultured different cell lines of At-lantic salmon at different temperatures. For instance, SSP-9, ASG-10, and ASG-13 were incubated at 20℃, whereasASHe grew well at 26℃ (Rodriguez Saint-Jean., 2014; Pham., 2017b; Gjessing., 2018). In the study, ASF cells could grow in the temperature range of 10℃–28℃ and exhibited optimal growth at 20℃. Atlantic sal- mon is a cold-water fish with comfortable temperatures for growth ranging from 15℃ to 20.5℃ and a critical ther- mal threshold of 27.8℃, which covers the optimum tem- perature of the cultured cells, suggesting the consistency of cell growth environmentand(Beaupré., 2020).
The typical chromosomal number of ASF cells (2n=58) was identical to that of Atlantic salmon but did not coin- cide with other cell lines isolated from Atlantic salmon, such as SSP-9 (2n=48) and AS (2n=52) (Sánchez., 1993;Pendás., 1994; Rodriguez Saint-Jean., 2014). The chromosome number in fish cell lines undergoing a degree of cell transformation does not always coincide with that of the intact host species (Schneider, 1973). These re- sults indicate that ASF cells maintain their ploidy nature.Epithelial cells exhibit proliferative and migratory abilities, and the epithelial nature of the ASF cells was validated by successfully closing the scratch within 7 days (Gjessing., 2018).
Fish cell lines are commonly used in virology research (Jyotsna., 2019). In the present study, the suscepti- bility of ASF cells to RGNNV and VHSV was determined. Significant CPEs and amplification of the RGNNVgene demonstrated that ASF cells were susceptible toRGNNV. Furthermore, the result of qRT-PCR and electronmicroscopy assays confirmed the proliferation of RGNNV in ASF cells. NNV is a potentially dangerous pathogen of Atlantic salmon (Korsnes., 2005). However, no cell line from Atlantic salmon is sensitive to NNV. Our results indicated that the ASF cell line could be used for NNV isolation and identification. Similar to ASG-10 and ASG- 13 cell lines derived from the gill tissue of Atlantic sal- mon (Gjessing., 2018), ASF cells showed high sus- ceptibility to VHSV. The fin is a route of entry for VHSV (Montero., 2011). Thus, ASF cells can be a usefultool for studying VHSV infection in a tissue-specific manner.
Fish cell lines are important tools for studying antiviral mechanisms in fish cells. However, not all cultured fish cellsare sensitive to immunostimulation (Rodriguez Saint-Jean., 2014; Pham., 2017a, 2020). IFN plays a key role in innate immune response induced by virus infection. Virus invasion induces IFN expression, and then activates the expression of downstream IFN-stimu- lated genes (ISGs), such as Mx-1, Viperin andISG15, to resist the virus (Lu., 2021). In the present study, we detected the transcript levels ofandto deter- mine whether ASF cells could be used to elucidate the mechanism of host-virus interaction (Zeng., 2016; Corrales., 2017). Our results showed that VHSV and RGNNV could significantly alter host cellularandtranscriptions in ASF cells, indicating that the ASF cell line might be a useful tool for studying the mechanism of host-virus interaction. Upon VHSV infection, the expres- sion levels ofandwere significantly upregu- lated, which were in accordance with those in Atlantic sal- monand in BASSf cells derived from the bulbus arteriosus of Atlantic salmon (Lovy., 2013; Pham., 2017a). By contrast, RGNNV infection significantly sup- presses the expression ofand its downstream factor, which play crucial roles in repressing NNV replica- tion (Wu., 2016,2010). Previous study also showed that antiviral genes might exhibit opposite expression pat- terns when challenged with different viruses. For instance, TNF-a2 and IL-12 p40-c were upregulated post infectious pancreatic necrosis virus and infectious salmon anaemia virus infection, but downregulated during salmonid alpha- virus infection in the salmonid cell line TO (Nerbovik., 2017). As the first line of defense against virus infection, the type-I IFN response was activated to elicit antiviral responses by inducing the expression of ISGs. Meanwhile, viruses have evolved various strategies to suppress the ac- tivation of host type-I IFN responses and thus survive in host cells (Chan and Gack, 2016; Lei., 2020). We spe- culated that RGNNV escapes the innate immune defense by inhibiting the IFN response in ASF cells. Further stud- ies are needed to clarify the mechanism by which NNV evades the cellular antiviral responses in ASF cells.
5 Conclusions
A cell line derived from the caudal fin of Atlantic sal- mon, designated as ASF, was established and characterized. ASF cells are susceptible to VHSV and RGNNV, and thus belong to VHSV- and RGNNV-supportive invitromes. VHSV and RGNNV infections induced different cellular responses in ASF cells. Taken together, our results indicate that the ASF cell line can serve as a usefultool for study- ing the pathogenesis of fish viruses and elucidating the me- chanism of host-virus interaction.
Acknowledgements
This work was supported by the China Postdoctoral Sci- ence Foundation (No. 2019M653152), the Pearl River S&T Nova Program of Guangzhou (No. 201806010047), the Na- tional Natural Science Foundation of China (No. 31771587),Fundamental Research Funds for the Central Universities (No. 19lgpy102), and the Natural Science Foundation of Guangdong Province (No. 2019A1515110842).
Aamelfot, M., McBeath, A., Christiansen, D. H., Matejusova, I., and Falk, K., 2015. Infectious salmon anaemia virus (ISAV) mucosal infection in Atlantic salmon., 46:120,DOI:10.1186/s13567-015-0265-1.
Beaupré, J., Boudreault, J., Bergeron, N. E., and St-Hilaire, A., 2020. Inclusion of water temperature in a fuzzy logic Atlantic salmon () parr habitat model., 87:102471,DOI:10.1016/j.jtherbio.2019.102471.
Bols, N. C., Pham, P. H., Dayeh, V. R.,and Lee, L. E. J., 2017. Invitromatics, invitrome, and invitroomics: Introduction of three new terms forbiology and illustration of their use withthe cell lines from rainbow trout.–,53: 383-405,DOI:10.1007/s11626-017-0142-5.
Brudeseth, B. E., Wiulsr?d, R., Fredriksen, B. N., Lindmo, K., L?kling, K. E., Bordevik, M.,., 2013. Status and future perspectives of vaccines for industrialised fin-fish farming., 35 (6):1759-1768,DOI:10.1016/j.fsi.2013.05.029.
Chan, Y. K., and Gack, M. U., 2016. Viral evasion of intracel- lular DNA and RNA sensing., 14 (6):360-373,DOI:10.1038/nrmicro.2016.45.
Collet, B., Collins, C., and Lester, K., 2018. Engineered cell lines for fish health research., 80:34-40,DOI:10.1016/j.dci.2017.01.013.
Corrales, L., Matson, V., Flood, B., Spranger, S., and Gajewski, T. F., 2017. Innate immune signaling and regulation in cancer immunotherapy., 27 (1):96-108,DOI:10.1038/cr.2016.149.
Dharmaratnam, A., Kumar, R., Valaparambil, B. S., Sood, N., Pradhan, P. K., Das, S.,., 2020. Establishment and cha- racterization of fantail goldfish fin (FtGF) cell line from gold- fish,forpropagation of Cyprinid herpes virus-2 (CyHV-2)., 8:e9373,DOI:10.7717/peerj.9373.
Emmenegger, E. J., Moon, C. H., Hershberger, P. K., and Kurath,G., 2013. Virulence of viral hemorrhagic septicemia virus (VHSV) genotypes Ia, IVa, IVb, and IVc in five fish species., 107 (2):99-111,DOI:10.3354/dao02671.
Eriksson-Kallio, A. M., Holopainen, R., Koski, P., Nousiainen,A., Koskinen, H., Kause, A.,., 2020. Susceptibility of rain- bow trout to three different genogroups of infectious pancreatic necrosis virus., 141:103-116,DOI:10.3354/dao03512.
Gjessing, M. C., Aamelfot, M., Batts, W. N., Benestad, S. L., Dale, O. B., Thoen, E.,., 2018. Development and characteriza- tion of two cell lines from gills of Atlantic salmon., 13 (2):e0191792, DOI:10.1371/journal.pone.0191792.
Gjessing, M. C., Krasnov, A., Timmerhaus, G., Brun, S., Afanas- yev, S., Dale, O. B.,., 2020. The Atlantic salmon gilltranscriptome response in a natural outbreak of salmon gill pox virus infection reveals new biomarkers of gill pathology and suppression of mucosal defense.,11:2154,DOI:10.3389/fimmu.2020.02154.
Harmache, A., LeBerre, M., Droineau, S., Giovannini, M., and Bremont, M., 2006. Bioluminescence imaging of live infected salmonids reveals that the fin bases are the major portal of entry for Novirhabdovirus., 80 (7):3655-3659,DOI:10.1128/JVI.80.7.3655-3659.2006.
Ito, T., Kurita, J., Mori, K., and Olesen, N. J., 2016. Virulence of viral haemorrhagic septicaemia virus (VHSV) genotype III in rainbow trout., 47:4,DOI:10.1186/s13567-015-0303-z.
Jenberie, S., Pe?aranda, M. M. D., Thim, H. L., Styrvold, M. B., Strandskog, G., J?rgensen, J. B.,., 2020. Salmonid alpha- virus subtype 3 induces prolonged local B cell responses in Atlantic salmon () after intraperitoneal infection., 11:1682,DOI:10.3389/fimmu.2020.01682.
Jia, P., Chen, X., Fu, J., Yi, M., Chen, W., and Jia, K., 2020. Near-Complete genome sequence of a fish nervous necrosis virus isolated from hybrid grouper in China., 9 (15): e01453-19,DOI:10.1128/mra.01453-19.
Jin, Y., Datsomor, A. K., Olsen, R. E., Vik, J. O., Torgersen, J. S., Edvardsen, R. B.,., 2020. Targeted mutagenesis of ?5 and ?6 fatty acyl desaturases induce dysregulation of lipid me- tabolism in Atlantic salmon ()., 21 (1):805,DOI:10.1186/s12864-020-07218-1.
Jyotsna, Vijayakumar, P., Ravi, M., Sudhakaran, R., and Rajas- waminathan, T. J. A., 2019. Development and characteriza- tion of a skin cell line (SGA) from the mosquitofishand its susceptibility to fish Betanodavirus., 520:734778,DOI:10.1016/j.aquaculture.2019.734778.
Kim, K. I., Lee, U. H., Cho, M., Jung, S. H., Min, E. Y., and Park,J. W., 2020. Transcriptome analysis based on RNA-seq of com-mon innate immune responses of flounder cells to IHNV, VHSV,and HIRRV., 15 (9):e0239925,DOI:10.1371/journal.pone.0239925.
Korsnes, K., Devold, M., Nerland, A. H., and Nylund, A., 2005. Viral encephalopathy and retinopathy (VER) in Atlantic sal- monafter intraperitoneal challenge with a noda- virus from Atlantic halibut., 68 (1):7-15,DOI:10.3354/dao068007.
Kwon, W. J., Choi, J. C., Hong, S., Kim, Y. C., Jeong, M. G., Min,J. G.,., 2020. Development of a high-dose vaccine formu- lation for prevention of megalocytivirus infection in rock bream()., 38 (51): 8107-8115, DOI:10.1016/j.vaccine.2020.11.001.
Le, Y., Li, Y., Jin, Y., Jia, P., Jia, K., and Yi, M., 2017. Estab- lishment and characterization of a brain cell line from sea perch,.–, 53 (9):834-840,DOI:10.1007/s11626-017-0185-7.
Lei, V., Petty, A. J., Atwater, A. R., Wolfe, S. A., and MacLeod, A. S., 2020. Skin viral infections: Host antiviral innate im- munity and viral immune evasion., 11:593901,DOI:10.3389/fimmu.2020.593901.
Li, J., Jia, P., Chen, X., Lai, M., Jin, F., Liu, W.,, 2019. Es- tablishment and characterization of a fin tissue cell line derived from silver pomfret,., 42 (10):1391-1399,DOI:10.1111/jfd.13059.
Lovy, J., Piesik, P., Hershberger, P. K., and Garver, K. A., 2013. Experimental infection studies demonstrating Atlantic salmon as a host and reservoir of viral hemorrhagic septicemia virus type IVa with insights into pathology and host immunity., 166 (1-2):91-101,DOI:10.1016/j.vetmic.2013.05.019.
Lozano-Mu?oz, I., Mu?oz, S., Díaz, N. F., Medina, A., Bazaes,J., and Riquelme, C., 2020. Nutritional enhancement of farm- ed salmon meat via non-GMO: Eico- sapentaenoic acid (EPA, 20:5 n-3), docosapentaenoic acid (DPA, 22:5 n-3) and vitamin D3 for human health., 25 (20): 4615,DOI:10.3390/molecules25204615.
Lu, X., Zeng, J., Jia, K., and Yi, M., 2021. Antiviral activities of sea perch type I and type II IFNs against RGNNV and their dif-ferent roles in antigen presentation., 534:736314, DOI:10.1016/j.aquaculture.2020.736314.
Martin, S. A., Taggart, J. B., Seear, P., Bron, J. E., Talbot, R.,Teale, A. J.,., 2007. Interferon type I and type II res-ponses in an Atlantic salmon () SHK-1 cell line bythe salmon TRAITS/SGP microarray.,32 (1):33-44,DOI:10.1152/physiolgenomics.00064.2007.
Montero, J., Garcia, J., Ordas, M. C., Casanova, I., Gonzalez, A., Villena, A.,., 2011. Specific regulation of the chemokine response to viral hemorrhagic septicemia virus at the entry site., 85 (9):4046-4056,DOI:10.1128/jvi.02519-10.
Munro, E. S., McIntosh, R. E., Weir, S. J., Noguera, P. A., Sandi- lands, J. M., Matejusova, I.,., 2015. A mortality event in wrasse species (Labridae) associated with the presence of vi- ral haemorrhagic septicaemia virus., 38 (4):335-341,DOI:10.1111/jfd.12237.
Nerbovik, I. G., Solheim, M. A., Eggestol, H. O., Ronneseth, A., Jakobsen, R. A., Wergeland, H. I.,., 2017. Molecular clo- ning of MDA5, phylogenetic analysis of RIG-I-like receptors (RLRs) and differential gene expression of RLRs, interferons and proinflammatory cytokines afterchallenge with IPNV, ISAV and SAV in the salmonid cell line TO., 40 (11):1529-1544,DOI:10.1111/jfd.12622.
Pendás, A. M., Morán, P., and García-Vázquez, E., 1994. Organi-zation and chromosomal location of the major histone cluster in brown trout, Atlantic salmon and rainbow trout., 103 (2):147-152,DOI:10.1007/bf00352324.
Pham, P. H., Misk, E., Papazotos, F., Jones, G., Polinski, M. P., Contador, E.,., 2020. Screening of fish cell lines for pis- cine orthoreovirus-1 (PRV-1) amplification: Identification of the non-supportive PRV-1 invitrome., 9 (10):833, DOI:10.3390/pathogens9100833.
Pham, P. H., Tong, W. W. L., Misk, E., Jones, G., Lumsden, J. S.,and Bols, N. C., 2017a. Atlantic salmon endothelial cells from the heart were more susceptible than fibroblasts from the bul- bus arteriosus to four RNA viruses but protected from two viruses by dsRNA pretreatment., 70:214-227,DOI:10.1016/j.fsi.2017.09.001.
Pham, P. H., Vo, N. T., Tan, E. J., Russell, S., Jones, G., Lums- den, J. S.,., 2017b. Development of an Atlantic salmon heart endothelial cell line (ASHe) that responds to lysophos- phatidic acid (LPA)., 53 (1):20-32,DOI:10.1007/s11626-016-0077-2.
Quillet, E., Dorson, M., Aubard, G., and Torhy, C., 2007.assay to select rainbow trout with variable resistance/suscep-
tibility to viral haemorrhagic septicaemia virus., 76 (1):7-16,DOI:10.3354/dao076007.
Robin, T., Capes-Davis, A., and Bairoch, A., 2020. CLASTR: The Cellosaurus STR similarity search tool–A precious help for cell line authentication.,146 (5):1299-1306,DOI:10.1002/ijc.32639.
Rodriguez Saint-Jean, S., González, C., Monrás, M., Romero, A., Ballesteros, N., Enríquez, R.,., 2014. Establishment and characterization of a new cell line (SSP-9) derived from At- lantic salmonthat expresses type I ifn., 85 (5):1526-1545,DOI:10.1111/jfb.12503.
Samsing, F., Rigby, M., Tengesdal, H. K., Taylor, R. S., Farias, D., Morrison, R. N.,., 2021. Seawater transmission and infection dynamics of pilchard orthomyxovirus (POMV) in Atlantic salmon ()., 44 (1): 73-88,DOI:10.1111/jfd.13269.
Sánchez, L., Abuín, M., and Amaro, R., 1993. Cytogenetic cha- racterization of the AS cell line derived from the Atlantic sal- mon (L.)., 64 (1):35-38,DOI:10.1159/000133556.
Schneider, I. J. T. C., 1973..Aca- demic Press, New York, 788-790,DOI:10.1016/B978-0-12-427150-0.50172-8.
Wu, Y. C., Lu, Y. F., and Chi, S. C., 2010. Anti-viral mechanism of barramundi Mx against betanodavirus involves the inhibi- tion of viral RNA synthesis through the interference of RdRp., 28 (3):467-475,DOI:10.1016/j.fsi.2009.12.008.
Wu, Y. C., Tsai, P. Y., Chan, J. C., and Chi, S. C., 2016. Endo- genous grouper and barramundi Mx proteins facilitated the clearance of betanodavirus RNA-dependent RNA polymerase., 59:110-120,DOI:10.1016/j.dci.2016.01.012.
Zeng, M., Chen, S., Wang, M., and Chen, A., 2016. Advances in avian antiviral innate immune effectors., 32 (5):627-633.
Zhang, W., Jia, K., Jia, P., Xiang, Y., Lu, X., Liu, W.,, 2020.Marine medaka heat shock protein 90ab1 is a receptor for red-spotted grouper nervous necrosis virus and promotes virus in- ternalization through clathrin-mediated endocytosis., 16 (7):e1008668,DOI:10.1371/journal.ppat.1008668.
Zhang, W., Jia, P., Liu, W., Li, Y., Yi, M., and Jia, K., 2018. Functional characterization of tumor necrosis factor receptor-associated factor 3 of sea perch () in in-nate immune., 75:1-7,DOI:10.1016/j.fsi.2018.01.039.
Zhang, W., Li, Z., Xiang, Y., Jia, P., Liu, W., Yi, M.,., 2019. Isolation and identification of a viral haemorrhagic septicae- mia virus (VHSV) isolate from wild largemouth bassin China., 42 (11):1563-1572,DOI:10.1111/jfd.13078.
December 10, 2020;
February 3, 2021;
June 1, 2021
? Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2022
. E-mail: jiakt3@mail.sysu.edu.cn
(Edited by Qiu Yantao)
 Journal of Ocean University of China2022年4期
Journal of Ocean University of China2022年4期
- Journal of Ocean University of China的其它文章
- Complete Mitochondrial Genome of Myra affinis (Decapoda:Brachyura: Leucosiidae) and Its Phylogenetic Implications for Brachyura
- Multisource Target Classification Based on Underwater Channel Cepstral Features
- Joint Model of Wind Speed and Corresponding Direction Based on Wind Rose for Wind Energy Exploitation
- Elastic-Wave Reverse Time Migration Random Boundary-Noise Suppression Based on CycleGAN
- Identification, Phylogeny and Expressional Profiles of Peptidoglycan Recognition Protein (PGRP) Gene Family in Sinonovacula constricta
- Molecular Characterization,Expression Pattern and Transcriptional Regulation of Figla During Gonad Development in Japanese Founder(Paralichthys olivaceus)
