Effects of Ultraviolet (UV) Radiation on Outdoor-and Indoor-Cultured Prorocentrum lima, a Toxic Benthic Dinoflagellate
CHEN Heng, HUANG Kaixuan, LIU Shasha, and LU Songhui
Effects of Ultraviolet (UV) Radiation on Outdoor-and Indoor-Cultured, a Toxic Benthic Dinoflagellate
CHEN Heng#, HUANG Kaixuan#, LIU Shasha, and LU Songhui*
,,,510362,
The effects of different types of ultraviolet (UV) radiation (UVR, wavelength=280–400nm) and light intensities on cell growth, pigment composition, UV-absorbing compounds (UVACs), and chlorophyll(Chl) fluorescence were studied in dinoflagellatecultured outdoors for 16 days and indoors for 18 days. In the outdoor experiment, UVA radiation (320–400nm) increased the growth rate of this dinoflagellate when solar light intensities were<12%; decreased growth rates were observed when intensities were>12%. Exposure to UVB radiation (280–320nm) alleviated the negative effects of UVA. In the indoor experiment, UVA and low doses of UVB enhanced growth rates. Addition of low doses of UVB to UVA exposure resulted in higher contents of Chland photoprotective pigments compared with UVA exposure only. The results of both experiments showed that UVB is the primary signal of UVAC synthesis. High-dose UVB exposure accelerated growth rates when UVAC contents were maintained at high levels, suggesting that the latter plays a key role in UVR damage protection. Furthermore, the repair rate was enhanced by UVB exposure after 16 days of culture. This study confirms the positive effects of UVA and UVB on the growth of, with the latter enhancing the photoprotective and recovery pathways of the species.
ultraviolet radiation; benthic dinoflagellates; positive effect;; UV-absorbing compounds
1 Introduction
Ultraviolet radiation (UVR) is a component of the solar radiation spectrum and can be divided into two bands at the Earth’s surface, including UVA (wavelength, 320–400nm) and UVB (wavelength, 280–320nm). Excess UVR is considered a harmful environmental factor for living organisms (Rastogi and Sinha, 2009). It can damage many biological systems because it induces the formation of cy-clobutane pyrimidine dimers in DNA, damages the photosynthetic apparatus, and decreases photosynthetic and growth rates (Villafa?e., 2004). Moreover, as a consequence of ozone layer depletion, increased UVB levels can decrease primary productivity and alter phytoplankton population structures (Gao., 2007, H?der., 2015).
Marine benthic dinoflagellate assemblages are essential participants in marine ecosystems (Faust, 1991). Research on harmful benthic dinoflagellates, which produce toxins that can threaten the health of marine organisms and humansthe food chain (Mangialajo., 2008; Luo., 2017), has steadily increased over the last decade. As epi-phytic or benthic species, these organisms are normallyfound on bottom substrates, such as rocks, macroalgae, co-rals, and seagrass, to which they attach and proliferate (Shears., 2009). The results of numerous field investigations indicate that benthic dinoflagellates are widely distributed in intertidal and subtidal zones where solar ir-radiance can vary from low levels to over 1000μmolpho-tonsm?2s?1(Cohu and Lemée, 2012). Similar to other epiphytes, benthic dinoflagellates inevitably experience high light irradiance due to variations in solar irradiation, which can be attributed to column mixing, tidal changes, and diurnal variation. Toxin-producing benthic dinoflagel-lates are mainly distributed in temperate and tropical regions, where they undergo complex solar radiation exposure patterns (Gómez., 2005; Ferrier-Pagès., 2007).
Marine autotrophic organisms have evolved photoprotective mechanisms to resist photodamage from excess light and UVR. The non-photochemical quenching (NPQ) process is an effective photoprotective strategy that allows such organisms to dissipate excess light energy and protect their photosynthetic apparatus. NPQ dissipation is related to the activity of the xanthophyll cycle and achi-evedde-epoxidation of photoprotective xanthophyll pig-ments, which, in dinoflagellates, are mainly diadinoxanthin and diatoxanthin (Briantais, 1994; Venn., 2006). Another photoprotective method commonly employed is the synthesis of ultraviolet-absorbing compounds (UVACs), which are mainly composed of mycosporine-like amino acids (MAAs) in marine algae (Sinha., 1998). These compounds have a high UVR extinction coefficient and a characteristic UV absorption peak of 310–360nm (Banaszak., 2006). MAAs are involved in the resistance of autotrophic organisms to UVR damage and can be induced by high light or UVR levels (Taira and Taguchi, 2017). The inherent and induced contents of MAAs are species-specific. An investigation of over 110 marine algal species indicated that dinoflagellates and haptophytes exhibit higher MAA synthesis rates than other algal groups (Jeffrey., 1999).
Some researchers speculate that benthic dinoflagellates can synthesize high concentrations of UVACs to resist UV damage. Thus, in the present study,, a globally distributed and toxic benthic dinoflagellate species (Nagahama., 2011; Luo., 2017), was selected to study the effects of different light and UVR intensities. The objective of this work is to understand the photoprotective mechanisms of harmful benthic dinoflagellates in response to excess light and UVR intensities.
2 Materials and Methods
(SD4) was isolated from Hainan Island, Hainan Province, China (Zhang., 2015), and cultured at the Research Center for Harmful Algae and Marine Biology, Jinan University. The strain was maintained at 25℃ in f/2 medium (Guillard and Ryther, 1962) under irradiation by a white light-emitting diode (LED) at 100μmol photonsm?2s?1with a 12h:12h light:dark period.
2.1 Outdoor Experiments
Algal cells in the exponential growth phase were diluted to 500cellsmL?1 in f/2 culture medium and placed into quartz tubes (diameter, 2cm; height, 20cm). Two ir-radiation treatments were then applied. In the first set of treatments, different light intensities (3%, 6%, 12%, 25%, 50%, and 100% solar radiation) were achieved by using different layers of neutral filters to cover the tubes (one layer reduces the light intensity in the treatment by approximately 50%). In the second set of treatments, diffe-rent UVR intensities comprising three kinds of photosynthetic active radiation (PAR), PA (PAR+UVA), and PAB (PAR+UVA+UVB), were achieved using different cutoff filters. The tubes containing the algae were transferred to a control bath (Eyela, Japan) with circulating water at 25℃±1℃ and cultured for 16 days (Fig.1A).
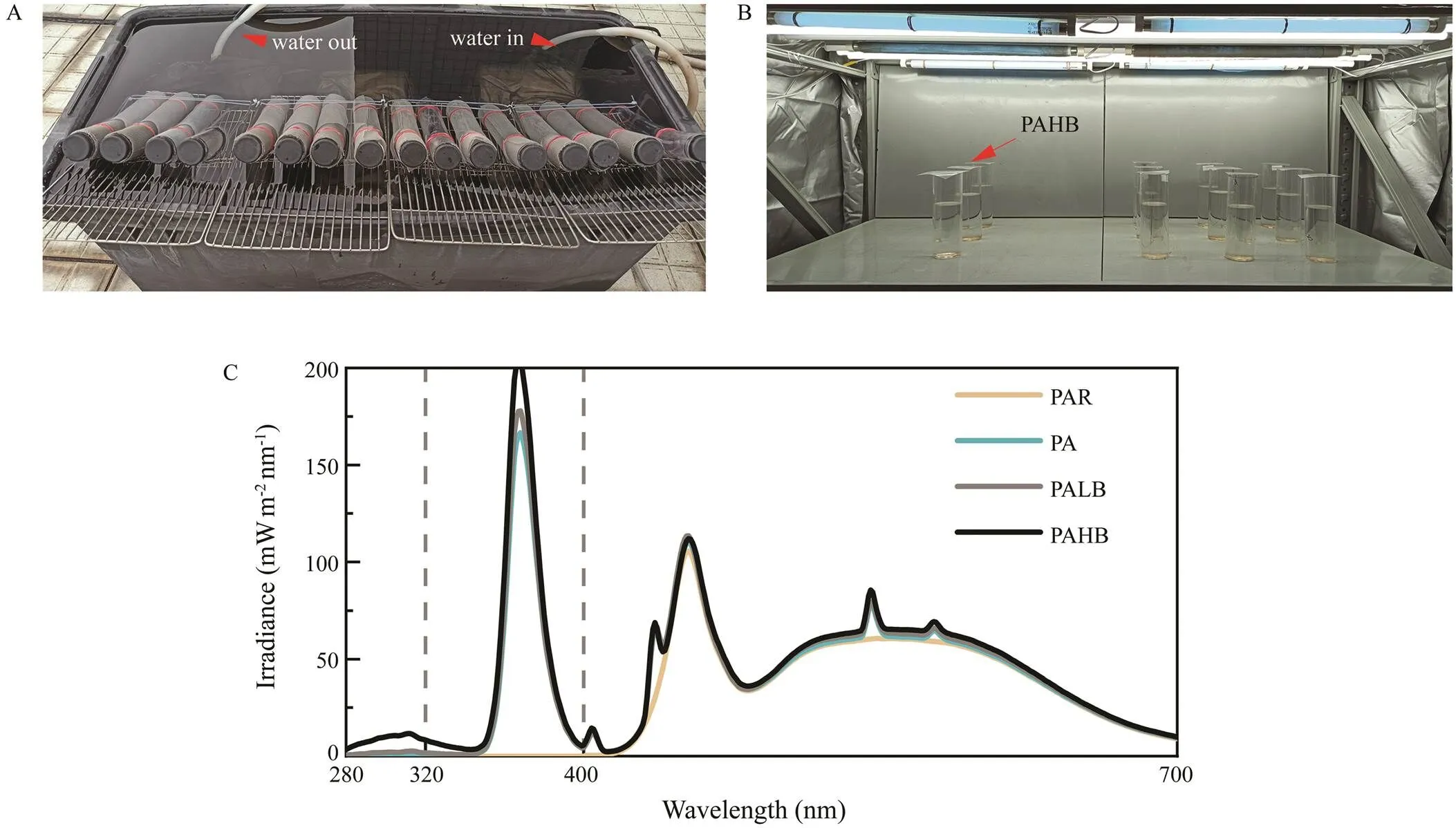
Fig.1 (A) In the outdoor culture, quartz tubes (diameter, 2cm; height, 20cm) containing P. lima were wrapped in different UV cutoff filters and neutral filters and immersed in a black water tank. The tank was connected to a control bath with circulating waterfor temperature control. The inlet and outlet water pipes are indicated by red arrows. Three such water tanks were used for the outdoor experiment. (B) In the indoor culture, P. lima was placed in quartz tubes (diameter, 4cm; height, 20cm) wrapped in different UV cutoff filters and cultured under artificial light. Two UVB lamps were wrapped by different layers of neutral filter to provide two levels of UVB irradiance. (C) Light spectra detected in the indoor cultures. UVA, UVB=ultraviolet A, ultraviolet B; PAR=photosynthetically active radiation; PA=PAR+UVA; PALB=PAR+UVA + UVB (0.05Wm?2); PAHB=PAR+UVA+UVB (0.3Wm?2).
2.2 Indoor Experiments
The algae were cultured at 25℃ indoors for 18d with exposure to PAR (100μmolphotonsm?2s?1), PA (PAR+UVA, UAV=4.2Wm?2or 21μmol photonsm?2s?1), PALB (PA+low UVB, UVB=0.05Wm?2or 0.25μmol photonsm?2s?1), or PAHB (PA+high UVB, UVB=0.3Wm?2or 1.5μmol photonsm?2s?1) to clarify the effects of UVB on(Fig.1B). The PAR, UVA, and UVB light sources were LED (Opple, China), TL-D (Philips, Germany), and G15TBE (Sankyo Denki, Japan), respectively. The spectrum of each treatment was measured by a spectrometer (Hopoocolor, China; Fig.1C).
2.3 Solar Irradiance and UVR Treatments
The outdoor cultures received solar radiation and were monitored by QSI2100 (Biospherical Instruments Inc., USA) and PMA2100 (Solar Light, USA) devices, which record-ed solar PAR, UVA, and UVB levels every 1 or 5min. Dif-ferent cutoff filters were used for the UVR treatments: 1) PAR–the quartz tubes were covered with 395nm cutoff filters (Ultraphan, Digefra, Munich, Germany) transmitting wavelengths>395nm; 2) PA–the quartz tubes were covered with 320nm cutoff filters (Montagefolie, Folex,Dreieich, Germany) transmitting wavelengths>320nm; and 3) PAB–the quartz tubes were covered with 295nm cutoff filters (Ultraphan, Digefra) transmitting wavelengths>295nm.
2.4 Measurement of Specific Growth Rates
The samples were fixed with Lugo’s solution, and the cell numbers were counted under an optical microscope (CX31, Olympus, Japan). The growth rate () was calculated as follows:
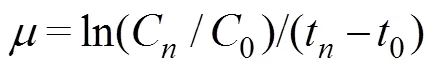
whereCand0represent the final and initial cell concentrations (cellsmL?1), respectively. Theof each treat-ment was measured in sextuplicate.
2.5 Measurement of UVAC and Pigment Concentrations
Exactly 15mL of cell culture medium was obtained be-fore and after the treatments and filtered through What-man GF/F filters. The filtrates were extracted by methanol (3mL) overnight at 4℃ and then centrifuged for 10min at 1500×(Eppendorf, Germany). Absorbance measure-ments of the supernatant were obtained within the wavelength range of 280–700nm using a scanning spectropho-tometer (U-3900, Hitachi, Japan). UVAC concentrations were expressed as the peak optical density at 310–370nm (Dunlap and Yamamoto, 1995). Chlorophyll(Chl) con-centrations were calculated following the method of Porra (2002). Pigment samples from the indoor experiments wereextracted by acetone and measured by a high-performance liquid chromatographic system (Agilent 1100, USA) with a C18 column (4.6mm, 180mm; Waters, USA). Pigment separation was performed following the method of Barlow. (1993). Chlorophyll and carotenoids were detected by absorbance at 440nm. Pigment peaks were iden-tified by comparing their retention times with those of the standards.
2.6 Measurement of Damage and Recovery Rates
After outdoor acclimation, the algal cultures were trans-ferred to small quartz tubes (10mL) at a density of less than 20μg Chlper mL and dark-adapted for 15min. The tubes were then covered with filters to conduct P, PA, and PAB treatments. A 1200W xenon lamp (HMI, Osram, Ger-many) was used as the UVR source. The lamp was adjusted to provide specific intensities of PAR (2000μmolphotonsm?2s?1), UVA (36Wm?2or 180μmolphotonsm?2s?1), and UVB (5.2Wm?2or 26μmolphotonsm?2s?1), whichare similar to the corresponding intensities observed at noon on a sunny day. After 30min of exposure to an artificial solar irradiance lamp, photochemical efficiency (Y’) and NPQ were measured by a Phyto-PAM device (Walz, Germany) every 3 or 15min, and the exposure–response curves (ERCs) were created. The ratio ofwas calculated by dividing the-value of different light intensities of solar irradiance (3%–50%) with the-value of 100% solar irradiance (Guan and Li, 2017). The rate of UVR-in-duced damage to the photosynthetic apparatus (, min?1) and corresponding repair rate (, min?1) were estimated ac-cording to previous studies (Lesser., 1994) as follows:
Y’/Y0Y’,
whereY’and0are the-values at timetand0, respectively.
2.7 Data Analysis
All experiments were performed in triplicate. Differences among the treatments were tested by one-way ANOVA (Tukey’s test) with SPSS 16.0. A confidence le-vel of 95% was used in all analyses.
3 Results
3.1 Long-Term Effects of Different UVR and Solar Light Intensities
During the 16d of acclimation under solar irradiance, the average intensities of PAR, UVA, and UVB radiation were 475μmolphotonsm?2s?1, 2.52Wm?2(12.6μmolphotonsm?2s?1), and 0.3Wm?2(1.5μmolphotonsm?2s?1), respec-tively. Two phases were defined according to the weather conditions during the experiment. Phase 1, which includ-ed the first 20d, represented rainy or cloudy conditions and featured a relatively low level of solar irradiance, as shown in Fig.2A.The relative cell densities observed among the different treatments were similar in this phase (shown in Fig.1B).The doses of solar irradiance in Phase 2, which includeddays 11–16 of the experiment, were approxi-mately 2–4 times higher than the average values in Phase 1 (Fig.2A). Rapid growth and variations in cell densities among the different treatments were observed in Phase 2 (Fig.2B).
The relationships betweenand percentage of solar irradiance under the conditions of P, PA, and PAB were cal-culated with formula=+×exp(×). In all cases, the formula fit the relationship (2>0.95, Fig.2C). The initial slopes of the curves under PA (0.0070±0.0007) and PAB (0.0065±0.0005) were higher than that under P (0.0029±0.0002;<0.05, Fig.2C).
Under 3%–12% solar irradiance, which is lower than thelight saturation point, UVA negatively inhibited algal growth,indicating that UVA radiation enhances growth under light-limited conditions (Fig.2D). With light intensities >12% so-lar irradiance, UVA positively whereas UVB negatively af-fected the algal growth (Fig.2D). This finding suggests that UVA inhibits algal growth whereas UVB may moderate this inhibition under high solar irradiance.
As shown in Fig.3, photosynthetic pigments wavelengths of 640–700nm with a peak at 665nm. The highest optical densities at 665nm in the P, PA, and PAB treatments occurred at solar irradiances of 25%, 12%, and 50%, respec-tively. Absorption by UVACs occurred at wavelengths of 280–400nm with peaks at 334 and 365nm. The optical densities of UVACs under the P, PA, and PAB treatments consistently increased according to the percentage of solar irradiance.
Fig.4A illustrates the relationship between Chland UVACs and the percentage of solar irradiance under different UVR treatments. When the light intensities were >12% solar irradiance, the cellular contents of Chlunder the PA treatment were all lower than those under the P and PAB treatments (<0.05) and no significant difference between P and PAB was found (<0.05). Associations between the contents of UVACs and percentage of solar irradiance under different UVR treatments were fittedusing linear equations (2>0.95, Fig.4B). The slope of thePAB model (0.034±0.002) was approximately three timesgreater than those of the PA (0.013±0.002) and PAR (0.012±0.001) models, which indicates that UVB may be the main radiation band inducing the synthesis of UVACs.
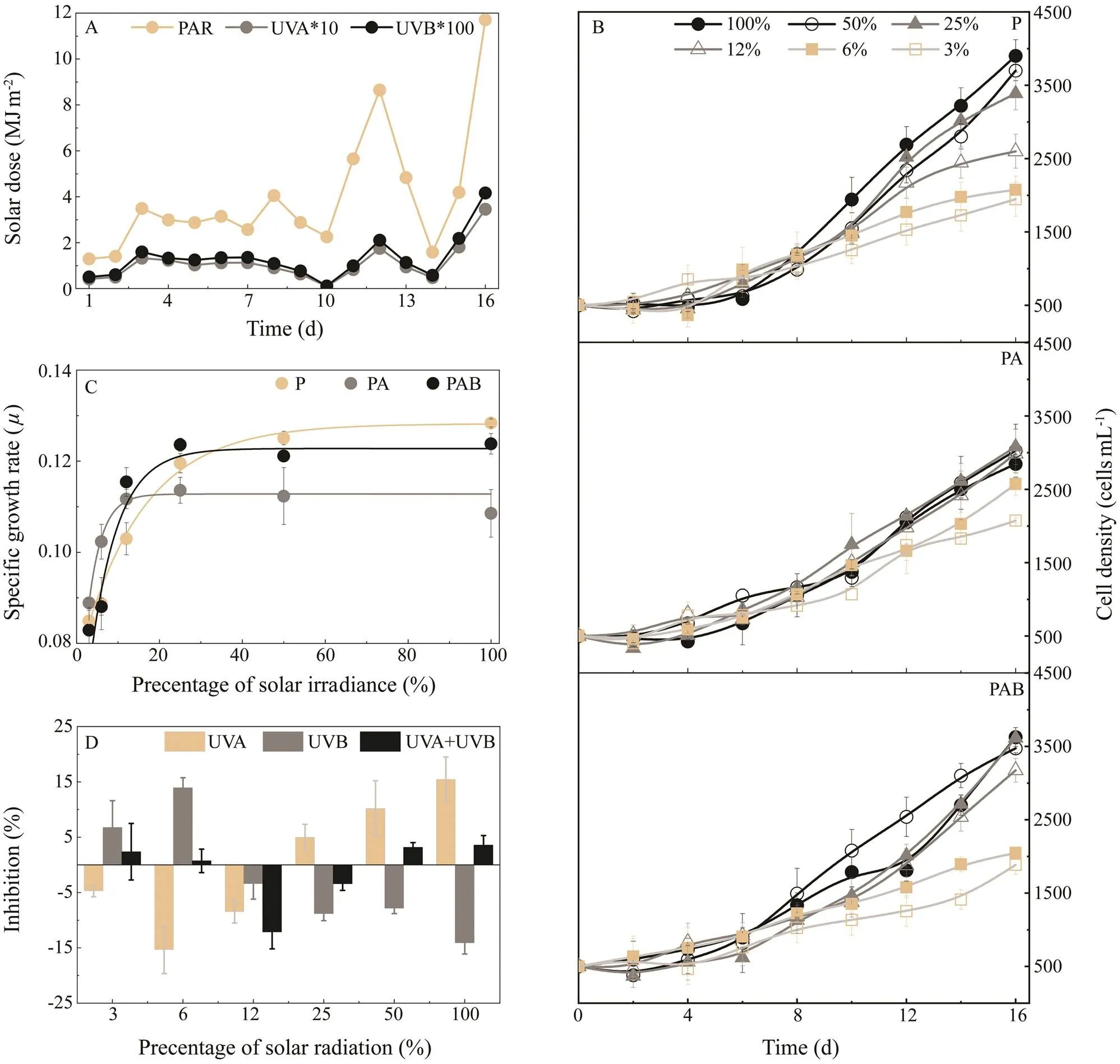
Fig.2 (A) Doses of solar radiation of PAR (400–700nm), UVA (320–400nm) and UVB (280–320nm) during the 16-d ex-periment. (B) Cell density throughout the experiments under different light intensities (3%–100% solar light) and diffe-rent UVR exposures (PAR, PA, PAB). (C) Specific growth rate (μ) of PAR, PA and PAB on day 16 as a function of acclimated light intensity (3%–100% solar irradiance). (D) Inhibition of different UV wavebands (UVA, UVB, and UVA+UVB) on μ under different acclimated light intensities. Vertical bars indicate standard deviations (n=6). PAR means photosynthetically active radiation;PA means PAR with UVA, PAB means PAR with UVA and UVB, UV means ultraviolet.

Fig.3 Spectral absorption characteristics after 16 days of acclimation to various light intensities under the (A) PAR, (B) PA, and (C) PAB treatments. PAR=photosynthetically active radiation, PA means PAR with UVA, PAB means PAR with UVA and UVB, UV=ultraviolet.
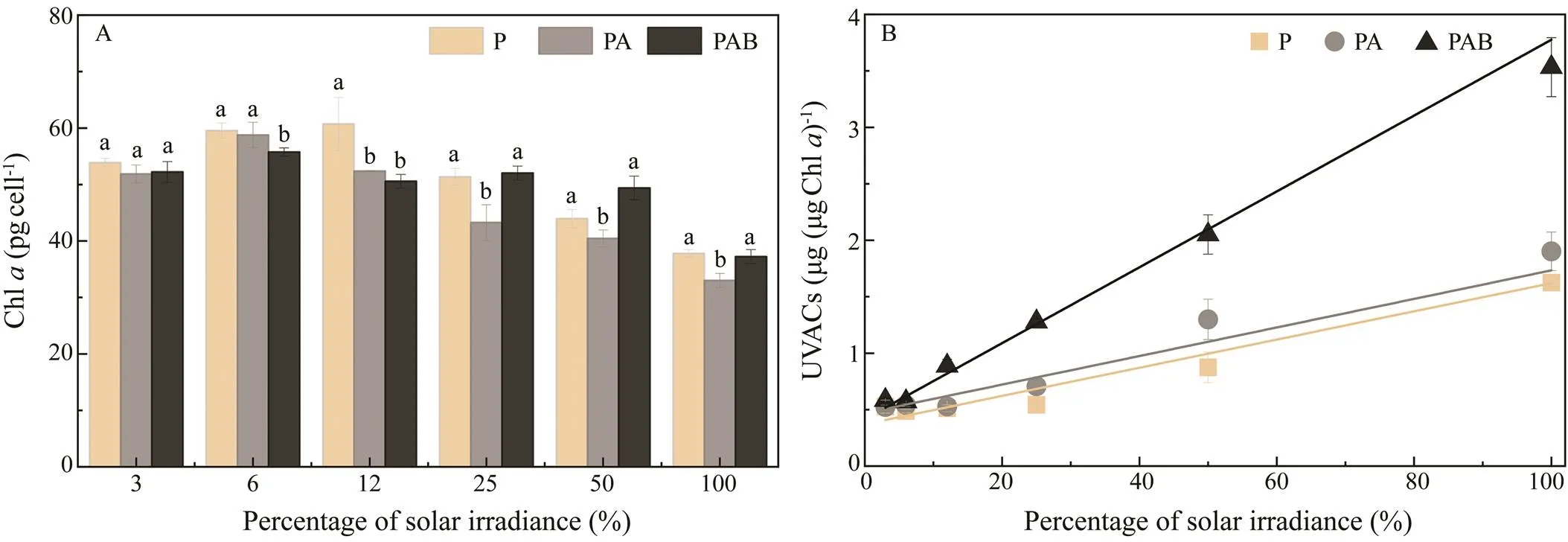
Fig.4 Effect of different UVR and solar light intensities on the contents of (A) Chl a and (B) UVACs. Vertical bars indicate standard deviations (n=3). Different lowercase letters between two bars indicate a statistical difference within treatments. Chl a means chlorophyll a; UVACs means ultraviolet-absorbing compounds; PAR means photosynthetically active radiation; PA means PAR with UVA, PAB means PAR with UVA and UVB, UV means ultraviolet.
3.2 Short-Term Effects of Different UVR and Solar Irradiance Intensities
As shown in Fig.5, the-value ratio and solar irradi-ance percentage were positively correlated, suggesting that photoacclimation occurs within the 16-day treatment period.samples grown at 100% solar irradiance under different UVR treatments (P, PA, or PAB) showed low-er decreases in effective photochemical efficiency than those grown at other solar irradiance percentages (3%–50%). In addition, the ratio of-values at other light intensities to that at 100% solar irradiance was less than 1 at most points of time.
The relationship between NPQ and light intensity was fitted by an exponential equation (2>0.95, Fig.6A). NPQ values remained steady at solar irradiances greater than 25%. The NPQ values of the P and PAB treatments were significantly higher than that of PA when solar irradiance intensities were >25% (<0.05).
Theand correspondingof each treatment were estimated according to the ERCs (Figs.6B, C). The results showed that different levels of solar irradiance do not af-fectunder different UVR treatments (>0.05, Fig.6C). By contrast, thevalues observed at solar irradiances of 50% and 100% under the PA treatment were significantly lower than those under the P and PAB treatments (<0.05, Fig.6B). Thus, the ratio ofto(/) with 100% irradi-ance under the PA treatment was significantly lower than those under the P and PAB treatments (<0.05, Fig.6D).

Fig.5 Plots of the effective photochemical efficiency (Y’) ratio of X to Y’ under 100% solar irradiance. X indicates the ratio of Y’ at solar irradiances of 50%, 25%, 12%, 6%, and 3%. Y’ratios for the (A) PAR, (B) PA, and (C) PAB treatments. Vertical bars indicate standard deviations (n=6). PAR means photosynthetically active radiation;PA means PAR with UVA, PAB means PAR with UVA and UVB, UV means ultraviolet.

Fig.6 Plots of (A) NPQ, (B) repair rate (r, min?1), (C) damage rate (k, min?1), and (D) r/k ratio under the PAR, PA, and PAB treatments with artificial light exposure as a function of the acclimated light intensity. Vertical bars indicate standard deviations (n=6). NPQ means non-photochemical quenching; PAR means photosynthetically active radiation; PA means PARwith UVA, PAB means PAR with UVA and UVB, UV means ultraviolet.
3.3 Evidence of the Effect of UVR on Indoor Acclimation
During indoor acclimation, the cell densities in the PA treatment were significantly higher than those in PAR on days 12 and 15 (<0.05, Fig.7A), confirming that UVA addition can enhance the growth of. The highest cell density and growth rate were observed under low UVB addition (PALB; Figs.7A, B). The growth rate under PAHB was not significantly different compared with that under PAR (Fig.7B). No difference in cell density for PAHB was noted between days 3 and 6. Then, following day 6, the cell density increased (>0.05, Fig.7A). Similar growth patterns were also observed with 100% PAB treatment during outdoor acclimation (Fig.2B). In this experiment,samples showed growth stagnation from day 10 to day 12. After day 12, they showed growth acceleration with increasing solar dosage.
The concentration of UVACs in the PAHB treatment increased from 0.09 (day 0) to 1.63μgμg?1Chl(day 6; Fig.7C), which might result in the stagnation of growth of PAHB. The highest concentration of UVACs (2.76μgμg?1Chl) among different UVR treatments was observed in PAHB on day 18. The concentration of UVACs under PALB (0.94μgμg?1Chl) was significantly higher than those under PA (0.46μgμg?1Chl) and PAR (0.35μgμg?1Chl;<0.05).exposed to PALB revealed the highest contents of Chland photoprotective pigments, particularly diadinoxanthin (Fig.7F), diatoxanthin (Fig.7G), and β-carotene (Fig.7I). The enhanced UVB (PAHB) treat-ment showed the lowest contents of pigments (<0.05, Figs.7D–I).
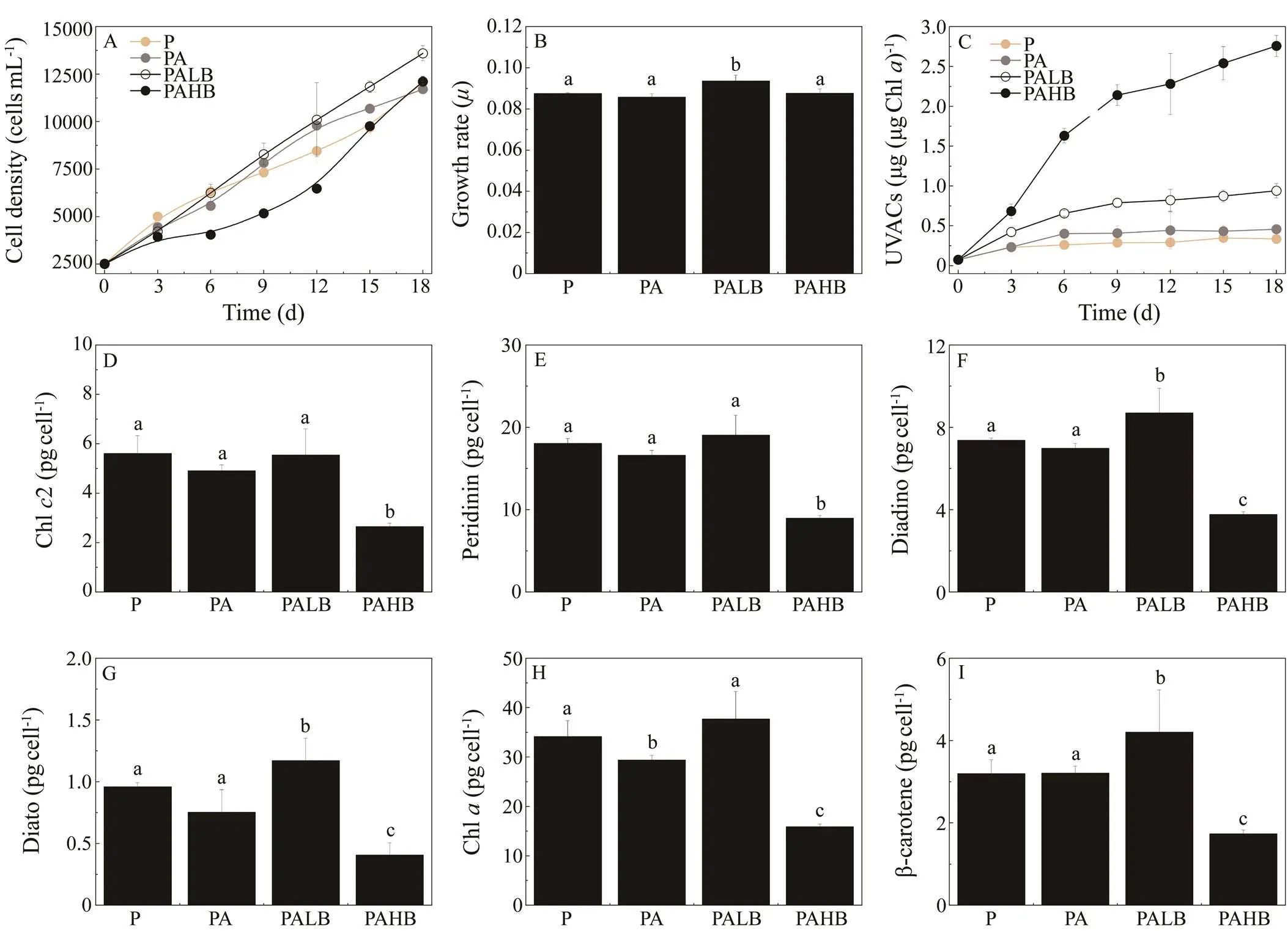
Fig.7 (A) Cell densities, (B) growth rates, (C) UVAC contents, and (D–I) pigments accumulated in indoor conditions under PAR, PA, PALB, and PAHB treatments. Vertical bars indicate standard deviations (n=3). Different lowercase letters between bars indicate a statistical difference within treatments. Diadino means diadinoxanthin; diato means diatoxanthin; UVACs meansultraviolet-absorbing compounds; PAR means photosynthetically active radiation; PA means PAR with UVA, PALB means PAR with UVA and low UVB (0.05Wm?2), PAHB means PAR with UVA and high UVB (0.3Wm?2), UV means ultraviolet.
4 Discussion
The present study investigated the combined effects of light and UVR intensities onin the long-and short-term scales. The simulated conditions represented different vertical distributions of algae exposed to solar light,., those occurring during low tide and at noon in intertidal flats. The results indicate thatnot only endures high solar irradiance and UVR, but also has a complex strategy for responding to or even benefiting from UVR.
UVA significantly enhanced the growth when solar light levels were <12%,and significantly decreased the growth when solar light levels were >12% (Fig.2B). The positive effect of UVA on algal growth has been widely reported in several species, including(Xu and Gao, 2010),(Guan., 2016), andand(Ehling-Schulz, 1997). Xu and Gao (2016) investigated macroalgae from different families and found that the majority of the species can utilize UVA for photosynthesis. However, in some situations UVA restrictions can accelerate the growth.It has been reported that the enhancements in UVA are common in limited-light conditions, such as cloudy days, and carbon fixation is mainly driven by longer UVA wave-lengths (340–400nm)(Mengelt and Prézelin, 2005). In the present study, because the UVA waveband used in the in-door experiments was 340–400nm, growth under PA in-creased, even when the UVA irradiance was >4Wm?2(Fig.1C).
Surprisingly, UVB addition significantly increased growth compared with that under PA with >12% solar irradiance (Fig.2B). UVB radiation is considered a negative factor in the growth and photosynthesis of photoautotrophic orga-nisms. However, several assays have reported a beneficial effect of UVB radiation on the performance of photosynthesis. The marine brown alga, for ex-ample, exhibits low photosynthesis recovery rates under UVB depletion and high recovery rates under UVB after sunlight-induced photoinhibition (Flores-Moya., 1997).Another study suggested that UVB may be involved in the simultaneous impairment and recovery of photosynthesis (Flores-Moya., 1999). Enhancements in recovery induced by UVB have also been observed in several other macroalgae species, including seaweeds(Rhodophyta)(Xu and Gao, 2010) and(Chlorophyta)(Figueroa.,2009), freshwater macroalgae (Bautista-Saraiva., 2018), and other aquatic plants (Hanelt., 2006). Many studies have revealed that the most positive effects of UVB are on tropical and subtropical species that experience in-tense solar radiation exposure, indicating that the UVB strategy may be used to adapt to high solar irradiance. Thestrain in the present study was isolated from Hainan Province, which is located in a tropical region (Zhang., 2015).
UVB exposure promotedand the corresponding. Thus, the/ratio ofunder PAB is higher than that under PA in high-light exposure conditions. The/ratio represents the turnover rate of the D1 protein, which is the main UVR damage target protein and influences the activity of PSII and degree of photoinhibition in cells. Hanelt. (2006) reported that reductions inunder PA treatment cause the highest inhibition ofduring high light exposure, which may be alleviated in cells acclimated by UVB addition. Greenberg. (1989) found that the turnover rate of the D1 protein under UVB exposure is higher than that measured under PAR illumination alone.
UVB may also play a role in stimulating and maintaining photoprotective compounds (Figueroa., 2009).Chland photoprotective pigments were readily degraded in the presence of UVA in both indoor and outdoor experiments (Figs.4A and 7C). UVA had no significant effect on the ratio of UVACs to Chl, suggesting thatdoes not efficiently protect Chl, the most important photosynthetic pigment, under UVA radiation. However, the contents of UVACs were significantly enhanced by UVB, indicating that UVB is the main UVAC induction band in. Moreover, the contents of dia-dinoxanthin and diatoxanthin, the main xanthophyll cycle pigments in dinoflagellates, were enhanced by low UVB in the indoor experiments. Xanthophyll pigments not only contribute to NPQ but may also help stabilize thylakoid chloroplasts and ROS quenching (Havaux and Niyoji, 1999; Brini, 2017). Acclimation under low doses of UVB pro-mote the synthesis of β-carotene, which is known as an efficient antioxidant compound (Rakhimberdieva., 2004). Research indicates that the accumulation of β-ca-rotene can prevent photosynthetic damage from UVA or blue light (White and Jahnke, 2002). The pigment results obtained in the present study support the hypothesis that low doses of UVB may stimulate the cellular photoprotective pathway to ameliorate the damage caused by UVR exposure.
The mechanism by which UVB facilitates the growth and photosynthesis of algae remains unknown. One possible mechanism might be related to the production of UVACs (mainly MAAs) under UVB enhancement. Growth rates are accelerated by high contents of UVACs, which implies that MAAs play a pivotal role in the growth ofunder UVR irradiance. High concentrations of MAAs in cells provide efficient photoprotection against UVR-in-duced damage. Approximately 30% of the photons striking the photosynthetic apparatus are reportedly restrained by MAAs in cyanobacteria (Garcia-Pichel., 1993). Taira and Taguchi (2017) applied several PAR radiation intensities and found different concentrations of MAAs in the cells of the dinoflagellate. Ex-tensive UVR-induced damage was observed under the con-dition of low MAA content induced by low PAR; by contrast, high UVR tolerance was observed in the presence of high MAA contents. On the other hand, MAAs may function as antenna compounds that capture UVR energy into the photosynthetic pathway. In the haptophyte, some MAA species, such as shinorine, Gly, and mycosporine-Gly/Val, are able to transmit absorbed UVR energy to Chlthe photosynthetic pathway (Moi-san and Mitchell, 2001). Whether marine algae use short UV wavelengths (., UVB) for photosynthesis is yet un-clear (Neori., 1986; Holm-Hansen., 1993).
5 Conclusions
This study confirmed that UVA and UVB have positive effects onand help the species accommodate different light conditions. When light is limited,can utilize UVA and UVB to supply extra energy for growth. Under intense light,may use UVB as a signal to induce greater photoprotection by increasing the contents of UVACs and photoprotective pigments,as well as enhancing the recovery rate required to reduce UVR damage.
Acknowledgements
The work was supported by the National Natural Science Foundation of China (Nos. 41706126, 41876173 and 41606176).
List of Abbreviations
UV, Ultraviolet; UVA, ultraviolet A; UVB ultraviolet B; UVR, Ultraviolet radiation; PAR, photosynthetically active radiation; PA, PAR photosynthetically active radiation with ultraviolet A; PAB, photosynthetically active radiation with ultraviolet A and B; PALB, photosynthetically active radiation with ultraviolet A and low B; PAHB, pho-tosynthetically active radiation with ultraviolet A and high B; Chl, chlorophyll; Chl2, chlorophyll2; UVAC, ultraviolet-absorping compound; NPQ, non-photochemi-cal quenching; MAAs, mycosporine-like amino acids(MAAs); Y’, photochemical efficiency; ERCs, exposure-response curves;, damage rate;, repair rate; Diato, diatoxanthin; Diadino, Diadinoxanthin.
Banaszak, A. T., Santos, M. G. B., LaJeunesse, T. C., and Lesser, M. P., 2006. The distribution of mycosporine-like amino acids (MAAs) and the phylogenetic identity of symbiotic dinoflagellates in cnidarian hosts from the Mexican Caribbean., 337 (2): 131-146.
Barlow, R. G., Mantoura, R. F. C., Gough, M. A., and Fileman, T. W., 1993. Pigment signatures of the phytoplankton composition in the northeastern Atlantic during the 1990 spring bloom., 40 (1-2): 459-477.
Briantais, J. M., 1994. Light-harvesting chlorophyll-complex requirement for regulation of Photosystem II photochemistry by non-photochemical quenching., 40(3): 287-294.
Brini, F., 2017. Photosynthesis under stressful environmental con-ditions: Existing challenges.In:. Singh., eds., Studium Press, In-dia, 68-91.
Cohu, S., and Lemée, R., 2012. Vertical distribution of the toxic epibenthic dinoflagellatescf.,andin the NW Mediterranean Sea., 53 (3): 373.
Dunlap, W. C., and Yamamoto, Y., 1995. Small-molecule antioxidants in marine organisms: Antioxidant activity of mycosporine-glycine., 112 (1): 105-114.
Ehling-Schulz, M., Bilger, W., and Scherer, S., 1997. UV-B-in-duced synthesis of photoprotective pigments and extracellular polysaccharides in the terrestrial cyanobacterium., 179 (6): 1940-1945.
Faust, M. A., 1991. Morphology of ciguatera-causing(Pyrrophyta) from widely differing sites., 27 (5): 642-648.
Ferrier-Pagès, C., Richard, C., Forcioli, D., Allemand, D., and Shick, M. P. M., 2007. Effects of temperature and UV radiation increases on the photosynthetic efficiency in four scleractinian coral species., 213 (1): 76-87.
Figueroa, F. L., Korbee, N., Carrillo, P., Medina-Sánchez, J. M., Mata, M., and Bonomi, J., 2009. The effects of UV radiation on photosynthesis estimated as chlorophyll fluorescence in(Chlorophyta) growing in a high moun-tain lake (Sierra Nevada, Southern Spain)., 68 (2): 206-216.
Flores-Moya, A., Hanelt, D., Figueroa, F. L., Altamirano, M., Vi-?egla, B., and Salles, S., 1999. Involvement of solar UV-B ra-diation in recovery of inhibited photosynthesis in the brown alga(Hudson) Lamouroux., 49 (2-3): 129-135.
Gao, K., Li, G., Helbling, E. W., and Villafane, V. E., 2007. Va-riability of UVR effects on photosynthesis of summer phytoplankton assemblages from a tropical coastal area of the South China Sea.,83 (4): 802-809.
Garcia-Pichel, F., and Castenholz, R. W., 1993. Occurrence of UV-absorbing, mycosporine-like compounds among cyanobacterial isolates and an estimate of their screening capacity., 59 (1): 163-169.
Gómez, I., Figueroa, F. L., Huovinen, P., Ulloa, N., and Morales, V., 2005. Photosynthesis of the red algaunder natural solar radiation in an estuary in southern Chile., 244 (1-4): 369-382.
Greenberg, B. M., Gaba, V., Canaani, O., Malkin, S., Mattoo, A. K., and Edelman, M., 1989. Separate photosensitizers mediate degradation of the 32-kDa photosystem II reaction center protein in the visible and UV spectral regions., 86 (17): 6617-6620.
Guan, W. C., and Li, P., 2017. Dependency of UVR-induced pho-toinhibition on atomic ratio of N to P in the dinoflagellate., 164 (2): 31.
Guan, W. C., Chen, H., Wang, T. G., Chen, S., and Xu, J. T., 2016. Effect of the solar ultraviolet radiation on the growth and fluo-rescence parameters of., 40 (1): 83-91 (in Chinese with English abstract).
Guillard, R. R. L., and Ryther, J. H., 1962. Study of marine plank-tonic diatoms: I.Hustedt, and(Cleve) Gran., 8 (2): 229-239.
H?der, D. P., Williamson, C. E., W?ngberg, S. ?., Rautio, M., Rose, K. C., and Gao, K. S., 2015. Effects of UV radiation on aquatic ecosystems and interactions with other environmental factors., 14 (1): 108-126.
Hanelt, D., Hawes, I., and Rae, R., 2006. Reduction of UV-B ra-diation causes an enhancement of photoinhibition in high light stressed aquatic plants from New Zealand lakes., 84 (2): 89-102.
Havaux, M., and Niyogi, K. K., 1999. The violaxanthin cycle protects plants from photooxidative damage by more than one mechanism., 96 (15): 8762-8767.
Holm-Hansen, O., Helbling, E. W., and Lubin, D., 1993. Ultravio-let radiation in Antarctica: Inhibition of primary production.,58 (4): 567-570.
Jeffrey, S. W., MacTavish, H. S., Dunlap, W. C., Vesk, M., and Groenewoud, K., 1999. Occurrence of UVA-and UVB-absor-bing compounds in 152 species (206 strains) of marine microalgae., 189: 35-51.
Lesser, M. P., Cullen, J. J., and Neale, P. J., 1994. Carbon uptake in a marine diatom during acute exposure to ultraviolet B radiation: Relative importance of damage and repair., 30 (2): 183-192.
Luo, Z., Zhang, H., Krock, B., Lu, S., Yang, W., and Gu, H., 2017.Morphology, molecular phylogeny and okadaic acid production of epibenthic(Dinophyceae) species from the northern South China Sea., 22: 14-30.
Mangialajo, L., Bertolotto, R., Cattaneo-Vietti, R., Chiantore, M., Grillo, C., and Lemee, R., 2008. The toxic benthic dinoflagel-late: Quantification of proliferation along the coastline of Genoa, Italy., 56 (6): 1209-1214.
Mengelt, C., and Prézelin, B. B., 2005. UVA enhancement of carbon fixation and resilience to UV inhibition in the genusmay provide a competitive advantage in high UV surface waters., 301: 81-93.
Moisan, T. A., and Mitchell, B. G., 2001. UV absorption by my-cosporine-like amino acids inKarsten induced by photosynthetically available radiation., 138 (1): 217-227.
Nagahama, Y., Murray, S., Tomaru, A., and Fukuyo, Y., 2011. Spe-cies Boundaries in the toxic dinoflagellate(Dinophyceae, Prorocentrales), based on morphological and phylogenetic characters., 47 (1): 178-189.
Neori, A., Vernet, M., Holm-Hansen, O., and Haxo, F. T., 1986. Relationship between action spectra for chlorophyllfluorescence and photosynthetic O2evolution in algae., 8 (3): 537-548.
Porra, R. J., 2002. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophyllsand., 73 (1-3): 149-156.
Rakhimberdieva, M. G., Stadnichuk, I. N., Elanskaya, I. V., and Karapetyan, N. V., 2004. Carotenoid-induced quenching of the phycobilisome fluorescence in photosystem II-deficient mutant ofsp., 574 (1-3): 85-88.
Rastogi, R. P., and Sinha, R. P., 2009. Biotechnological and in-dustrial significance of cyanobacterial secondary metabolites., 27 (4): 521-539.
Shears, N. T., and Ross, P. M., 2009. Blooms of benthic dino-flagellates of the genus; An increasing and ecolo-gically important phenomenon on temperate reefs in New Zea-land and worldwide., 8(6): 916-925.
Sinha, R. P., Klisch, M., Gr?niger, A., and H?der, D. P., 1998. Ul-traviolet-absorbing/screening substances in cyanobacteria, phy-toplankton and macroalgae., 47 (2-3): 83-94.
Taira, H., and Taguchi, S., 2017. Cellular mycosporine-like ami-no acids protect photosystem II of the Dinoflagellatefrom ultraviolet radiation damage., 174: 27-34.
Venn, A. A., Wilson, M. A., Trapidd-rosenthal, H. G., Keely, B. J., and Douglas, A. E., 2006. The impact of coral bleaching on the pigment profile of the symbiotic alga,., 29 (12): 2133-2142.
Villafa?e, V. E., Buma, A. G., Boelen, P., and Helbling, E. W., 2004. Solar UVR-induced DNA damage and inhibition of pho-tosynthesis in phytoplankton from Andean lakes of Argentina., 161 (2): 245-266.
White, A. L, and Jahnke, L. S., 2002. Contrasting effects of UV-A and UV-B on photosynthesis and photoprotection of beta-carotene in twospp., 43 (8): 877-884.
Xu, J. T., and Gao, K. S., 2010. Use of UV-A energy for photo-synthesis in the red macroalga., 86 (3): 580-585.
Xu, J. T., and Gao, K. S., 2016. Photosynthetic contribution of UV-A to carbon fixation by macroalgae., 55 (3): 318-322.
Zhang, H., Li, Y., Cen, J. Y., Wang, H. L., Cui, L., Dong, Y. L., and Lu, S. H., 2015. Morphotypes of(Di-nophyceae) from Hainan Island, South China Sea: Morpholo-gical and molecular characterization., 54 (5): 503-516.
April 13, 2020;
May 27, 2020;
November 10, 2020
? Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2021
#The two authors contributed equally to this work.
. E-mail: lusonghui1963@163.com
(Edited by Qiu Yantao)
 Journal of Ocean University of China2021年3期
Journal of Ocean University of China2021年3期
- Journal of Ocean University of China的其它文章
- Effects of Nitrogen Sources and Concentrations on the Growth of Different Phytoplankton Taxa
- Mathematical Proof of the Synthetic Running Correlation Coefficient and Its Ability to Reflect Temporal Variations in Correlation
- Risk Assessment of Marine Environments Along the South China Sea and North Indian Ocean on the Basis of a Weighted Bayesian Network
- Assessment of the Tidal Current Energy Resources and the Hydrodynamic Impacts of Energy Extraction at the PuHu Channel in Zhoushan Archipelago, China
- Changes in the Photosynthetic Pigment Contents and Transcription Levels of Phycoerythrin-Related Genes in Three Gracilariopsis lemaneiformis Strains Under Different Light Intensities
- Influence of Environmental Conditions on the Sound Velocity Ratio of Seafloor Surficial Sediment
