Changes in the Photosynthetic Pigment Contents and Transcription Levels of Phycoerythrin-Related Genes in Three Gracilariopsis lemaneiformis Strains Under Different Light Intensities
CAO Xuexue, WANG Haitao, ZANG Xiaonan, LIU Zhu, XU Di, JIN Yuming,ZHANG Feng, and WANG Zhendong
Changes in the Photosynthetic Pigment Contents and Transcription Levels of Phycoerythrin-Related Genes in ThreeStrains Under Different Light Intensities
CAO Xuexue#, WANG Haitao#, ZANG Xiaonan*, LIU Zhu, XU Di, JIN Yuming,ZHANG Feng, and WANG Zhendong
,,266003,
Threestrains,including wild type and high-temperature-resistant cultivars 981 and 2007,were studied with the changes in their photosynthetic pigment contents and related gene transcription levels under different light intensities (10, 60, 100, and 200μmolm?2s?1). The threestrains had the following photosynthetic pigments with high-to-low contents: phycoerythrin (PE), phycocyanin (PC), allophycocyanin (APC), and chlorophyll(Chl). Among the three strains, cultivar 981 had the highest PE content, followed by cultivar 2007. The PC and APC contents were similar among the three strains, but they were higher in cultivars 981 and 2007 than in the wild type. The Chlcontents in the threestrains were equal. A low light intensity (10μmolm?2s?1) promoted photosynthetic pigment accumulation inand improved the relative PE gene transcription (and) in a short period (≤6d). A high light intensity decreased the PE content. PebA and PebB, which catalyzed phycoerythrobilin synthesis, showed no compensatory upregulation at a low light intensity among the strains except for the wild typeAt a high light intensity, transcription levels ofandin the three strains were upregulated. This study provided an experimental basis for elucidating the photosynthesis ofAs key genes of algal growth, photosynthesis-related genes served as useful gene markers for screening elite varieties with good traits in breeding. Cultivar 2007 was superior to cultivar 981 in terms of maintaining high pigment levels in a wide range of light intensities, which is the most suitable for aquaculture.
; light intensity; photosynthetic pigment; PE; gene transcription
1 Introduction
(Rhodophyta, Gigartinales,Gracilariaceae) is a red alga composed of agar (Freile- Pelegrín and Murano, 2005) and other important bioac- tive substances, such as polysaccharides and phycobili- proteins. It is widely grown in aquaculture and used in chemical and food industries.cultivars 981 and 2007 are high-temperature-resistant strains (., from wild type to cultivar 981 and to cultivar 2007). They have the advantages of significantly enhanced tensile strength,fast growth, high agar content, and good anti-adversity per-formance compared with wild-type. Cul- tivar 2007 is bred from cultivar 981 andisconsidered su- perior because of its fast growth rate, high-quality agar, and high tolerance to increasing temperatures (Meng., 2009).
Oxygenic photosynthesis is the main reaction used by cyanobacteria, algae, and plants. In this process, light en- ergy is converted into chemical energy. In photosynthesis, light-harvesting pigment complexes transfer light energy to the reaction center for the primary charge-separation re- action and participate in energy dissipation and state transitions in the light protection mechanism (Haldrup., 2001; Rochaix, 2007). There are many kinds oflight-har- vesting pigment complexes. For example, the light-har- vesting pigment system of red algae consists of chloro- phyll(Chl) protein complexes and phycobilisomes, which are composed of colored phycobiliproteins and non- pigmented linker proteins (Wu., 2016).
Phycobilisomes are supramolecular complexes that playan important role in the photosynthesis of red algae by absorbing and transmitting light energy and promoting pho-tosynthesis (Maccoll, 1998). Phycobilisomes possess a classof major light-harvesting proteins called phycobiliproteinsthat efficiently transfer captured light energy to Chl(Mac- coll and Guard-Friar, 1987). Phycobiliproteins are classified into phycoerythrin (PE), phycocyanin (PC), and allo- phycocyanin (APC) according to the specific spectrum and composition of their chromophores (Gantt, 1980). PE is one of the most important light-harvesting proteins in red al- gae.is rich in PE,which gives the algae a red appearance. PE has unique optical, antitumor, and antivirus properties and other important physiological func- tions (Huang., 2017). Thus, it can be used in medi- cine, food, and other industries.
PE consists of three subunits, namely, α, β, and γ (Fic- ner., 1992). Apophycobiliproteins are colorless and must be combined with phycobilins to present an optical activity. The chromophores of PEs include phycoerythro- bilins (PEBs) and phycourobilins (PUBs). The fluores- cence chromophore of PE is PEB. PUBs function in energy transfer. PEB biosynthesis requires two subsequent two- electron reductions. The first reduction reaction is cata- lyzed by the ferredoxin oxidoreductase PebA (15,16-dihy- drobiliverdin), which reduces the Δ15,16 double bond of biliverdin IX α, which is a PEB precursor in red algae. The second reduction reaction is catalyzed by the PEB ferredoxin oxidoreductase PebB, which reduces the A-ring 2,3,3(1),3(2)-diene structure of 15,16-dihydrobiliverdin to yield PEB (Dammeyer and Frankenbergdinkel, 2006). Thus,(used to synthesize the α and β protein subunits of apo-PE),,and(involved in PEB synthesis) are key genes in the synthesis of optically active PE in. Therefore, their expression patterns under different light conditions should be studied to elucidate the molecular mechanism of optically active PE synthesis.
In this study, the effects of changes in short-term light intensity on the PE, PC, APC, and Chlcontents of threestrains were determined and compared. The changes in the expression of four key genes associ- ated with PE synthesis were examined and compared with the changes in pigments, as PE is an important light-har- vesting protein complex in red algal photosynthesis and is also the most sensitive protein complex to light intensity fluctuations. Specifically, variations in the transcription le- vels of the α and β protein subunits of PE and two genes involved in the synthesis of the PEB moiety, namely,and, under different light intensities were determin- ed. This study provided an experimental basis for the in- depth understanding of the biosynthesis of optically active PE and a theoretical and technical basis for the screen- ing of algae with strong agronomic traits.
2 Materials and Methods
2.1 Experimental Materials
Wild-typewascollected from Fushan Bay, Qingdao, China (E120.4, N36.1). Cultivars 981 and 2007, which are laboratory-bred varieties, were obtained from a farm area in Nan’ao Island, Shantou, China (E117.1, N23.4). The algae were washed with filtered seawater to remove contaminating algae and surface impurities and then placed in 1L beakers with natural sea water that was replaced twice a week.
After the algae were allowed to adapt to laboratory cul- ture conditions and grew well, 24g (fresh weight) of al- gae was placed in 4500mL Erlenmeyer flasks (with 1/3 volume of filtered and boiled seawater) and precultured in a light incubator (Ningbo Jiangnan Instrument Factory GXZ type) for 4d at 23℃, salinity 33, pH 8.0, 60μmolm?2s?1light intensity, and 12:12 light:dark photoperiod.
2.2 Experimental Design and Cultivation
After the preculture, the algae were placed in illumina- tion incubators at light intensities of 10, 60, 100, and 200μmolm?2s?1. The other conditions were the same as the pretreatment conditions. Relevant tests were carried out on days 0, 2, 4, 6, and 8. A light intensity of 60μmolm?2s?1under the preculture growth conditions was chosen as the control condition.
2.3 Research Methods
2.3.1 Determination of photosynthetic pigment contents
In this procedure, 0.25g of the fresh material sample was ground in liquid nitrogen after surface moisture was removed. Then, 4mL of PB buffer (50mmolL?1, pH 5.5) was added. Algal cells were ruptured (crushed for 2s and paused for 3s for a total time of 10min) by using an ul- trasonic crusher, and the samples were maintained in an ice bath. Frozen autolysis was performed at ?20℃ for 2h. Then, the samples were thawed rapidly at 35℃ in a wa- ter bath and centrifuged at 13000and 4℃ for 20min. The supernatants were composed of the initial phycobi- liprotein crude extracts. Afterward, 4mL of 90% (v/v) ace- tone was added to the crude extracts in the dark for 20min and centrifuged at 10000for 10min to obtain the crude chlorophyll extracts. The process was carried out at 0–4℃ in the dark. For each group, measurements were made on three parallel samples. The absorbance of phyco- biliprotein was measured at 498.5, 614, and 651nm. The APC, PC, and PE contents were calculated using the fol- lowing equations (Kursar., 1983). The unit was gramsper milliliter converted to milligrams per gram (fresh weight of algae according to the amount of each sample).

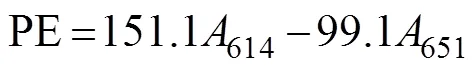
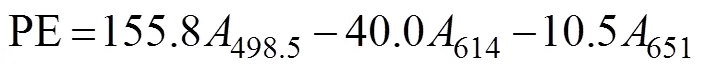
The absorbance of chlorophyll was measured at 630, 645, and 662nm. The Chlcontent was determined as follows (UNESCO, 1966):

2.3.2 RNA extraction and reverse transcription
Total RNA was extracted with an OMEGA Plant RNA kit R6827 at a fixed time (15:00 daily) to avoid any pho- toperiod effect and immediately reversely transcribed to cDNA by using a PrimeScriptTMRT reagent kit with gDNA Eraser (Takara, Japan).
2.3.3 Quantitative real-time PCR
Three pairs of specific primers were designed accord- ing to the cloned PE β-subunit gene(Genbank acces- sion number: MH724192), the PE α-subunit gene(Gen- bank accession number: MH724191), and the ferredoxin oxidoreductase genes(Genbank accession number: MH715937) and(Genbank accession number: MH 725905) by using Primer Premier. The most suitable pri- mers for quantitativereal-time PCR (TaKaRa SBYR?Pre-mix Ex TaqTM II) were shown in Table 1. The actin geneand the transcription factor genewere selected as internal reference genes.
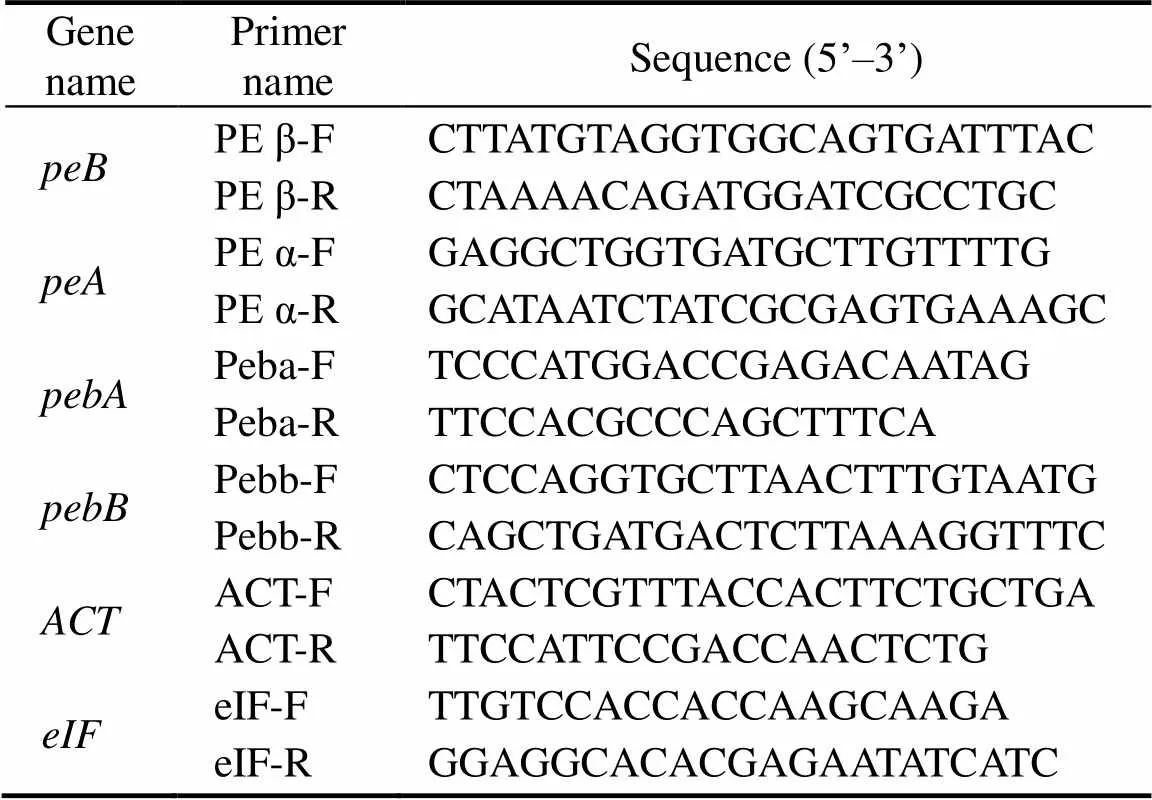
Table 1 Primer sequences of peB, peA, pebA, pebB, ACT,and eIF forreal-time PCR
Notes: The PCR reaction system of real-time PCR is composed of the following reagent in 20μL total volume; dH2O, 8.4μL; SYBR?Premix Ex Taq II, 10μL; (Tli RNaseH Plus; 2× conc.); primer 1 (10μmolL?1), 0.3μL; primer 2 (10μmolL?1), 0.3μL; and template, 1μL.
The cDNA stock solution obtained through reverse trans- cription was diluted 100-fold and used as a template, and this procedure for each sample was repeated three times.
The following conditions were set for real-time PCR am- plification: 95℃ for 30s, followed by 40 cycles of 94℃ for 5min, 55℃for 20s, 72℃for 20s.
2.4 Data Analysis
The relative transcription levels of the genes in each treatment group were analyzed using the 2?ΔΔCtmethod (Livak and Schmittgen, 2001). The final data was display- ed in the form of log10(RQ) in which RQ was the relative quantitation. The mean Ct values ofandwere used for ΔCt, and the gene transcription level at 60μmolm?2s?1was set as the control. Excel 2007 and Origin 8.5 were used for data processing and graphics analysis. ANOVA was conducted to examine significant differences, and the significance level was set at<0.05.
3 Results
3.1 Effect of Light Intensity on Photosynthetic Pigment Contents
3.1.1 Effect of light intensity on the photosynthetic pigment content of wild-type
Table 2 showed the contents of various photosynthetic pig-ments of wild-typeunder different light intensities measured every other day for 8d. ThePE con- tent ((0.83±0.31)mgg?1) was significantly higher than the APC ((0.16±0.08)mgg?1), PC ((0.16±0.07)mgg?1), and Chl((0.14±0.05)mgg?1) contents in wild-type(light intensity: 60μmolm?2s?1; time: 0d). The PE content was regulated by light. At a low light in- tensity (10μmolm?2s?1), the PE content significantly de- creased (<0.05) on day 2. As time was prolonged, the PE content began to increase. The PE content at 10μmolm?2s?1was higher than at other light intensities after 6d,which might increase the amount of light captured for algal growth. At a high light intensity (200μmolm?2s?1), the PE content gradually decreased over time. This finding implied that high light intensities might inhibit PE accumulation in wild- type. The APC and PC contents showed a similar trend. The Chlcontent was stable within a range of 0.14–0.15mgg?1.

Table 2 Photosynthetic pigment contents of wild G. lemaneiformis under different light intensities
3.1.2 Effect of light intensity on the photosynthetic pigment content of Cultivar 981
Table 3 showed the photosynthetic pigment contents of cultivar 981 under different light conditions monitored for 8d. The most abundant photosynthetic pigment of culti- var 981 was PE ((1.81±0.19)mgg?1), which was much high-er than PC ((0.42±0.02)mgg?1), APC ((0.28±0.04)mgg?1),and Chl((0.17±0.02)mgg?1). The PE content of culti- var 981 gradually decreased as the light intensity increased. A low light intensity promoted an increase in the PE con- tent, whereas a high light intensity inhibited PE accumu- lation. The contents of the other threepigments did not change remarkably over time under different light intensities.

Table 3 Photosynthetic pigment contents of cultivar 981 under different light intensities
3.1.3 Effect of light intensity on the photosynthetic pigment content of Cultivar 2007
The photosynthetic pigment content of cultivar 2007 did not change substantially under different light intensities for8d (Table 4). On day 0, at a low light intensity, the PE con- tent ((1.49±0.31)mgg?1) was the highest, followed by the PC ((0.36±0.08)mgg?1), APC ((0.27±0.06)mgg?1), and Chl((0.15±0.04)mgg?1) contents. The PE content of cultivar 2007 was relatively stable compared with that of the wild type and cultivar 981. At a low light intensity (10μmolm?2s?1), the PE content slightly increased over time, thereby com- pensating for the reduced light capture to meet the growth needs. The PE content of cultivar 2007 remained stable or even increased at a high light intensity. The APC, PC, and Chlcontents remained relatively stable for 8d at the four light intensities. Thus, cultivar 2007 appeared insensitive to light intensity and maintained high pigment contents in the entire range. This finding might be directly related to its fast growth and strong resistance to adverse conditions.
Table 4 Photosynthetic pigment contents of cultivar 2007 under different light intensities

3.2 Transcription Analysis of PE Genes Under Different Light Intensities
Genesandencode two major subunits, namely, α and β of PE. The gene transcription level at 60μmolm?2s?1was set as the control. The transcription patterns ofandwere similar (Fig.1). The maximum gene trans- cription level at each light intensity was reached on day 6. A low light intensity appeared to be conducive to higher relative transcription levels ofandof the wild type. The changes of transcription levels of the PE α and β subunits were similar to the change in the PE content.

Fig.1 Changes in the PE contents and peA and peB transcription in wild-type G. lemaneiformis under different light intensities.
For cultivar 981, the genes of α and β of PE were ge- nerally upregulated at a low light intensity on the first 2 days, subsequently downregulated, and upregulated again on day 8 (Fig.2). The upregulation and downregulation of the genes were based on the comparison of values (<0.05), and the difference was significant. In general, the transcription levels ofandin cultivar 981 changed slightly and more uniformly than those of the wild type under different light intensities. The changes in the PE content were similar to the changes of transcription levels of the PE α and β subunits, especially at a low light inten- sity.

Fig.2 Changes in the PE contents and peA and peB transcription of cultivar 981 under different light intensities.
For cultivar 2007, the transcription levels of the genes encoding the α and β subunits of PE were mostly upregu- lated during the first 6 days at the three light intensities (10, 100, and 200μmolm?2s?1). On day 8, their levels de- creased compared with that of the control group (60μmolm?2s?1). On day 2, the upregulation level at a low light in- tensity was significantly higher than that at a high light in- tensity (<0.05). The trends of the change in the PE con- tent and transcription levels of the PE α and β subunits weresimilar, especially at a low light intensity of 10μmolm?2s?1.

Fig.3 Changes in the PE contents and peA and peB transcription of cultivar 2007under different light intensities.
3.3 Transcription Levels Analysis of pebA and pebB Under Different Light Intensities
The relative transcription levels ofandinwild-typeunder different light intensities were measured over time(Fig.4). Transcription ofwas upregulated under both low and high light intensities compared with that of the control group at 60μmolm?2s?1until day 6, and it declined significantly at a high light in- tensity (60μmolm?2s?1) on day 8 (<0.05). Conversely, thetranscription level only slightly changed.
As shown in Fig.5, the relative transcription levels ofandof cultivar 981 decreased at a low light in- tensity. Conversely, their expression levels increased under high light conditions. The patterns ofandtran- scription levels under different light conditions over time were similar.

Fig.4 Changes in the pebA and pebB transcription of wild-type G. lemaneiformis under different light intensities.
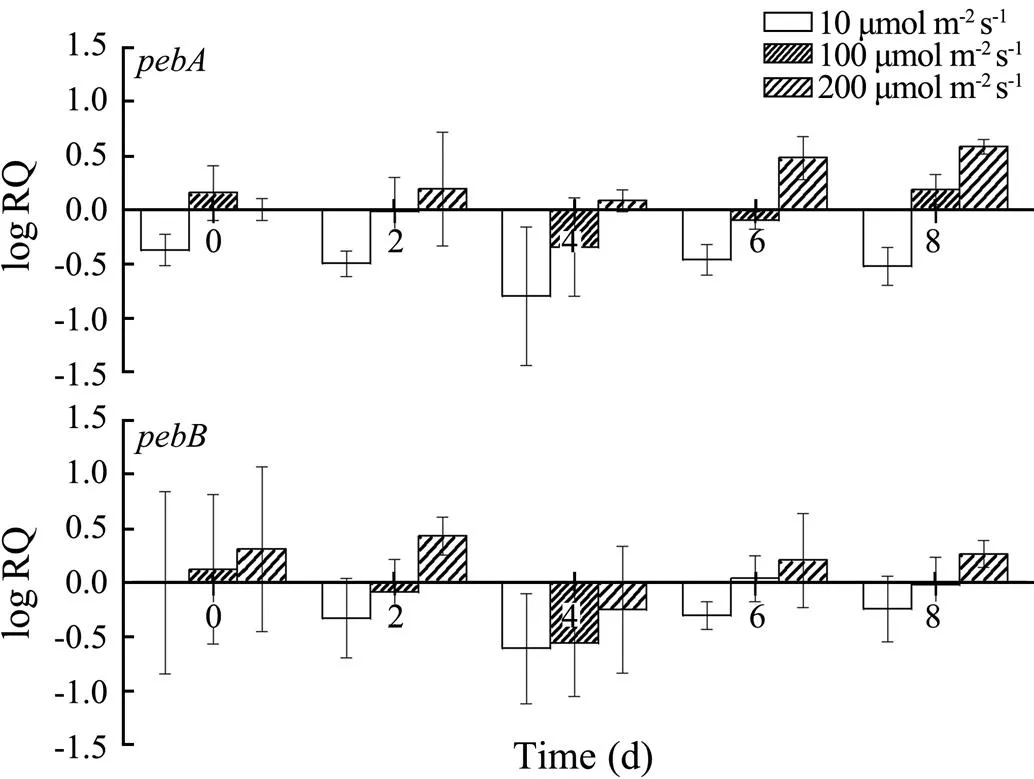
Fig.5 Changes in the pebA and pebB transcription of cultivar 981 under different light intensities over time.
At high light intensities, theandexpression levels of cultivar 2007 increased. By contrast, their levels decreased at low light intensities (Fig.6).
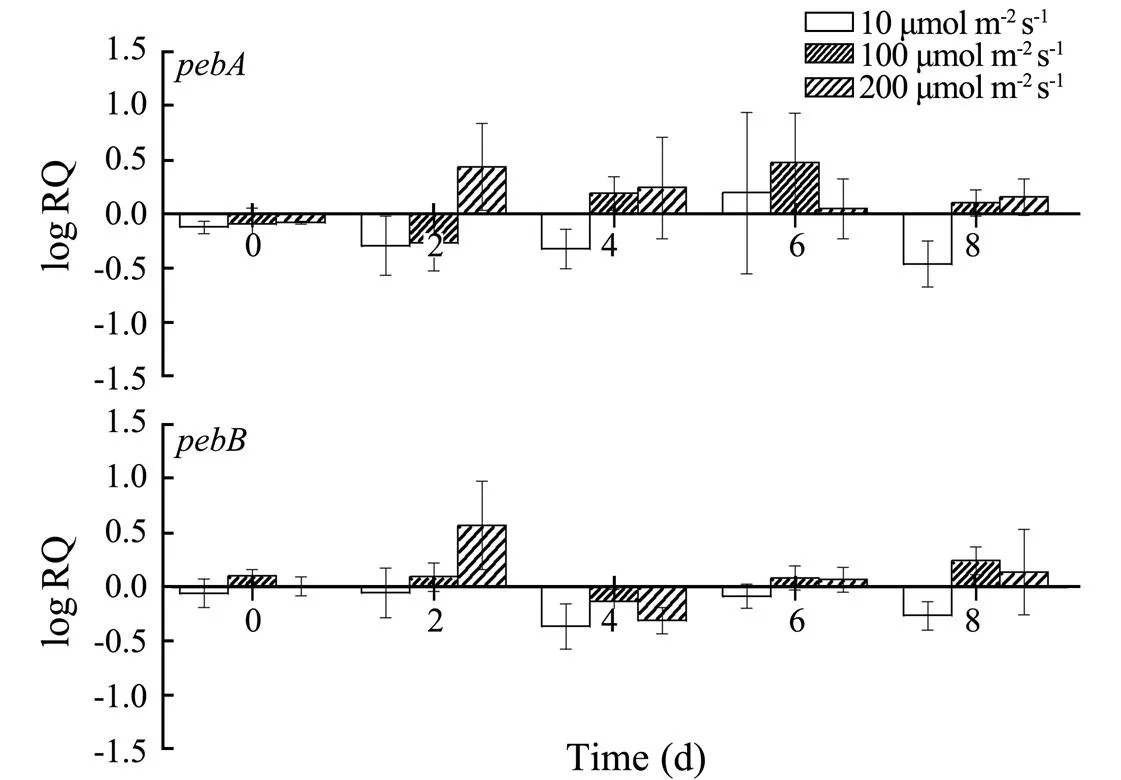
Fig.6 Changes in the pebA and pebB transcription of cultivar 2007 under different light conditions.
4 Discussion
Light intensity directly affects photosynthesis. The ab- sorption and utilization of light energy are positively cor- related as long as light intensity is within the tolerance le- vel of plants. When light intensity exceeds a certain level, plants will absorb excessive light energy, and the unused light energy will lead to photoinhibition and a decreasedlight energy conversion rate. In red algae, long-term expo- sure to a high light intensity may damage phycobilipro- teins and Chl(Sommers, 2013). In our study, the photo- synthetic pigment contents of wild type and cultivar 981decreased as light intensity increased tofour different levels. This observation was similar to those in other studies on algae,., the PE, PC, and Chlcon- tents ofdecrease as light intensity in-creases (Ak and Yücesan, 2012). Our study further reveal- ed that the PE content of cultivar 2007 did not decrease at a light intensity of 200μmolm?2s?1because of its good per- formance at a high light intensity. Under low light condi- tions, plants increase the amount of light energy captured through physiological and biochemical regulation to main- tain the normal photosynthetic efficiency (Zhang.,2003). A high PE content accumulates under low light con- ditions to compensate for the reduced light level (Talarico and Maranzana, 2000; Marinho-Soriano, 2012). In our re- search, the PE content of wild-typewas regulated by light, and it increased significantly at a low light intensity, thereby compensating for the decreased light energy to meet the growth requirements. This observation is also consistent with the important role of PE in thephotosynthesis of. The PE contents of cul- tivars 981 and 2007 were higher than those of the wild type, but the highest PE content was found in cultivar 981. Under the different light conditions, the PE content of cul- tivar 2007 remained relatively stable, and the short-term high and low light intensities favored PE accumulation. This observation may be associated with the fast growth rate and good tolerance of cultivar 2007 to a high light in- tensity (Chen., 2009; Meng., 2009). The short- term (8d) changes in light intensity slightly affected cul- tivar 2007.
PE is located at the periphery of phycobilisomes and directly absorbs light energy. Phycobilisomes can adapt to environmental changes by constantly adjusting their struc- ture, so they can efficiently transfer the absorbed light energy. PE levels can also vary through the addition of more PE units to phycobilisomes and the formation of additional phycobilisomes, which can affect photosynthe- sis by altering the energy transfer efficiency (Grossman., 1993). A change in illumination inevitably leads to a variation in the transcription levels of PE subunit genes and other related genes. In the present study, at light in- tensities of 10, 60, 100, and 200μmolm?2s?1, the transcrip- tional changes in,,, andof wild-type, 981, and 2007were measuredreal- time PCR. The results showed that the changes in the two PE subunit genes (α and β) were similar under the dif- ferent light conditions, indicating that they were likely co- ordinated. This result was consistent with the fact that the gene loci of the α and β subunits are connecteda short linker in the genome, thereby allowing them to be trans- cribed synchronously and co-participate in the assembly of PE. The modifications in the transcription levels of the PE α and β subunits were similar to the change in the PE content, especially under low light conditions. This find- ing indicated that the PE α and β subunit genes were stably transcribed and translated to PE. The variations in the transcription levels of the PE genes of cultivar 981 and 2007 were lower than those of the wild type under diffe- rent light conditions. These findings were consistent with their more stable PE levels under various light conditions.
The transcription trends ofandunder dif- ferent light conditions were similar, indicating a coordi- nated expression, which was different from the transcrip- tion trends of PE subunit genes. Overall,andonly changed slightly under different light conditions, and the gene transcription levels increased as the light inten- sity increased. No compensatory upregulation was found inandtranscription levels at a low light inten- sity. By contrast,andof the three strains were upregulated at a high light intensity. The transcriptions ofandwere not remarkably inhibited by a high light intensity and did not reach a high level even at 200μmolm?2s?1. This result indicated that the change in the content of PE with light intensity was mainly due to the variation in the expression level of genes encoding PE rather than a variation in PEB synthesis.
5 Conclusions
The highest PE content was observed in cultivar 981, followed by that in cultivar 2007, whereas the lowest PE content was found in the wild type. The effects of chang- ing light intensity on the transcription of PE and related genes in cultivars 981 and 2007 were less than those in the wild type. Cultivar 2007 was superior to cultivar 981 in terms of maintaining high pigment levels in a wide range of light intensities, and can adapt to different light intensities. Thisfinding was consistent with the fast growth rate and high ad- versity-tolerance of cultivar 2007 described in other stud- ies (Chen., 2009; Meng., 2009). Thus, among thethree strains studied in this research, cultivar 2007was the most suitable for aquaculture.
Acknowledgements
This research was supported by the National Natural Sci- ence Foundation of China (No. 31872555), the China Agri- culture Research System (No. CARS-50), and the Key Pro-gram of Science and Technology Innovation Ningbo (No. 2019B10009). Critical comments and support were pro- vided by Dr. John van der Meer.
Ak, I., and Yücesan, M., 2012. Effect of light intensity on the pigment composition of(Rhodophyta)., 21: 2126-2131.
Chen, W. Z., Di, X. U., Wang, L. G., Meng, L., Hong, D. U., and Zhang, X. C., 2009. Preliminary study on economic charac- teristics and agar characteristics of two new strains of., 39 (3): 437-442 (in Chinese with English abstract).
Dammeyer, T., and Frankenbergdinkel, N., 2006. Insights into phycoerythrobilin biosynthesis point toward metabolic chan- neling., 281: 27081-27089.
Ficner, R., Lobeck, K., Schmidt, G., and Huber, R., 1992. Isola- tion, crystallization, crystal structure analysis and refinement of B-phycoerythrin from the red algaat 2.2 A resolution., 228: 935- 950.
Freile-Pelegrín, Y., and Murano, E., 2005. Agars from three spe- cies of(Rhodophyta) from Yucatán Peninsula., 96: 295-302.
Gantt, E., 1980. Structure and function of phycobilisomes: Light harvesting pigment complexes in red and blue-green algae., 66: 45-80.
Grossman, A. R., Schaefer, M. R., Chiang, G. G., and Collier, J. L., 1993. The phycobilisome, a light-harvesting complex re- sponsive to environmental conditions., 57: 725.
Haldrup, A., Jensen, P. E., Lunde, C., and Scheller, H. V., 2001. Balance of power: A view of the mechanism of photosynthe- tic state transitions., 6: 301-305.
Huang, X., Zang, X., Wu, F., Jin, Y., Wang, H., Liu, C., Ding, Y., He, B., Xiao, D., and Song, X., 2017. Transcriptome sequen- cing ofto analyze the genes re- lated to optically active phycoerythrin synthesis., 12: e0170855.
Kursar, T. A., Van, D. M. J., and Alberte, R. S., 1983. Light-har- vesting system of the red alga: II. Phy- cobilisome characteristics of pigment mutants., 73: 361-369.
Livak, K. J., and Schmittgen, T. D., 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method., 25: 402-408.
Maccoll, R., 1998. Cyanobacterial phycobilisomes., 124: 311.
Maccoll, R., and Guard-Friar, D., 1987..Inc., Boca Raton, 218pp.
Marinho-Soriano, E., 2012. Effect of depth on growth and pig- ment contents of the macroalgae., 22: 730-735.
Meng, L., Xu, D., Chen, W. Z., and Zhang, X. C., 2009. Selection and characterization of a new strain of., 39 (S1): 94- 98 (in Chinese with English abstract).
Rochaix, J. D., 2007. Role of thylakoid protein kinases in pho- tosynthetic acclimation., 581: 2768-2775.
Sommers, E., 2013. A Look Into the Past: Photo-Physiology of Cyanobacterial Mat Pigments in Alpena, Michigan and Lake Huron Sinkholes. Presentation, Annis Water Resource Institute (AWRI).
Talarico, L., and Maranzana, G., 2000. Light and adaptive re- sponses in red macroalgae: An overview 1., 56: 1-11.
Wu, F., Zang, X., Zhang, X., Zhang, R., Huang, X., Hou, L., Jiang, M., Liu, C., and Pang, C., 2016. Molecular cloning ofand heterodimeric bilin lyase activity analysis of CpcU and CpcS for attachment of phycocyanobilin to Cys-82 on the β-subunit of phycocyanin inFACHB314., 21: 357.
Zhang, Y., Feng, Y., Feng, Z., and Cao, K., 2003. Morphological and physiological acclimation to growth light intensities in., 29: 206-214.
June 1, 2020;
June 28, 2020;
December 22, 2020
? Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2021
#The two authors contributed equally to this work.
. E-mail: xnzang@ouc.edu.cn
(Edited by Qiu Yantao)
 Journal of Ocean University of China2021年3期
Journal of Ocean University of China2021年3期
- Journal of Ocean University of China的其它文章
- Effects of Nitrogen Sources and Concentrations on the Growth of Different Phytoplankton Taxa
- Mathematical Proof of the Synthetic Running Correlation Coefficient and Its Ability to Reflect Temporal Variations in Correlation
- Risk Assessment of Marine Environments Along the South China Sea and North Indian Ocean on the Basis of a Weighted Bayesian Network
- Assessment of the Tidal Current Energy Resources and the Hydrodynamic Impacts of Energy Extraction at the PuHu Channel in Zhoushan Archipelago, China
- Influence of Environmental Conditions on the Sound Velocity Ratio of Seafloor Surficial Sediment
- Quantitative Evaluation of the Larval Density of Sea Potato Acaudina spp. with Mitochondrial Molecular Marker
