硬脂酸鋅制造、可持續(xù)性和全生命周期數(shù)據(jù)的建立
Michael Schiller 嚴(yán)晴 嚴(yán)一豐
(1,HMS Concept e.U.,Am Huegel 1,9601 Arnoldstein,Austria;2.深圳市志海實(shí)業(yè)有限公司,深圳,518102)
1.Background
Fatty acids,C16-18,zinc salts(CAS:91051-01-3;Synonyms:Zinc distearate,Zinc stearate)belongs to a group of substances called Zinc soaps,which are widely used.Zinc stearate is mainly used in polymers industry as a stabilizing component (e.g.in PVC,PE,PS)where it contributes to enhance and maintain the properties of the articles made from these polymers.It is also used as lubricant,mould release agent and anti-tack agent for rubber.Zinc stearate is further used in paints,lacquers and varnishing industry as a sanding and flatting agent,in the building industry as a hydrophobic agent in plaster systems,in the paper,pulp,board and textile industry as a hydrophobic agent,in the cosmetics and pharmaceuticalindustry,chemicalindustry,metal industry and other applications.The main types of use categories of zinc stearate can be characterised as non-dispersive and use resulting in inclusion into or onto matrix.
Already in 1995 the total production volume of Zinc stearate in the EU was nearly 25000 mt which were reached more than 30000 mt in 1997[2].
Zinc stearate consists of natural fatty acids and Zinc.Fatty acids are natural constituents of the human body and essential components of a balanced human nutrition.Fatty acids are generally judged as not representing a risk to human health.Zinc as essential trace element is also vital.The non-toxicity of Zinc stearate is proved by extensive testing data which showed no adverse health effects[2].
The database for environmental effects,ecotoxicology and toxicology of Zinc distearate is extensive,allowing a robust evaluation of its hazard properties.However,it is interesting enough that no life cycle assessment(LCA)of this substance is available today.This paper is an attempt to create data on which a LCA which might be created close to reality by considering its raw materials,production,packaging and transportation under the view point of sustainability.
2.Consideration of process and raw materials
Zinc salts can be produced in different ways[3].One way is the neutralisation of the acid(HAc)by a Zinc oxide(ZnO resp.Zn(OH)2);Eq.1.

Based on this reaction mechanism three production ways are possible:
-Reaction 1:a direct and nearly dry conversion of the acid and Zinc compound without solvent(e.g.water)in a high speed mixer. (According to the knowledge of the authors Zinc oxide is mainly used for the production of Zinc stearate.)
-Reaction 2:a melt process at temperatures depending on the melt temperature of the metal soap and the acid which is applicable when Zinc stearate is produce in combination with other metal soaps like Zinc,Magnesium or Lead stearate or laurate.
-Reaction 3:a precipitation reaction in two steps;Eq.2&Eq.3.In a 1ststep an alkaline(mainly Sodium)salt is formed.In a 2ndstep a solution of the soluble metal salt(e.g.Zinc chloride)is added.In the case of reaction 4 an alkaline salt is formed as by-product (e.g.Sodium chloride),which is dissolved in water.
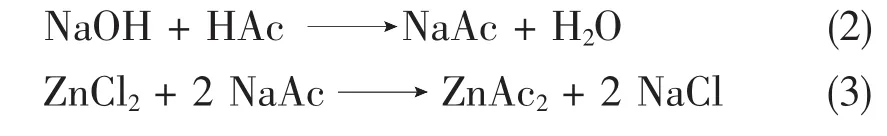
The variation of the fatty acid source and of the different processes is complicating the preparation of an LCA of Zinc stearate.The chemically pure stearic acid is a saturated fatty acid with C18-carbon chain.The IUPAC name is octadecanoic acid.It is a waxy solid, and its chemical formula is CH3(CH2)16CO2H.Its name is derived from the Greek word σ τε'αρ "stéar",which means tallow.Stearic acid is one of the most common saturated fatty acids found in nature following palmitic acid.The socalled and commercially available Sstearic acid occurs in many animal and vegetable fats and oils.It is more abundant in animal fat(<30%)than vegetable fat(<5%).Commercially available stearic acid is prepared by treating these fats and oils with hydrogen for conversion of the unsaturated fatty acids to the saturated at a high pressure and temperature and followed by the hydrolysis of saturated triglycerides.The resulting mixture of fatty acids with different chain length (Table 1)is then distilled.Byproducts are mainly glycerine,sulphuric acid contaminated pre-purification waste and some shorter chain fatty acids.Already Table 1 is showing that based on the source (either tallow or palm oil)a wide variation of chain length of the fatty acid is available which makes the preparation of an LCA for zinc stearate very difficult.Nevertheless,the author will try to create for an LCA for Zinc stearate based on:
-Zinc oxide which is assumed to be the standard raw material
-One based tallow (assumed molecular weight 274 g/mol)
-Another one based on palm oil assumed molecular weight 269 g/mol
-Based on the reaction in Eq.1 which is assumed to be the easiest,cheapest and probably the most used for the production of zinc stearate in PVC industry.
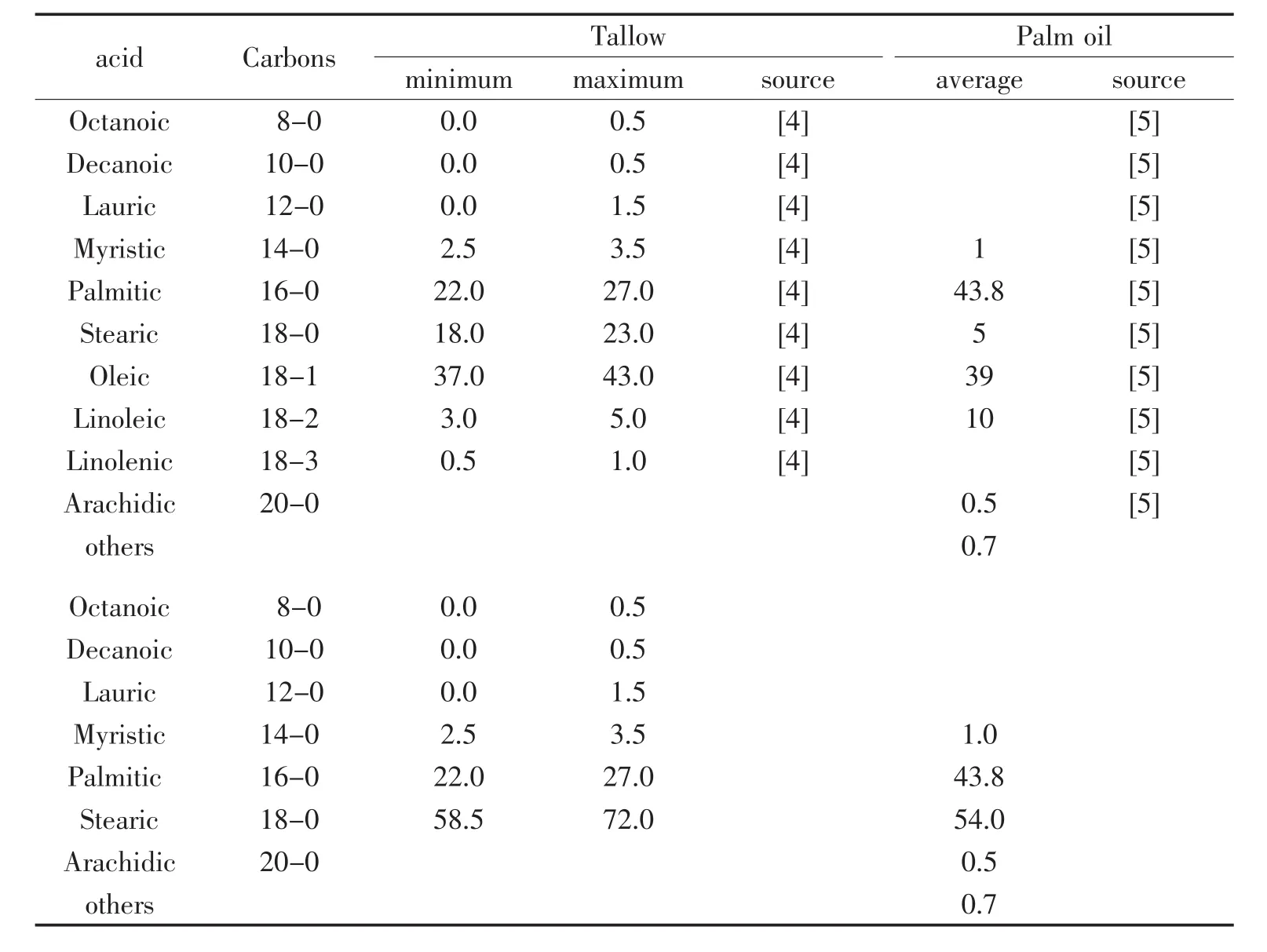
Tab.1 Composition of the fatty acids in tallow and palm oil and the resulting saturated fatty acids after hydrogenation in percent
3.Input and output flows for 1000 kg(1 mt)of zinc stearate based on tallowInput flows
According to Eq.1
-133.13 kg zinc oxide and
-896.31 kg tallow based stearic acid are necessary.29.44 kg water are formed as a by-product.However,in some cases are “catalysts” are used like <1%of formic acid,acetic acid,hydrochloric acid or water.This might be neglected in this consideration.
We will assume the same average transportation is 500 km maximum by heavy trucks probably 40 mt per shipment[6].
Regarding tallow-based stearic acid the transportation is more difficult to judge.Some zinc stearate producers are also producing stearic acid like e.g.Peter Greven[7].In such cases the transportation is<1 km.Other producers of zinc stearate are purchasing stearic acid from different source.In these cases we can assume a maximum seaway distance of 15000 km[8]plus 1000 km by heavy trucks.The second category of producers is the minority.We will as-sume that only 10% (or even less)of all Zinc stearate which is produced in Europe is produced by manufacturers who are not producing stearic acid themselve.
The final Zinc stearate is either packed in 40 paper bags(25 kg each)or in 2 bigbags(PP;500 kg each).For the paper bags is one wooden pallet per mt necessary.In the case of the bigbags are 2 wooden pallets are used per mt.We can assume that the ratio is 50%25 kg paper bags and 50%bigbags.Regarding the wooden pallets we can assume that the pallets of Zinc oxide and of stearic acid are re-used for this purpose and only 0.5 wooden pallet
Even if we focus in this paper on the reaction in Eq.1 it will require some compromises regarding energy input.The 1st obstacle is that zinc stearate can be slightly acidic or neutral or slightly overbasic.The reaction time will be increased in this order and according to the reaction time the consumed electrical energy will be increased.Furthermore,the reaction time is also depending on the quality and reactivity of the zinc oxide and the fatty acid (like particle size,degree of carbonatisation of Zinc oxide),presence or absence of catalyst,on reaction temperature,mixer speed,mixer volume,filling grade of mixer,mixer tools,insulation of reactor etc.;see e.g.According to Eq.1 about 3%water is formed during the reaction.This requires a drying step for the reaction product before packaging.The drying process can be done in many different ways with different sources of energy.Last but not least a milling step depending on the particle size of the raw product and the final product might be necessary…However,there are many small things which will influence the energy input.There is no real and practical chance to elicit these data from single producers…Due to this misery the authors will create (Tab.2)and calculate 2 different scenarios regarding the energy input and its final influence on the LCA of zinc stearate.We assume in scenario:
-1 a comparably short reaction time and no addition of water or any catalyst which might represent the minimum energy input during production of zinc stearate
-2 a similar process without the addition of any catalyst or water but with longer reaction time compared to scenario 1.
In these scenarios we assume that the reaction is finished at 100℃.So we have to heat up the reaction mixture from 20℃ to 100℃ which is the boiling temperature of water added as a catalyst resp.formed during the reaction.The specific heat capacity of zinc stearate was not available.So,we used that of the raw materials,both stearic acid and zinc oxide.We also neglected the exothermic energy of the reaction which would reduce the total energy on one hand.On the other hand we also nearly neglected the energy loss of the reactor to environment which would increase the energy balance.
We can conclude while comparing scenario 1 and 2 that the reaction time has an significant influence on total energy input

Tab.2a Used energy values taken from literature Value Unit Value Unit Source
Output flows
According to Eq.1 29.44 kg water are formed as a by-product while producing 1 mt zinc stearate.This might be either condensed and going the waste water system or directly to air.During the reaction of stearic acid and zinc oxide no direct emissions of SOx and NOx are occurring in theory.(Indirect emissions of power input are possible and probable.)No emissions of Carbon dioxide are expected.

Tab.2b Different theoretical scenarios of energy input for the production of 1 mt tallow-based Zinc stearate based of the data in Tab.2a
Other emissions to air might be a very small amount of the raw materials and the final zinc stearate.These emissions can be extremely low and next to nothing when state-of-art filter technology is installed.We can assume that it is maximum 96 g zinc stearate per mt product①We assume a plant with a production of 10000 mt/year.Furthermore,we assume that the emissions are at the top limit of“TA Luft”Chapter 5.2.1[17]with a mass flow of 0.2 kg/hr.(Chapter 5.2.5 “Organic substances in dust form” says:To treat as total dust.The dust containing in the exhaust emissions in exhaust shall not exceed the following values:Mass flow rate: 0.20 kg/h or Mass concentration: 20 mg/m3 Even if they meet or falls below a mass flow rate of 0.20 kg/h in the exhaust gas must not exceed the mass concentration of 0.15 g/m3.Assuming a maximum mass flow of 0.2 kg/h,200 days production per year and 24 hrs per day it will result in 960 kg emission per 10000 mt product resp.96 g per 1 mt product..In reality it might be factor 10 or even 100 lower.There is in theory no direct emission to soil because it is produced in nearly dust free closed systems.The flooring is covered by concrete.Any possible dust will be collected by either brushing or wetting with water and following brushing or by cleaning with water which is going to the waste water system.So,the wheels for forklift trucks and the shoes of workers can transfer are an extremely small amount of raw materials However,there is a theoretical possibility that zinc stearate is going to a waste water system.The solubility of zinc stearate is very limited (0.9 mg/L resp.0.9 g/m3at 20°C[15]).Even if we assume that our theoretical 10000 mt plant was treating 100 m3waste water per with 200 days operation per year the emission of Zinc stearate to water would be 18 kg per year in total or 1.8 g per mt product in maximum.However,“Stearic and palmitic acid as such are readily biodegradable,although the degradability can be inhibited by the formation of insoluble salts (e.g.calcium,magnesium and zinc distearates),that are not readily biodegradable.This is confirmed in a OECD 301D study”[16].
Regarding packaging materials this means of zinc oxide:
-0.133 wooden pallets which can be re-used
-0.133 bigbag (PP)or 5.33 paper bags maybe with PP inside(25 kg)②We can assume 50%bigbags and 50%25 kg bags which are going to waste..
Regarding packaging materials this means for Stearic acid based on tallow:
-0.896 wooden pallets which can be re-used
-0.896 bigbag(PP)or 35.85 woven PP bags(25 kg)③We can assume 50%bigbags and 50%25 kg bags which are going to waste..
Small amounts of the raw material will remain in the packaging.We can assume that this amount is 0.1%④This is the maximum value which is allowed according to law[18]or even lower[18].This results:
-133 g zinc oxide and
-896 g tallow based stearic acid will maximally remain in the packaging.
Depending on the country these PP bigbags and the paper bags will be incinerated and end in a landfill.The stearic acid will be converted to carbon dioxide and water during combustion and also during biodegradation in a landfill.Zinc oxide will not be calcinated.It might react with other inorganics from other waste If the waste incinerator is either producing steam for heating or electricity the recovered energy of the packaging material and the stearic acid might improve the Carbon dioxide food print slightly.However,neither the emission to soil,water or are result in a real environmental problem because:
-stearate acid was found to be readily biodegradable[16].
-Zinc stearate finally degrades zinc carbonate,carbon dioxide and water according to Eq.4.
-The carbon dioxide formed during degradation does not increase the carbon food print because the stearate source is bio-based and renewable.
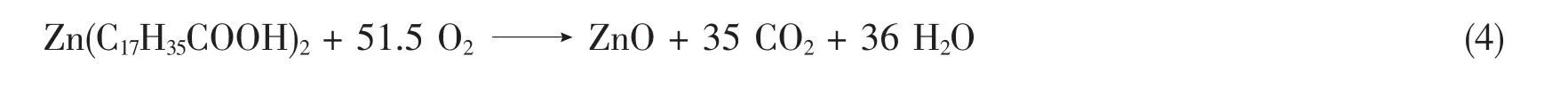
4.Input and output flows for 1 mt of Zinc stearate based on palm oil
Input flows
According to Eq.1
-135.35 kg Zinc oxide and
-894.58 kg palm oil based stearic acid are necessary.
These figures are very close to that of tallow based Zinc stearate.The input regarding packagings and transportation of the raw material are assumed to be the same as in paragraph 3.1.In Tab.3 we calculated the energy input under as the same conditions as in Tab.2b but only for scenario 1 to get a information about the minimum energy input.The energy input of a palm oil based Zinc stearate in scenario 1 is less than 0.6%higher compared to a tallow based Zinc stearate produced under the same conditions.

Tab.3 Theoretical scenario of energy input for the production of 1 mt palm oil based Zinc stearate based of the data in Tab.2a
5.Consideration of the kinetics of the reactions
This paragraph focuses on the kinetics of the reactions will synthesis or production.That’s a little bit complexes because several reactions will occur at the same time.This is independent on the type of production.In this article we’ll focus on the direct synthesis based on stearic acid and zinc oxide(ZnO).The entire time of reaction also depends on the temperature.Independently on the temperature we assume that following reactions occur:
In a 1st step ZnO will react with Stearic acid(HSt)and form a monobasic zinc stearate(ZnSt(OH));Eq.4.
When we plot the conversion of ZnO,HSt and ZnSt(OH)we will see the the percentage of ZnO and HSt will decrease while the percentage of ZnSt(OH)will start to increase at the beginning of the synthesis.
The monobasic zinc stearate ZnSt(OH)will react with another HSt and form zinc stearate(ZnSt2).Water is formed as a byproduct;Eq.5.

The consequence in the above mentioned plot of conversion vs time is
-an increase of the percentage of zinc stearate and-the formation of a maximum and a later decrease in the percentage of ZnSt(OH);Fig.1.What we can see in Fig.1 too is that:
-a small amount of free fatty acid will stay over time.It’s roughly 2%in Fig.1.

Fig.1 Simplified plot of the conversion of ZnO,HSt,ZnSt(OH)and zinc stearate
-there will be a very small amount of ZnO and ZnSt(OH)and
-the content of Zinc stearate will be~97%when the reaction is stopped at this time.
However,we assume that there is minimum another reaction is occurring.Two molecules of ZnSt(OH)might react together and form ZnO plus water and Zinc stearate;Eq.6.
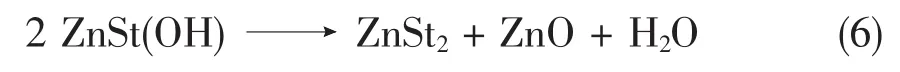
In theory this should be an equilibrium but at elevated temperaturesthewaterwillleavethe reactor/reaction system and this willpush the equilibrium to the right side.The plot of the conversion vs.time is shown in Fig.2.The free fatty acid(FFA)is lower than in Fig.1 and the content of zinc stearate is~98%.The rest is ZnO and ZnSt(OH).We are not sure about other competing side reactions in this process which we didn’t consider yet.

Fig.2 Plot of the conversion of ZnO,HSt,ZnSt(OH)and zinc stearate
In the following partwe’llconsiderthe melt process.The melting point of zinc stearate is about 120℃ .So,we’llhave to process athigher temperatures,e.g.at 130℃ .That’s a kind of optimum.A higher temperature will result in shorter reaction time butthe productmightstartto discolour.A 2nd problem is the formation of water due to Eq.5 and Eq.6.This will cause a foaming of the reaction mixture.If the reactor was filled too much an overflow of the mixture would be the result.
In order to avoid this the ZnO might be give in e.g.5 portions each 10 minutes;Fig.3.The content of zinc stearate will be slightly lower,the FFA,the content of ZnSt(OH)and ZnO will be slightly higher compared to Fig.2
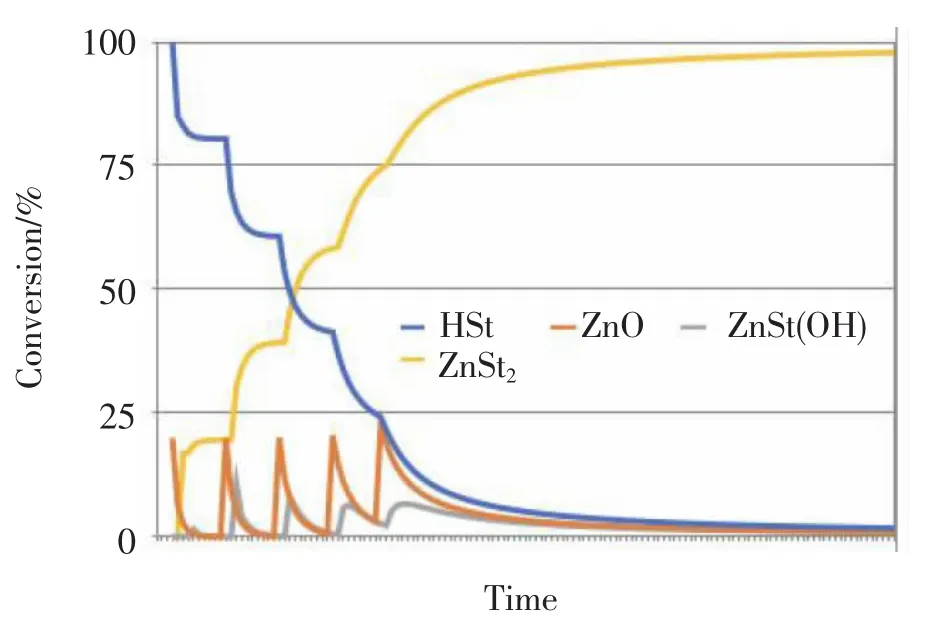
Fig.3 Plot of the conversion of ZnO,HSt,ZnSt(OH)and zinc stearate when the ZnO is added in 5 portions after 10 minutes each
If the target is to have a low FFA value excessive amounts of ZnO has to be added.
6.Other parameters
Trace elements,especially of heavy metals,might be interesting for the evaluation ofenvironmental impact.These are summarized in Tab.4.These trace metal can be emitted to environment in extremely low amounts due to the residues in packaging and emissions to soil,air and water.
7.The importance of free fatty acid
The content of FFA in zinc stearate is a very important parameter in the specification.It shall be<0.5%in the most cases.Otherwise the free fatty acid can cause problems like:
gasing when it react with basic substances like metal oxides and metal hydroxides in the formulation This might result in failures in electrical performance in cable insulation or in a reduced corner weld strength in window frame applications.Plate out in the die and in the calibration might be another unwished side effect.

Tab.4 Examples of analytical results of ICP analysis of several trace metals in stearic acid(palm oil based)and zinc stearate based on palm oil(BDL:Below detection limit)
8.Sustainability
Schiller and Everard[3]considered several metals soaps which were and are still used in PVC stabilization,assessing them using tools from the TNS Framework for sustainability;Tab.5.Beside cadmium and lead all other metals including sodium,magnesium,aluminum,calcium,lanthanum,barium and zinc have a high potential to become fully sustainable if the PVC product is recycled in a closed loop.
Tab.5 Overview about which metal soap has the best potential to become fully sustainable in 2011:breaching the SC,:can breach the SC,:will not breach the SC;left:metal,right:acid)

Tab.5 Overview about which metal soap has the best potential to become fully sustainable in 2011:breaching the SC,:can breach the SC,:will not breach the SC;left:metal,right:acid)
metal acid SC 1 SC 2 SC 3 SC 4 ∑Zn Stearic and oleic acid ....(..(.. .. ..( ..(..( ..(
9.Summary
It seems that it is very easy to produce zinc stearate but we worked out that it is more tricky to produce zinc stearate in the required quality and in an economic way.We have shown that zinc stearate can be produced based on different sources of stearic acid and which influence the raw material source and the way of production have on the energy consumption.We considered emissions,packaging,transportation…and we are absolutely sure that the generated data are a serious base to create a LCA of zinc stearate what is overwhelming the skills of the authors.However,we can summarise that zinc stearate is an important chemical and additive in plastic industry with a high potential of sustainability.

