Effect of Chain Length on the Dipolar Interaction of Morpholine with Primary Alkanols
PRIYA B PALANIAPPAN L(Department of Physics,Manonmaniyam Sundaranar University,Tirunelveli,TN 670,India;Physics Wing,D.D.E,Annamalai University,TN 60800,India)
Effect of Chain Length on the Dipolar Interaction of Morpholine with Primary Alkanols
PRIYA B1PALANIAPPAN L2,*
(1Department of Physics,Manonmaniyam Sundaranar University,Tirunelveli,TN 627012,India;2Physics Wing,D.D.E,Annamalai University,TN 608002,India)
Sound velocity,density,and viscosity values have been measured at T=303 K for four binary systems of morpholine+methanol,ethanol,1-propanol,and 1-butanol.From these data,acoustical parameters, such as adiabatic compressibility,free length,free volume,and internal pressure,have been estimated using the standard relations.The results are interpreted in terms of the molecular interaction between the components of the mixtures.The observed excess values in all the mixtures indicate that the molecular symmetry existing in the system is highly disturbed by the addition of morpholine molecules.The interaction energy terms of the statistical mixing are also verified for these binary systems,and the dipole-dipole interactions are found to be predominant and are greatly affected by the chain length of the primary alkanols.
Ultrasonic velocity;Acoustic parameter;Dipolar interaction;Interaction
[Article]
www.whxb.pku.edu.cn
1 Introduction
Molecular interactions between any two components have been the subject of numerous studies in the solution chemistry.An interesting aspect of the binary systems is the possibility of the enrichment of one interaction in the influencing shell of the other leading to the characteristic deviation of the solution properties from a linear dependence upon the mixture composition.To study the possible interactions between the components,various technique such as dielectric,ultrasonic etc.are widely used.These techniques are fundamentally related to the binding forces between the atoms or the molecules.
Exhaustive numbers of liquid mixture systems have been carried out in the field of ultrasonic characterization to ascertain their molecular interactions1.The variations observed and the related parameters throw much light upon the structural changes associated with the liquid mixtures having weakly interacting components as well as strongly interacting components.Of the various strongly interacting types,polar-polar components of different orientations are of particular interest2.In this category, one straight chain component(alcohol)and a ring structure component(morpholine)is expected to exhibit peculiar trends.
Exhaustive background about primary alcohols is largely available and their uses in various fields are very evident and need not be emphasized.In particular,for the industrial rectification column,alcohols are frequently used as entrainer/solvent to avoid the formation of azeotropes3.
Morpholine is a heterocyclic organic chemical compound belongs to a lower aliphatic secondary amine.It possess both amine and ether functional groups.The presence of amine makes morpholine to behave as a base4.It posses conformation and 98%of its puckering can be explained with an ideal cyclohexane chair form5.Gontrani et al.6have studied the conformation behavior of morpholine using X-ray diffraction studies.Jones et al.7have used morpholine for structure determination of selected macro molecules.
Morpholine finds wider application in diverse fields.The use of morpholine in pharmaceutical industry was studied extensively by different authors8,9using mass spectrometry and capillary electrophoresis.The ability of morpholine to improve the enzymatic hydrolysis of wheat straw was attempted by Li10and Zhao11et al.Morpholine is an extremely versatile chemical with many important applications such as an intermediate in the manufacture of rubber chemicals and optical brighteners,as a building block in anticancer drug linezolid,as chemical emulsifier in waxing of fruits,as a corrosion inhibitor in steam boiler systems,an additive for pH adjustment,as a solvent in many chemical reactions12,13,etc.
Enormous number of investigations was carried out by many researchers using ultrasonic techniques14-16in liquid mixtures having alcohol as one of the component.But still the survey of literature clearly shows that a systematic study of the morpholine with primary alcohols is not at all attempted.The present work thus deals with the measurement of ultrasonic velocity andcomputation of related parameters in the four binary systems,viz., morpholine in first four primary alcohols viz.,methanol,ethanol, 1-propanol,and 1-butanol at T=303 K and the analysis of interactions between the components thereby determining the influence of chain length and hydroxyl/amine group on interaction phenomenon.
2 Experimental
The mixtures of various concentrations in mole fraction were prepared by taking purified AR grade samples at T=303 K.The purity of liquids were checked by measuring the density values and are found to match well with the literature17-19values quoted for the same temperature.Fixed volumes of components were taken,mixed and the respective mole fractions were calculated. The density and viscosity were measured using a pycknometer and an Ostwald′s viscometer respectively with an accuracy of 3×10-5for density and 0.001 N?s?m-2for viscosity.The ultrasonic velocities in liquid mixtures were measured using an ultrasonic interferometer(Mittal Instruments,Chennai,India)working at 2 MHz frequency with an accuracy of±0.1 ms-1.
The calibration of the pycknometer was carried out with deionized,doubly distilled water.After standardization,the density of water at 303 K was observed to be 995.62 kg?m-3that closely match with the literature value(995.6473 kg?m-3)17,20.
In all the property measurements,the temperature was controlled within±0.01 K,using a constant temperature bath(INSERF Model IRI-016C,Chennai,India)and the temperature was monitored with a platinum resistance thermometer with an accuracy of±0.001 K and an uncertainty of±0.004 K.
Each observation was repeated for at least seven times and the average of the concurrent values was found and presented here. The maximum standard deviation for density data was found to be 0.682 and the respective standard error was 0.257.The maximum standard error for the reported viscosity data was 0.302 and for velocity data was 0.194.
The interferometer is a fixed frequency variable path type. Ultrasound of constant frequency is generated at the bottom of the cylindrical sample cell using quartz crystal and is sent into the medium of interest taken.The propagated waves after getting reflected at the reflector surface held at the top of the cell,again travels back through the same medium.These two waves forms stationary wave pattern and hence nodes and antinodes are formed in the medium.Afine micrometer screw is provided in the set up that can allow the finer movements of the reflector plate in the medium.Thus by moving the reflector plate for a fixed number of antinode(or node)positions,the distance moved for a known number of waves can be known.Use of frequency with this data will yield the sound velocity in the medium.
3 Theoretical aspects
Using the measured data of sound velocity,density,and viscosity,few acoustical parameters such as adiabatic compressibility (β),free length(Lf),free volume(Vf),and internal pressure(πi)and their excess parameters were calculated using the following standard expressions21:

where,KTis the temperature dependent constant having a value 199.53×10-8in M.K.S.system,k is a constant,equal to 4.28×109in M.K.S.system,independent of temperature for all liquids,Meff= Σximi,where,xiis the molar fraction and miis the molecular weight of ith component,AEstands for excess property of any given parameter,Aexpis the experimental value,andAidis the ideal value.
It is to be remembered that the adiabatic compressibility is the ease with which a system can be compressed.Among these various relations,Eq.(1)is simply the Newton-Laplace equation22that is applicable for any longitudinal wave motion in fluids.A larger value of adiabatic compressibility indicates the availability of more free space between the components.Lf,the free length is again a measure of the distance of separation of any two components.These two parameters basically depend on the interaction phenomenon and they picture the strength of the interaction23,24. However,the compactness be best revealed by free volume and internal pressure.
Free volume being a three dimensional entity represents the available volume in which the components can freely move.Again depends on the tendency of the medium whether it offers adhesive or cohesive forces on its components.This is revealed by the trend of the internal pressure25,26.
All these measured and the calculated parameters are based on the fact that there will be non-linear variations that is caused by the non-ideality of the systems.To establish this non-ideality,the ideal values have been calculated and their deviations from the experimental values,called as the excess values,are found.If these deviations tend to zero,then the system approaches the ideality and has negligible interactions,else the existence of appreciable interactions is confirmed.Further the energy due to various interactions existing in the system was attempted using statistical mechanics relations and is compared with the net observed interaction energy,so as to confirmthe conclusions arrived.
4 Results and discussion
Morpholine and primary alcohols were used in this work.Their structural forms are depicted in Scheme 1 and Scheme 2,respectively.The prepared mixtures of increasing mole fractions of morpholine are numbered serially,so as to present all the binaries in the same table that will offer a nice comparison of effect of chain length.The actual mole fraction value of morpholine in these serially numbered mixtures for all the four binaries taken up in the present study are listed in Table 1.In all the other tables and in the graphs,the mixtures are given in the corresponding serial numbers.

Scheme 1 Structural forms of morpholine

Scheme 2 Structural forms of primary alcohols

Table 1 Actual mole fraction values corresponding to the serial numbers
The measured values of density,viscosity and ultrasonic velocity for the morpholine-alcohol mixtures were given in Table 2. Calculated values of adiabatic compressibility,free length,free volume,and internal pressure for the chosen systems were given in Table 3.The respective excess values were depicted in Figs.1 to 4.The standard values required for the calculation of interaction energy terms were given in Table 4.Taking 1-propanol system as an illustration,the trend of major types of interactions along with the observed interaction energy in the experimental mixtures was depicted in Fig.5.Acomparison of chain length was attempted in the considered systems in Fig.6 by referring the apparent interaction energy trends.
The measured parameters viz.,density and sound velocity are observed to be of increasing nature with increasing mole fraction of morpholine in all the mixtures but non-linearly.Density(and also velocity)seems to increase with increase in chain length which is due to the existence of number of hydrocarbons.Increase in density with increasing mole fraction of morpholine may be attributed to the polar nature of both of the components involved.
As commented by Peters27,the higher density of a substance is a clear indication of its extent of intra molecular interactions; morpholine,being polar and more denser,has maximum intramolecular interaction than the other components.The existence of such high intra interactions of morpholine and the associative nature of alkanol are reflected in the observed increasing nature of sound velocity.Thus the addition of morpholine to alcohol is not affecting appreciably the existing high degree of intermolecular interactions.

Table 2 Values of density(ρ),viscosity(η),and ultrasonic velocity(U)at 303 K
As the alkanol molecules are chain structured rather than the ring structure,their effective molecular area will be larger and hence 1-butanol record high coefficient of viscosity values.The increase in the coefficient of viscosity with the order of alcohol indicates that higher members are less flexible for motion.Of the four primary alcohols considered here,1-butanol seems to be distinctly different from other three members of their family as it shows a reverse trend to that of other three members in viscosity variations.The hydroxyl group that offers polarity is common to all alcohol members in the present study.However the number of methyl groups which is hydrophobic in nature plays the significant role that it favourably interacts with non-polar molecules.Such groups are more in butanol and as both of the components involved are polar,the interactions are least pronounced in butanolsystem.This is reflected in the observed viscosity variations. Further,the addition of morpholine reduces the component area in 1-butanol and hence a continuous decrease in the coefficient of viscosity is observed.

Table 3 Values of adiabatic compressibility(β),free length(Lf),free volume(Vf),and internal pressure(πi)at 303 K
Apart from the frictional forces existing in the mixture,the coefficient of viscosity is influenced by many other factors.In addition to temperature and concentration,the closeness of components,their area of contact,the relative movement between the components are also important to consider for the observed variations.As the closeness and the relative motion are determined by the parameters like dipole moment,dielectric constant,electron affinity etc.,the area in contact is due to the size of the chain.As 1-butanol is having lengthiest chain among the considered molecules,it has larger contact area and hence its viscosity is higher than all other mixtures.Any further addition of morpholine,depletes 1-butanol molecule,and hence lessen the contact area and hence viscosity reduces with increase in mole fraction of morpholine in 1-butanol system.
However,for a given mole fraction of morpholine,it can easily be predicted that the resonating electrons of morpholine freely interact with the methyl or hydroxyl group of alkanol28,because the increase in chain length favours the increase in viscosity.On increasing the mole fraction of morpholine,the system is having more and more ring-structured molecules with side chains and depleted of linear molecules that leads to increase the existing particle-particle friction and thus viscosity shows continuously increasing trend but slowly and appreciably in first three members of alcohols.Such variations are not much pronounced in the 1-butanol system.All these trends clearly indicate the presence of intra and inter molecular interactions29,30.
The perusal of calculated parameters of adiabatic compressibility,intermolecular free length,free volume,and internal pressure for the present binary systems in Table 3 reveals that β and Lfbehave similar in nature whereas,Vfand πishow almost opposite trend to each other for methanol and butanol systems.
As morpholine molecules are basic in nature due to the presence of π electrons31,32,there are some possibilities of interactions between the alcohol and morpholine.Such interactions bring the components close to each other and hence β and Lfis of decreasing magnitude.
The extent of free volume offers the knowledge of type of interaction.In the present case,for methanol,a dip exists in Vf(or peak for πi)trend.As cited already,morpholine is better structured than alcohol and hence on increasing the mole fraction of morpholine,compactness increases in the system.In all other systems, especially at higher mole fractions,Vfshows a decreasing trend whereas πishows an increasing trend.However these two parameters are found to be of decreasing magnitude with chain length for any given mole fraction of morpholine.
As regards the strength of interaction,as revealed by β and Lf, and as regards the compactness(Vfor πi),the increase in chain length seems to offer a positive contribution to both.Morpholine being aromatic has electron delocalisation effects and it is always associated with temporary dipoles that lead to its resonating nature.Such delocalised electrons and the methyl or hydroxyl group of alkanol can mutually form induced dipoles.The interactions between the methyl group of alcohol which are hydrophobic and the resonating electrons of morpholine leads to dipole-dipole or induced dipole-dipole type.But the dipolar interactions between the alcoholic group which are hydrophilic and the resonating electrons of morpholine manifests as dispersive interactions that supports the existence of more space between the components. This is always attributed with a reduction of internal pressure as is noticed.
It is to be noted that the β and Lfshow similar variations,i.e., a decreasing trend with increase in mole fraction of morpholine as well as with increase in chain length of alcohol.As these parameters are basically analyzing the interaction mechanism and not the structural compactness,they are not expected to offer more information pertaining to the influence of chain length.
It is to be noted that the trend of parameters,in particular,the Vfand πi,with respect to morpholine mole fraction is similar especially for higher alkanols.But specific variations can be noticed as regards the chain length,i.e.,in a given range of mole fraction, the observed trend for one form is not the same as that for the other form.
To be clearer,least value of free volume is observed at 0.3 mole fraction in(S.No.6)methanol system and the same for 0.1 mole fraction in(S.No.2)ethanol whereas the pure component value is the least for other two alcohols.This suggests that free volume shifts towards lower mole fraction with increasing chain length. Further,the internal pressure shows a continuously decreasing trend especially for higher alcohols.Thus the observed variations in the calculated parameters with respect to the chain length suggest that the chain length has a significant role in improving the compactness.This suggestion reflects whether the chain is short or long(Scheme 2).Moreover,for a given mole fraction of morpholine,internal pressure shows a regular decrease with increase in chain length.Thus the availability of more methyl groups undergoes a disruptive interaction with the amino group of morpholine.This trend suggests that the dipolar interactions of lower alcohols are better structure makers than the higher alcohols.
The respective excess parameters always give excellent confirmations for the predictions offered by the study and suggestions33,34.In the present study,S.Nos.1&11 of mixtures indicates pure component and hence their excess value is zero.The presented excess parameters in Figs.1&2 for these binary systems reveal that βEand LfEexhibit almost similar variations for all four alcohol systems,i.e.,regular deviations are obtained for increase in chain length.However the magnitudes of these two excess parameters are negative which indicates the existence of strong interaction between the components.This is acceptable as both of the components involved are polar.
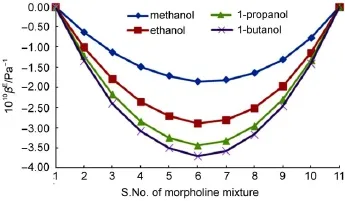
Fig.1 Variation of excess adiabatic compressibility at 303 K

Fig.2 Variation of excess intermolecular free length at 303 K
However,the estimated excess values in the present systems in a particular mole fraction indicate that the presence of extremum is an indication of the maximum interaction.At all other mole fractions,interaction is not so much pronounced.This suggests that the methyl or hydroxyl group of alcohol is fully saturated by the excessive morpholine molecules and so,the chances of inducement of dipoles are remote in this system35.As the experimental free length is lower than that expected,components are held more close,which is possible only by the existing strong type interactions in these alcohol systems.It is to be further noted that the interactions are in increasing order as the chain length increases from methanol to butanol.
The trends shown by VfEand πiEand in the mixtures(Figs.3& 4)are interesting that they present a different view.For all the mixtures considered,VfEis fully negative and reflect to the specific variation of chain length.The unanimous dip that occurs around S.No.6 of mixture in all the four systems clearly shows that the existing interactions are strong and are dipole-dipole type36.The chances for induced dipole-dipole is also there but their contribution is very little comparing to dipole-dipole type.This is in line with the observed positive trend in πiEvariations in methanol and butanol system.However the shift in variations to the negative magnitude for other two alcohols clearly conveys that other interactions like dispersive are dominating in the systems considered.As already cited the dispersive interactions are due to the hydrophobic nature of methyl group,which is more in ethanol and propanol than in methanol.It is still more in butanol,but enormous dispersions reappear as induced dipole or charge transfer type and hence they offer positive contributions and hence inbutanol,πiEis in positive trend.

Fig.3 Variation of excess free volume at 303 K
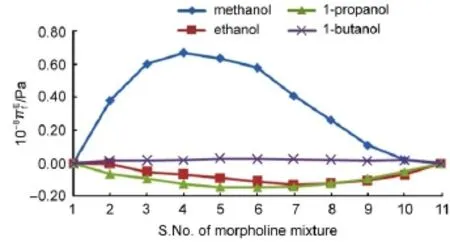
Fig.4 Variation of excess internal pressure at 303 K
For methanol and butanol systems,VfEis with negative magnitude but πiEis with positive magnitude.Also their trends behave exactly opposite to each other,i.e.,VfEfirst decreases and then increases,but πiEfirst increases and then decreases.Further at any given mole fraction of morpholine,the magnitude(of both VfEand πiE)is always higher for methanol than butanol.Such behaviour clearly reflects the dominating nature of hydroxyl group than that of alkyl group.It is to be remembered that the solubility of butanol in any polar solvent is less than that of other first three alcohols37which is due to the fact that the interaction of hydroxyl group is almost fully balanced by the interactions of alkyl groups.As the interactions due to hydroxyl group and alkyl group are of opposite in nature and hydroxyl group shows extraordinary predominance, methanol system has large interactions.This leads to very high internal pressure in methanol system as observed here.Further methanol is a weak acid and morpholine is a weak base4and thus the available free volume in methanol system is not much deviating from its ideal value as in Fig.3.
Another possible explanation can also be given for the observed trend of excess internal pressure.Internal pressure is a cohesive force,which is the result of attractive and repulsive forces between the molecules.In all the systems considered here,the hydro carbon chain length is the only varying parameter.This chain is perfectly linear in methanol and not so in other alcohols.The chain is very small in methanol and lengthier in butanol.It is well known that the hydrocarbon chain which is hydrophobic in nature can attract non-polar while the hydrophilic hydroxyl group attracts the polar part.As regards morpholine,the central ring which is remote access is nonpolar part&possesses conformation5,6and the side chains are polar and can be easily accessed by components.This reassures the possibility of very high interactions between the methanol and morpholine components that leads to very high πiE. In other alcohols the hydroxyl group interactions are counter balanced by the twist in the chain as well as by the presence of more alkyl groups and hence πiEare not much pronounced.
Further an overview of interaction energy of statistical mechanics,applied to these mixtures can provide a quantitative confirmation to the conclusion arrived.Evidences can be found in the literature38,39for the use of such equations to calculate and to interpret the deviations in the excess values.Israelachvili38has suggested the energy equations for various interactions existing in liquid mixtures.Based on these equations,Ababneh39made some assumptions to use these equations to the interaction studies and adopting these features for the present binary systems,the dipole-dipole interaction energy(Ed-d),the induced dipole-dipole interaction energy(Ei-d),and the dispersion interaction energy (Edisp)are found to be proportionate to 2φ1φ2μ12μ22,φ1φ2[μ12α2+ μ22α1],and φ1φ2α1α2[I1I2/(I1+I2)],respectively.Here φ1,φ2are the volume fractions;μ1,μ2are the dipole moments;α1,α2are the polarizabilities;and I1,I2are the ionisation constants of the respective components.The pure components values as found in literature are listed in Table 4.

Table 4 Values of dipole moment(μ),polarizability(α),and ionisation constant(I)of the experimental liquids at 303 K7-10,13
Being a very good polar molecule and having infinite solubility especially in alcohol systems,morpholine can frequently form temporary dipoles due to its resonance vibrations.Thus,few of the expected interactions include dipole-dipole,induced dipoledipole,dispersive type etc.Based on the criticism of Israelachvili38, the interaction energy for the interactions such as dipole-dipole, induced dipole-dipole and the dispersion has been calculated. Energy being the product of pressure and volume,the interaction energy represents the excess energy and hence the product of excess pressure and excess volume is a measure of the net observed interaction energy of the system.
As stated in the introduction section,in addition to the effect of chain length,the hydroxyl-amine group interaction is also to be attempted here.To unravel this intermolecular interaction between morpholine-alcohol,interaction energy terms have been calculated.For the study of hydroxyl-amine interactions,both components should be actively involved in the interaction mechanism. In methanol system,hydroxyl group predominate the alkyl group and this manifests as large intra-molecular interaction and less intermolecular.In butanol system,as stated already,the hydroxyl is almost totally balanced by alkyl and the component seems to exhibit very less inter molecular interactions.Thus either ethanol system or 1-propanol system is expected to offer pronounced intermolecular interactions.

Fig.5 Trend of various interaction energy terms in 1-propanol system at 303 K
However calculations were carried out for all the four systems and they are depicted in Figs.5&6.Fig.5 shows the various energy terms in a single system(1-propanol)whereas Fig.6 shows the sum total of various energies in different systems.As the calculations of various energy terms follow the same common trend with variations in magnitude in all the four systems,forsimplicity,only one system may be enough to present.As stated above,methanol and butanol systems are less suitable for intermolecular interactions,among the remaining two systems the bigger molecule 1-propanol is shown in Fig.5.

Fig.6 Trend of apparent interaction energy at 303 K
Fig.5 depicts these interaction energy terms for the illustrative system of morpholine+1-propanol.It is to be noted that the experimental and the dipole-dipole energy terms are in comparable magnitude whereas the induced dipole-dipole is in the order of 10-29kcal?mol-1(1 kcal?mol-1=4.187 kJ?mol-1)and the dispersion is of 10-18kcal?mol-1.In the present systems,the contributions due to the dispersion interactions may also be taken as appreciable,but that of induced dipole-dipole are extremely weak.This is in line with the observations of previous workers38,39. As the apparent interaction energy is a sum total of various interactions existing in the systems,it is found to be slightly of lesser magnitude than the predicted interaction energy terms.
Apparent interaction energy curves for all the systems are shown in Fig.6.It is unanimously found that the net interaction energy is positive for ethanol and 1-propanol systems whereas it is negative for other two systems.Further,the perusal of Fig.5 indicates that the major contribution to the interaction is by dipoledipole type.Fig.6 indicates that methanol system behaves like that of 1-butanol but the hydrophobic interactions are least in it.This again confirms the stronger interactions of the 1-butanol molecules.Considering the cases of ethanol and 1-propanol,variations are more haphazard for ethanol and the values are more positive, which indicates that the attachment of less number of methyl group has less dispersive interactions.
It is to be remembered that the actual interaction energy magnitude deviates much from the estimated magnitudes for all the systems.This may be attributed to the assumptions and approximations made in arriving the estimated values.However,it is highly clear that the dipolar interactions are the predominantly existing type and the chain length is found to have a sharp influence in the interaction phenomenon and compactness from methanol to butanol.
5 Conclusions
The present study clearly confirms the existence of specific interaction of strong magnitude in all the systems taken up here. The predominance of hydroxyl group over alkyl group in alcohols is evident and it largely affects the existing intermolecular interactions.Of the various interactions in the systems,dipoledipole type is predominantly noticed in the systems and ethanol system is having maximum degree of dipole-dipole interactions. Further,the chain length of alcohols and the conformation of ring in morpholine are found to have sharp influence in the interaction mechanism and compactness.
References
(1)Nithiyanantham,S.;Palaniappan,L.;Lenin,M.Journal of Bionanosciences 2015,9,4.doi:10.1166/jbns.2015.1312
(2)Palaniappan,L.Physica B 2008,403,3887.doi:10.1016/j. physb.2008.07.022
(3)Palaniappan,L.;Jaafar,M.S.Main Group Chemistry 2009,8, 89.doi:10.1080/10241220902977612
(4)https://en.wikipedia.org/wiki/morpholine(accessed Nov 20, 2015)
(5)Parkin,A.;Oswald,I.;Parsons,S.Acta Cryst.2004,B60,219.
(6)Gontrani,L.;Ramondo,F.;Caracciolo,G.;Caminiti,R. Journal of Molecular Liquids 2008,139,23.doi:10.1016/j. molliq.2007.10.006
(7)Jones,P.G.;Taouss,C.;Teschmit,N.;Thomas,L.Acta Cryst. 2013,B69,405.doi:10.1107/S2052519213013481
(8)Klampfl,C.W.Electrophoresis 2006,27(Special Issue),3. doi:10.1002/elps.200500523
(9)Torchilin,V.P.Nature Reviews Drug Discovery 2005,4,145. doi:10.1038/nrd1632
(10)Li,Q.;He,Y.C.;Xian,M.;Jun,G.;Xu,X.;Yang,J.M.;Li,L. Z.Bioresource Technology 2009,100,3570.doi:10.1016/j. biortech.2009.02.040
(11)Zhao,H.;Jones,C.L.;Baker,G.A.;Xia,S.;Olubajo,O. Journal of Biotechnology 2009,139,47.doi:10.1016/j. jbiotec.2008.08.009
(12)Nath,J.;Chaudhuri,M.K.Catalysis Letters 2009,133,388. doi:10.1007/s10562-009-0157-y
(13)Cheng,H.;Ma,H.Journal of Salt and Chemical Industry 2012,4,19.
(14)Haller,J.;Miecznik,P.;Kaatze,U.Chem.Phys.Lett.2006, 429,97.doi:10.1016/j.cplett.2006.07.088
(15)Baluja,S.;Parsania,P.H.J.Pure Appl.Ultrason 1997,19,36.
(16)Palaniappan,L.;Arul,G.J.Acoust.Soc.India 2000,28,393.
(17)Lide,D.R.Handbook of Chemistry and Physics,81st ed.; CRC Press:Boca Raton,FL,2000.
(18)Williams,G.The Theory of Resonance;John Wiley:New York,1979.
(19)Dean,J.A.Lange's Handbook of Chemistry,13th ed.;McGraw Hill International:New York,1987.
(20)Kell,G.S.J.Chem.Engg.Data 1975,20,97.doi:10.1021/ je60064a005
(21)Arul,G.;Palaniappan,L.Indian J.Pure Appl.Phys.2005,43, 755.
(22)Subrahmanian,B.Text Book on Sound,5th ed.;New Delhi:S. Chand Publishers,1998.
(23)Miecznik,P.;Golebiewski,Z.;Mielcarek,S.Fluid Phase Equilibria 2004,221,41.doi:10.1016/j.fluid.2004.04.016
(24)Sundharam,N.;Palaniappan,L.Indian J.Phys.2005,79,1173.
(25)Suryanarayana,C.V.;Kuppusamy,J.J.Acoust.Soc.India 1976,4,75.
(26)Suryanarayana,C.V.;Govindasamy,S.Acta Chim.Hung. 1960,25,341.
(27)Peters,E.I.Introduction to Chemical Principles,3rd ed.;CBS College Publishing:Philadelphia,1982.
(28)Palaniappan,L.Indian J.Phys.2001,75B,515.
(29)Vogel,I.Practical Organic Chemistry;Orient Longman: London,1978.
(30)Sasikumar,P.;Thiyagarajan,R.;Palaniappan,L.J.Indian Chem.Soc.2015,92,991.
(31)Ali,A.;Sabir,S.;Tariq,M.Acta Phys.-Chim.Sin.2007,23, 79.doi:10.1016/S1872-1508(07)60008-4
(32)Nikam,P.S.;Mehdi,H.J.Chem.Eng.Data 1988,33,165. doi:10.1021/je00052a032
(33)Palaniappan,L.;Devadoss,D.;Thairiyaraja,M.Indian J. Phys.2003,77B,679.
(34)Karthikeyan,V.;Palaniappan,L.Indian J.Phys.2005,79,153.
(35)Vadivel,S.;Palaniappan,L.Indian J.Phys.2005,79,1383.
(36)Nikam,P.S.;Kapade,V.M.;Hasan,M.Indian J.Pure Appl. Phys.2000,38,170.
(37)https://en.wikipedia.org/wiki/Alcohol(accessed Nov 20,2015)
(38)Israelachvili,J.N.Intermolecular and Surface Forces,2nd ed.; Academic Press:New York,1992.
(39)Ababneh,A.M.;Large,C.C.;Georghiou,S.Biophysical Journal 2003,85,1111.doi:10.1016/S0006-3495(03)74548-2
10.3866/PKU.WHXB201601081
August 7,2015;Revised:January 5,2016;Published on Web:January 8,2016.*Corresponding author.Email:lp_dde_au@yahoo.com

