羥烷基胺功能化離子液體吸收SO2的量子化學(xué)計(jì)算
李學(xué)良 陳潔潔 羅 梅 陳祥迎 李培佩
(合肥工業(yè)大學(xué)化學(xué)工程學(xué)院,可控化學(xué)與材料化工安徽省重點(diǎn)實(shí)驗(yàn)室,合肥 230009)
Ionic liquids(ILs)have attracted a great deal of interest in various fields[1-10],due to their outstanding characteristics such as negligible vapor pressure and stability.One of the most important properties is that ILs could be tailored and assembled by changing and adjusting the structures of their cations and anions, offering a key way in designing functional ILs for cyclic absorbents.Therefore,ILs can be the candidate solvents to realize the particular function for absorption,desorption,and separation of sulfur dioxide(SO2).The new ILs will provide a solution to solve the pollution problem of SO2from flue gas.Some ILs have been synthesized and studied for absorbing and desorbing SO2,including guanidinium[11-12],imidazolium[13-15]and ammonium[16]systems.Wu et al.[11]found that the molar absorption ratio of SO2:TMGL reached 1.7 while tetramethylguanidium lactate (TMGL)was exposed to pure SO2gas.Anderson et al.[15]reported that 1-n-hexyl-3-methylimidazolium bis(trifluoro-methylsulfonyl)imide([hmim][Tf2N])and 1-n-hexyl-3-methylpyridinium bis-(trifluoromethyl-sulfonyl)imide([hmpy][Tf2N])dissolved large amounts(up to 85%(molar fraction,x))of SO2.Yuan and co-workers[16]synthesized and studied nine kinds of ammonium ILs with high solubility of SO2at ambient pressure.
Owing to the interaction between ILs and SO2,the variation of IL structures and physicochemical properties occurred with the absorption,including charge transfer interaction,viscosity etc. Except for the preparation and absorption studies,some researchers have also paid attention to studying the interaction between ILs and SO2and the absorption mechanism.Ando et al.[17]reported that the Raman spectrum of 1-butyl-3-methylimidazolium bromide(BMIBr)-SO2clearly indicated a specific charge transfer interaction involving SO2and the halide.Barrosse-Antlle et al.[13]indicated that absorption of SO2caused a decrease in viscosity of 1-ethyl-3-methylimidazolium bis(trifluoromethanesufonyl)imide.Huang et al.[14]reported that the 1-butyl-3-methylimidazolium [BMIM]+and TMG cation-based ILs could physically absorb 1-2 mol SO2per mole of IL reversibly at ambient pressure and room temperature as excellent solvents.The results reported by Wu et al.[11]suggest that the TMGL can absorb SO2by both physical and chemical absorptions.Some researchers investigated novel IL for absorption/desorption in extensive fields, such as the poly(ionic liquid)[18],supported IL membranes[19],and combination with IL and porous materials[20-21],etc.
Theoretical calculation and simulation have provided an important opportunity[22-24]to study SO2absorption.Several excellent works have been reported with theoretical calculations for guanidinium and imidazolium cations performed mostly by molecular dynamics(MD)simulations.Ando et al.[17]investigated BMIBr-SO2by MD simulations,indicating the potential application of cations with substituents like—NH2,—NHR,and—NR2groups,as an efficient gas removal agent.Siqueira et al.[25]reported the MD results that the absorption of SO2with BMIBr resulted in the phase change from crystal to liquid with relatively low viscosity and high conductivity.Wang et al.[26]used a combination of ab initio calculations and OPLS parameters for developing an all-atom force field for the TMGL IL.Then they used quantum chemical calculations to investigate the interaction between SO2and TMGL,and obtained a deeper understanding of the factors that govern the high solubility of SO2in TMGL[27].
As analyzed above,many progresses have been made on the absorbing SO2process,and it was helpful for understanding the absorption behavior of ILs.However,some explanations about absorption mechanism[11,14]were different.Owing to few systematic studies on different ILs,further systematic and theoretical investigations are needed to understand the mechanism of absorption and desorption of SO2by choosing different cations and anions.Most kinds of ILs are in liquid state,and the physicochemical properties are different with the gas and solid.It may be difficult to exactly and reasonably explain the properties of ILs by gas state models.Therefore,we need to modify the models and construct liquid state models for quantum chemical calculation.With the development of computational chemistry,theoretical calculation and simulation will play a crucial role in absorption studies.Aswe know,the amine groupsare efficient electron donors[17]toward SO2,so we choose ammonium ILs as SO2absorbents and systematically study the effects of ILs with different cations with advanced and accurate method based on DFT.
The present work studied the ammonium ILs(cation:hydroxyalkyl ammonium,anion:acetate)and IL-SO2systems by quantum chemical calculations,achieving optimized geometry structures,analyzing the interaction,discussing thermodynamic properties,and investigating transition state etc.The theoretical investigation was made to obtain the important data and information,such as vibration information,energy characteristics,and electronic strutures.
1 Computational details
1.1 Computational method
All the calculations have been performed using the DMol3module[28-29]of the Materials Studio program.The minimumenergy geometry structures of the hydroxyalkyl ammonium ILs and IL-SO2were determined by combining generalized gradient approximations[30-31]and density functional theory(DFT)methods.Then the transition state was determined by synchronous transit method[32].Hirshfeld population analysis[33]was performed to analyze the charge transfer.Thermodynamic properties and vibrational spectrum were obtained by the nonlocal exchangecorrelation functional proposed by Perdew et al.[34].Double precision numerical basis sets combined with polarization functions were used to describe the valence electrons,while all-electron core treatment was utilized to describe the core electrons.A spinpolarized scheme was employed to deal with the open-shell systems.The transition state(TS)search was performed by linear synchronous transit(LST)/quadratic synchronous transit(QST) method,gaining the energy barrier of SO2absorption.Then the transition state was confirmed with the nudged elastic band (NEB)methods.Activation energy(Ea)and minimum energy path(MEP)of the absorption reaction can be acquired by LST/QST and NEB,respectively.Then the calculation results verified byexperiment[16]to show the validityoftheoretical calcu-lation.
1.2 Computation principle
The total energy(Et)of systems may be written as:

where ρ is the density of a system,T(ρ)and U(ρ)are the kinetic energy and classical electrostatic energy due to Coulombic interactions,respectively.Exc(ρ)includes all many-body contributions to the total energy,in particular the exchange and correlation energies.
The results of a vibration analysis or Hessian evaluation can be used to compute enthalpy(H),entropy(S),free energy(G),and heat capacity(Cp)as functions of temperature[35].
For deeply exploring the absorption reaction,gas state and liquid state models were constructed for hydroxyalkyl ammonium ILs and IL-SO2systems.The amorphous structure has been constructed as the liquid state model by Amorphous Cell(a module of Materials Studio)which is versatile suite of computational tools used to develop an understanding of molecular properties and behavior,especially for liquids and amorphous polymers.By observing the relationship between system structure and properties,we can obtain a more thorough understanding of the important molecular features,allowing us to better design new compounds or new formulations.The representative structures used in this study are optimized and presented in Fig.1.
Starting from reactants(ammonium ILs and SO2)and products(IL-SO2systems and acetic acid),the synchronous transit methods interpolate the pathway to find the transition state of absorption reaction.The LST and QST tools locate a maximum energy structure,but this maximum may not be the correct transition state.TS confirmation tool can be used to confirm that the transition state of absorption reaction does indeed connect the presumed reactant(R)and product(P).Byusing the LST method, an estimate of the transition state is generated by finding the highest point along the shortest line connecting‘R’and‘P’.The QST method further extends this by subsequently searching for a minimum along a line perpendicular to the previous one.The transition state structure was optimized to achieve the refined activation energy.By guessing the absorption MEP which connects two stable structures(‘R’and‘P’),the NEB method works to confirm the transition state and find the additional minima on the MEP.
2 Results and discussion
2.1 Molecule structure change after absorption
Scheme 1 shows the schematic structure of ILs for reacting with SO2.A variety of substitutent groups can be connected to the nitrogen atom,obtaining many kinds of ILs with different absorbing properties.
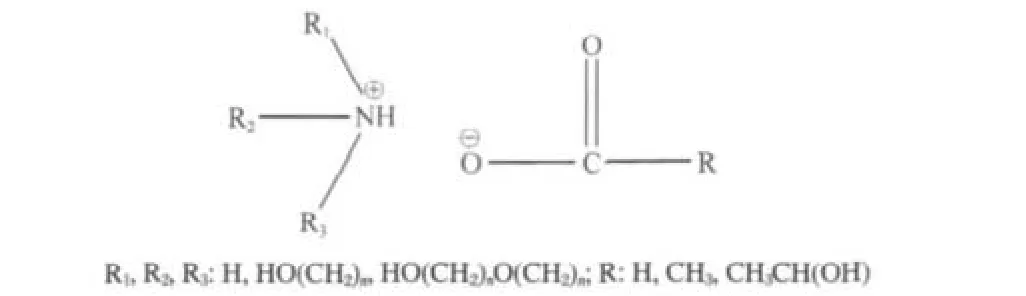
Scheme 1 Schematic structure of the ammonium ionic liquid
In this work,the substituent group on cation is hydroxyethyl, and all the anion is the acetate.The—NH2,—NHR,and—NR2groups of ammonium ILs,which are efficient electron donors toward SO2.The reversible absorption/desorption reactions of ILs with SO2can be expressed as the following reaction(x=1,2,3):
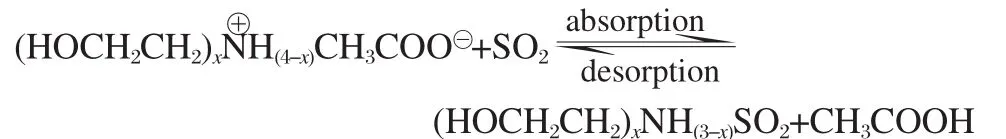
The structures of IL-SO2systems are fully optimized,obtaining stable structures and structural parameters.The representative optimized structure of absorption product in liquid state model,hydroxyalkyl ammonium-SO2[(HOCH2CH2)xNH(3-x)SO2] (x=1),was given in Fig.1,including the IL and acetic acid.
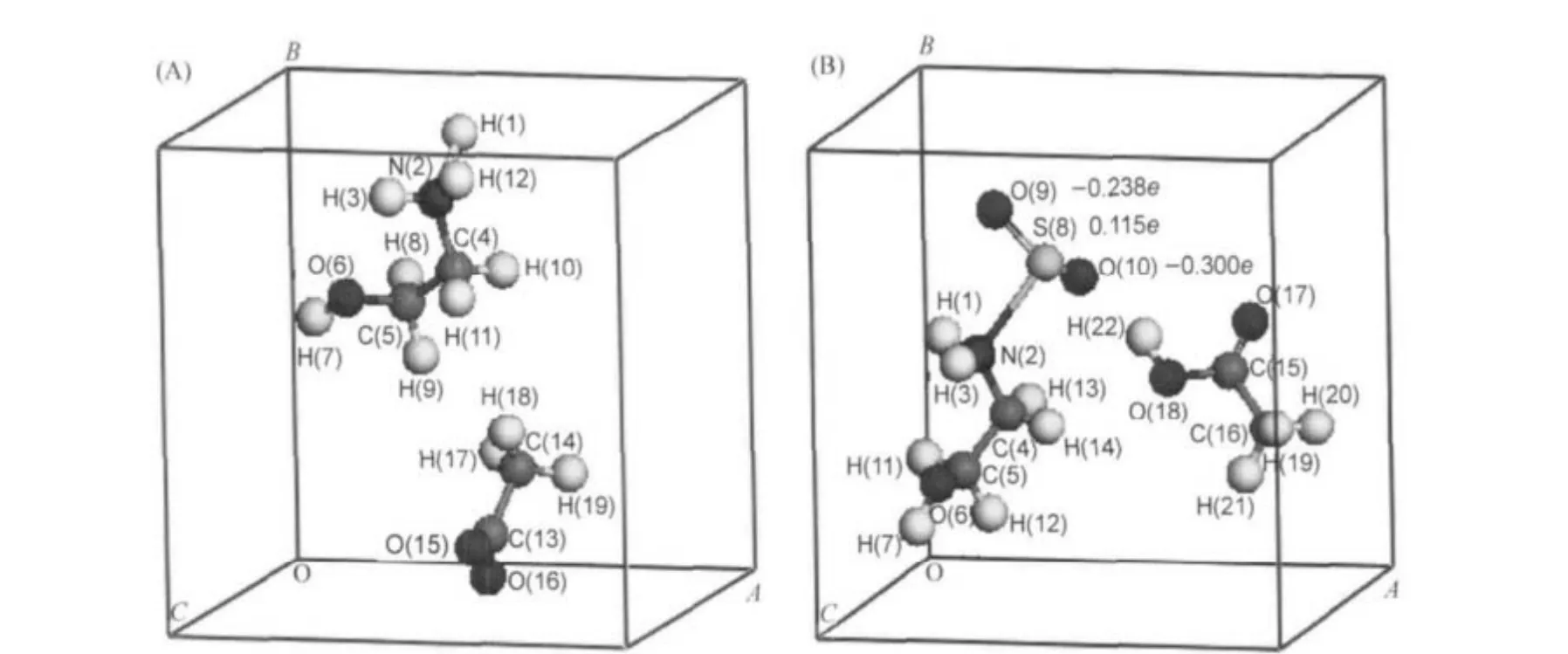
Fig.1 The representative optimized structures and charge distribution in SO2(A)primary ammonium acetate ionic liquid(a=0.600 nm,b=0.665 nm,c=0.665 nm,α=β=γ=90°); (B)HOCH2CH2NH2-SO2+CH3COOH(a=0.615 nm,b=0.695 nm,c=0.725 nm,α=β=γ=90°)
The charge distribution,average bond distances,and vibration spectrum data were computed and analyzed.In IL-SO2systems, the charge distribution has changed during the absorption reac-tion.The S—N bond forms between atom S and N with the average bond distance of 0.240 nm both in gas state and liquid state models.The S—N bond tends to be chemical combination, for its distance is between chemical bond length(0.184 nm)and the sum of van der Waals radius(0.334 nm).
The charge distribution of IL?SO2systems indicate that absorption reaction results in the transfer of negative charge from ILs to SO2both in gas and liquid state models.The geometry parameter of SO2and net charge transfer amount are exhibited in Table 1.Compared with isolated SO2molecule,the average distance ofS—O bond(lengthsoftwo S—O bondshave few difference)increases and the bond angle of O—S—O decreases in IL?SO2systems,which means that SO2molecules are absorbed on ILs and the nitrogen atom becomes the important active site.
From Table 1,in gas state model,it can be found that the average bond distance of S—O expands from 0.1490 to 0.1495 nm and the bond angle of O—S—O diminishes from 117.435°to 116.903°,with the number of substitutes on the N atom increasing.Meanwhile,the increase of substitute number results in much charge transfer from ILs to SO2.Therefore,the sequence of interaction intensity between ILs and SO2can be deduced as: (HO(CH2)2)3N?SO2>(HO(CH2)2)2NH?SO2>HO(CH2)2NH2?SO2.In liquid state model,the variation trend of geometry parameter is not the same as the results calculated from gas state model, while the interaction intensity is stronger for longer bond distance of S—O,smaller angle of O—S—O,and more charge transfer from ILs to SO2than the results in gas state model.
2.2 Thermodynamic properties
According to vibrational analysis and calculation,important thermodynamic properties of IL?SO2systems can be obtained. More information about the absorption reaction will be predicted and verified by analyzing the thermodynamic properties of these systems.In this study,we analyzed standard free energy change (ΔG?)and equilibrium constant of the absorption reaction.
The free energy of ammonium ILs is displayed in Fig.2 as the function of temperature.The ILs were investigated in gas state and liquid state models,while SO2was calculated only in gas state model.The free energy of all the ILs decreases with increasing the temperature.In general,it is found that the free energies of these ILs increase with the number of substitute groups on the nitrogen atom increasing at the same temperature both in gas state model and liquid state one,which follows the sequence:HO(CH2)2N+H3<(HO(CH2)2)2N+H2<(HO(CH2)2)3N+H(anion:acetate).However,in gas state model,the free energy ofammonium IL with primary amine group is larger than secondary ammonium IL when the temperature exceeds 900 K. Each IL in liquid state model has larger free energy than in gas state model,and the difference increases with increasing the temperature.
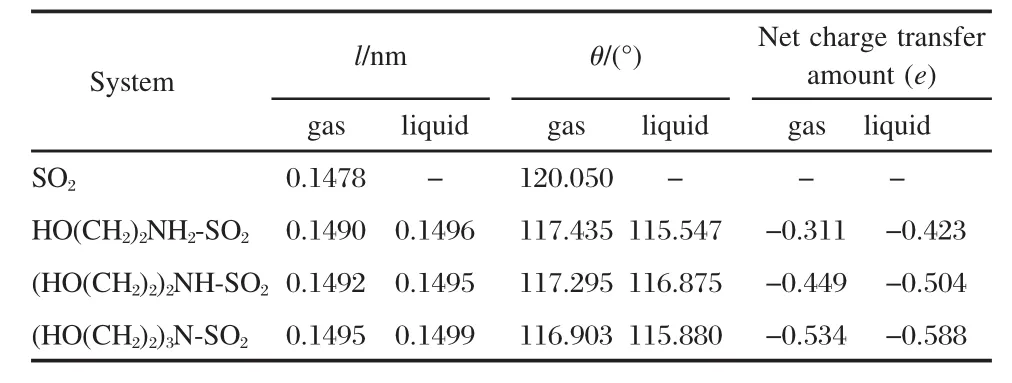
Table 1 Average distance of S—O bond(l),bond angle of O—S—O(θ),and net charge transfer amount of SO2
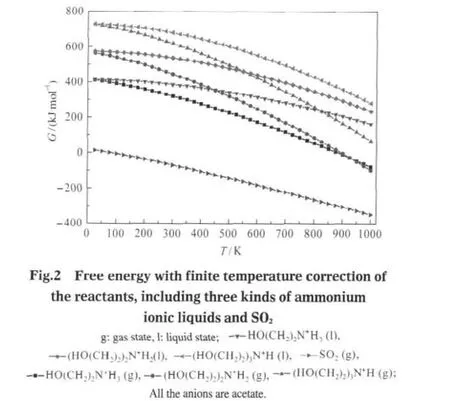
The free energies of IL?SO2systems(products)and acetic acid with finite temperature correction are presented in Fig.3.In order to reflect the interaction of condensed state,the acetic acid in liquid state was included in the amorphous cell structure of the absorption products,while in gas state model the geometry structure of acetic acid was optimized separately.The values of all the materials decrease with increasing the temperature.At the same temperature,the high molecular weight results in the large free energy in gas state model and liquid state one,except the primary ammonium IL?SO2system in liquid state when the temperature exceeding 900 K.

Based on the total energy at 0 K and the temperature correction values presented in Fig.2 and Fig.3,ΔG?of the absorption reaction can be calculated at different temperatures,where the total energy presented in the Supporting Information(available free of charge at http://www.whxb.pku.edu.cn)is the total electronic energy at 0 K.The thermodynamic parameters of hydroxyalkyl ammonium IL-SO2with gas state and liquid state models at 298.15 K are listed in Table 2.
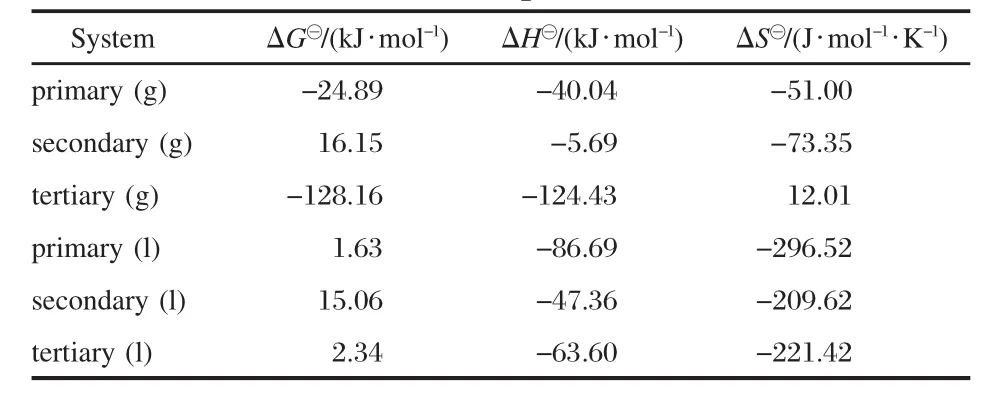
Table 2 Thermodynamic parameters(298.15 K)of the SO2 absorption reaction with hydroxyalkyl ammonium acetate ionic liquids
It can be found that the absorption reaction in gas state model can occur spontaneously at 298.15 K,except the IL with secondary amine group,whose ΔG?is 16.15 kJ·mol-1.The reaction between SO2and secondary ammonium IL(gas state)may spontaneously occur in the lower temperature.However,in the liquid state,the difference of ΔG?value is not significant for the reactions of these ILs with SO2molecule.And it is relatively easy for primary ammonium IL to react with SO2,and ΔG?is 1.63 kJ· mol-1in Table 2.The enthalpy change(ΔH)indicates that these absorption reactions are exothermic processes.Whether in gas state model or in liquid state one,the secondary ammonium IL absorbs SO2and generates the least thermal energy.Tertiary ammonium IL-SO2system in gas state exhibits the significant difference of thermodynamic properties among the systems,which may result from the complex molecular structure of the IL or the gas state model not fitted to show the properties of absorption reaction in the theoretical calculation.
According to the entire calculation data in Fig.4,it is found that the low temperature is good for absorption reaction.The absorption reaction occurs at low temperature.The temperature corresponding ΔG?=0 kJ·mol-1are 743.42,35.26,281.58, 220.00,and 281.57 K for primary(g),secondary(g),primary(l), secondary(l)and tertiary(l),respectively.Exceeding these temperatures,the hydroxyalkyl ammonium IL systems will tend to occur the desorption reaction,indicating that ammonium ILs can be used as recyclable solvent for absorbing SO2.But the desorption temperature of the secondary ammonium IL-SO2system (g)is too low to fit for desulfurization.Although the ΔG?of tertiary ammonium IL in gas state is the lowest at the same temperature,SO2molecule is difficult to escape from IL with increasing the temperature.Maybe SO2will be released in the vacuum environment,which is not very suitable for the industrial application.Therefore,the secondary ammonium IL is not the good one as regenerable SO2absorbent.It is revealed that the structures of ILs play an important role in determining the absorption properties of SO2.
Naturally,the experimental data can directly examine the validity and correctness of theoretical analysis about the absorption reaction of SO2.We choose primary ammonium acetate IL(2-hydroxyethylammonium acetate)as an example to examine the calculated results.The experiment data of 2-hydroxyethylammonium acetate can be found in Fig.3 of Ref.[16],presenting the equilibrium molar fraction of SO2in absorption reaction at 293.2,298.2,303.2,313.2,and 323.2 K[16].For comparing conveniently,theoretical equilibrium constant(K)was computed from ΔG?by the equation displayed as follows:

Then the equilibrium constants were converted to theoretical molar fraction of SO2(XSO2)by the following formulas based on the chemical equation (Scheme 1).The pressure of SO2keeps 101.3 kPa in the experiment,so pSO2/p?equals 1(p?is chosen as 101.3 kPa,which is consistent with the pressure in the exported results from software).
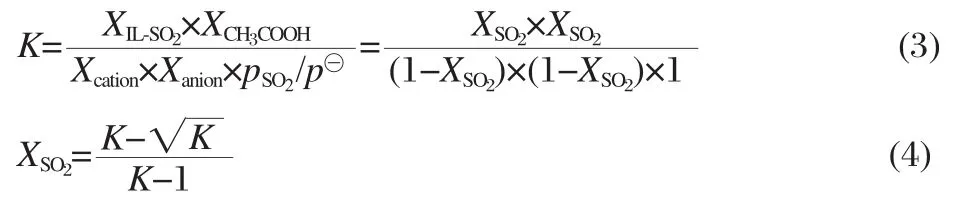
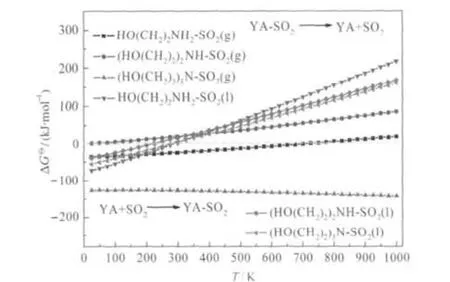
Fig.4 Standard Gibbs free energy changes of the reversible absorption/desorption reactionsY:(HOCH2CH2)x,A:NH(3-x),x=1,2,3
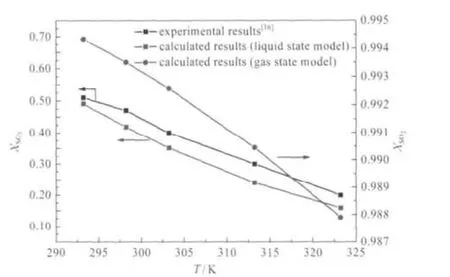
Fig.5 Comparison of molar fraction of SO2obtained in experiment and theoretical calculation in liquid state and gas state models for HO(CH2)2NH2-SO2system
Experiment data and theoretical values of XSO2are presented in Fig.5 at different temperatures.In order to conveniently verify the theoretical results,the activity coefficient for the substances in these absorption reactions are considered as‘1’.We found that the values of theoretical calculation with liquid state model were in good agreement with the experiment results,indicating the validity of theoretical analysis.The experimental molar fraction of SO2is a little higher than the theoretical value,which may be resulted from partial physical adsorption besides the chemical absorption.From the results mentioned above,it is concluded that the DFT functional and numerical basis set se-lected in the calculation was appropriate for calculating the absorption reaction of ammonium ILs with SO2in liquid state model,and the theoretical molar fraction of SO2at equilibrium state calculated in this work is successfully verified by experiment results.
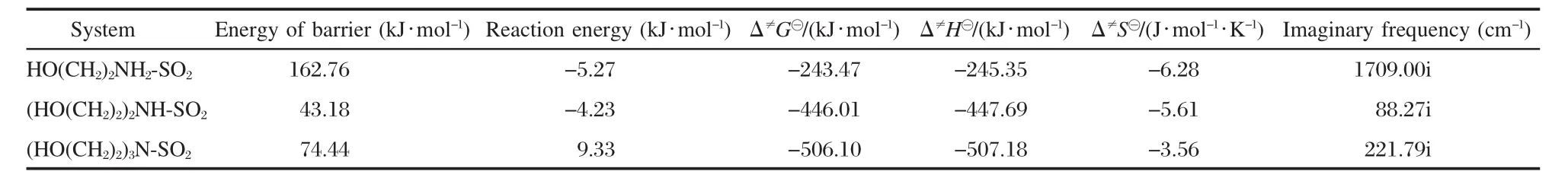
Table 3 Thermodynamic parameters(298.15 K)and the imaginary frequency of hydroxyalkyl ammonium IL-SO2systems
The differences between results from gas state model and experiment are also displayed in Fig.5.The molar fraction of SO2calculated with gas state model is much higher than the valuesobtained from liquid state model and experiment,and the difference becomes more obvious in relatively high temperature area.
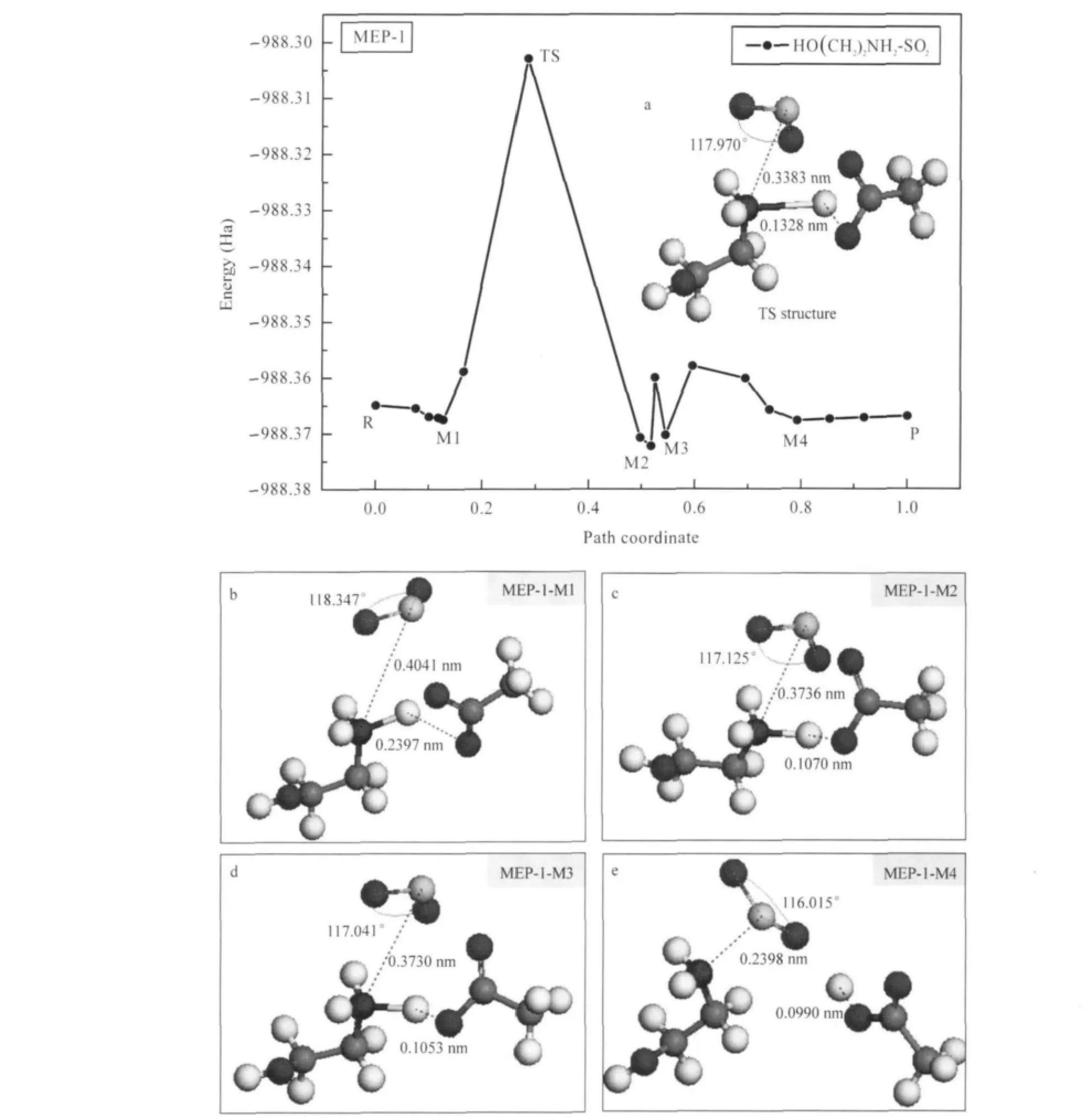
Fig.6 MEP-1 curve with the HO(CH2)2NH2-SO2system structure of the saddle point(a)and minimum energy points(b-e)based on reactant,1 Ha=2625.50 kJ·mol-1
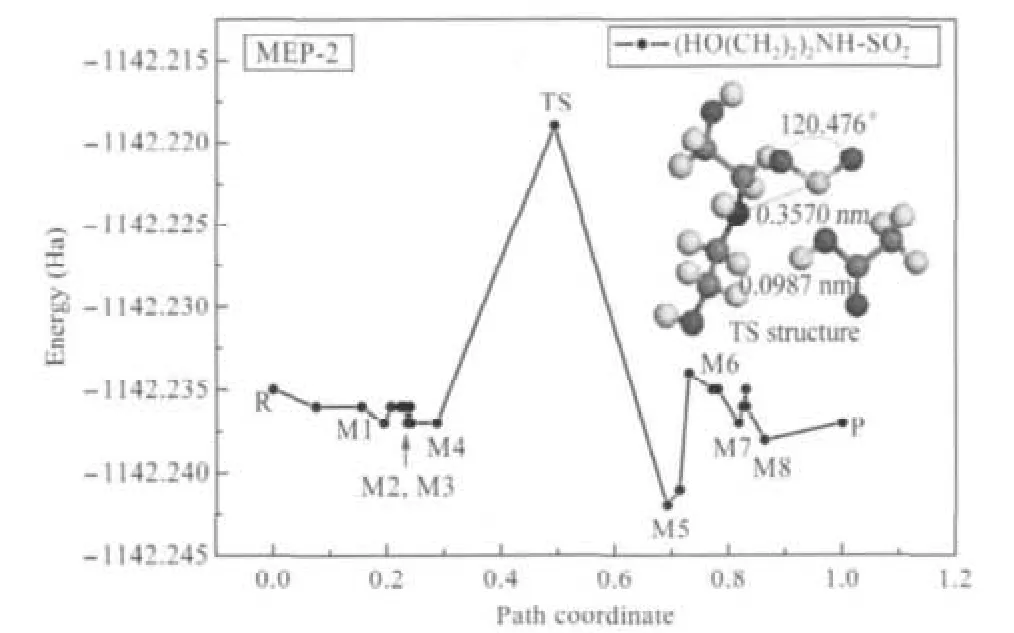
Fig.7 MEP-2 curve and the transition state structure (saddle point)of(HO(CH2)2)2NH-SO2systembased on product,minimum energy structures on MEP-2 presented in Supporting Information
Compared with the model of gas state,liquid state model is much fit for calculating the reaction of ammonium ILs with SO2. Liquid state model could well present the interaction between SO2and ammonium IL.The theoretical calculation depends on the model construction,and the reasonable model will achieve the valuable conclusion.
The values computed by DFT in this work are successfully verified by experiment results[16],and it indicates that the parameters and quality sets fit the absorption reaction of SO2with ammonium ILs,and computer simulation can be applied to predict the properties of new compounds and analyze the experimental results.The appropriate functional groups will be worked out for other series of ILs by computer simulation.
2.3 Transition state and reaction pathway
The energy barrier,thermodynamic parameters(298.15 K) and the imaginary frequency of the SO2absorption reaction are presented in Table 3.Owing to the stable structure of the cation in primary ammonium IL,the energy barrier of the absorption reaction is 162.76 kJ·mol-1.The absorption rate is determined by the activation energy,and it will take a long time to reach the equilibrium status for primary ammonium IL-SO2.
In order to confirm the reaction pathway,the MEP was worked out by NEB.For primary ammonium IL-SO2system,the MEP curve with the absorption structure of the saddle point(TS) and the structures with minimum energy(M)are shown in Fig.6. The results for secondary and tertiary ammonium IL-SO2systems are displayed in Figs.7 and 8.The structures with minimum energy on MEP-2 and MEP-3 can be found in Supporting Information file.
According to the structures of typical points on the MEP-1 in Fig.6,the marked distances of S—N and H—O decrease,and the bond angle of O—S—O declines during the absorption.The distances of S—N and H—O are 0.3383 and 0.1328 nm,respectively,and the bond angle of O—S—O is 117.970°in transition state structure with the highest energy on the MEP curve.The structural information of the minimum points is displayed below the MEP-1 plot.The distance of S—N changes from 0.4041 nm to 0.2398 nm,meanwhile,the distance of H—O changes from 0.2397 nm to 0.0990 nm,forming the chemical bonds.
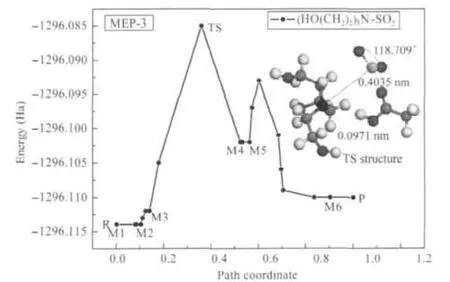
Fig.8 MEP-3 curve and the transition state structure (saddle point)of(HO(CH2)2)3N-SO2systembased on product,minimum energy structures on MEP-3 presented in Supporting Information
The activation energy(Ea)and Δ≠G?of absorption reaction with secondary ammonium IL are listed in Table 3.For Δ≠G?reaches -446.01 kJ·mol-1and Eais the lowest(43.18 kJ·mol-1)of these systems at 298.15 K,the absorption reaction can spontaneously occur and easily arrive at equilibrium state.But the ΔG?of the absorption reaction with this IL is the highest among three ILs in liquid state model.
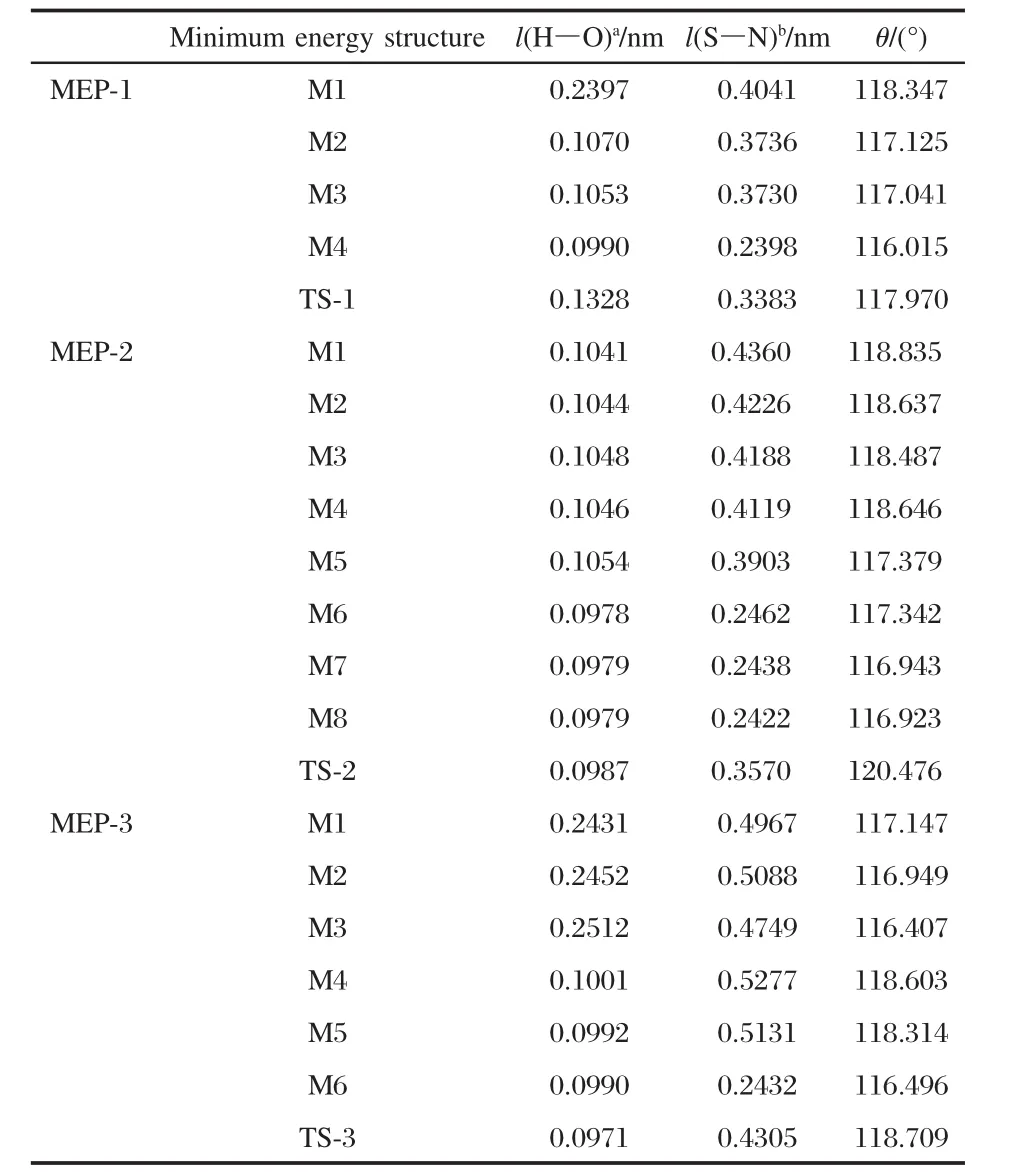
Table 4 The distance of H—O bond(l(H—O)),S—N bond (l(S—N))and bond angle of S—O—S(θ)of minimum energy structures and transition state structures on MEP curves
In Table 4,the distances between S and N in TS structures on the MEPs follow this sequence:TS-3>TS-2>TS-1.On the contrary,the distances between H and O follow the sequence:TS-1>TS-2>TS-3.The possible reason of two sequences is that the steric effect prevents SO2from absorbing.It is easy for the H ion to leave the N atom,but hard for N atom to bond with the S atom.The distance change of S—N in minimum energy structure on MEP-2 follows the same regular with that on MEP-1,diminishing from M1(0.2431 nm)to M8(0.0990 nm),while the distance change of H—O is fluctuation.
The distance changes of S—N and H—O in minimum energy structure on MEP-3 present relatively complexity in Table 4. The energy barrier of 74.43 kJ·mol-1results in relatively fast absorption through the reaction path.The structural parameters of the transition state are shown in Fig.8.The tertiary ammonium IL can desorb SO2with the temperature increasing as presented in Fig.4,and the ΔG?(2.34 kJ·mol-1,Table 2)and Δ≠G?(-506.10 kJ·mol-1,Table 3)indicate that this reaction can be easy to occur at the room temperature and ambient SO2pressure.Therefore,the calculated results demonstrate ammonium ILs should be the good candidate for regenerable SO2absorbent,and the one with tertiary amine group would be the best.
3 Conclusions
Quantum chemical calculations were performed for acquiring the information of the reversible reaction for absorbing/desorbing SO2,obtaining optimized geometry structures,charge distribution and energy characteristics,etc.
The hydroxyalkyl ammonium ILs react with SO2to form S—N bond and show the charge transfer from ILs to SO2.The absorption reaction results in the increase of S—O bond length and the decrease of O—S—O bond angle.The absorption mainly presents chemical action,proved by the charge distribution and the results of geometry analyses.The nitrogen atom of ammonium ILs plays a key role in the absorption reaction.
In general,the free energies of all the materials decrease with the increase of temperature and the decrease of molecular weight both in gas state and liquid state models.The absorption reaction occurs at low temperature.The temperature corresponding ΔG?= 0 kJ·mol-1are 281.58,220.00,and 281.57 K for primary(l),secondary(l),and tertiary(l),respectively.The hydroxyalkyl ammonium ILs can be used as regenerable solvents for absorbing SO2.Tertiary ammonium IL-SO2system in gas state model has the lowest ΔG?of all the systems.Furthermore,the theoretical results of XSO2(molar fraction of SO2)calculated in this work are in accord with experiment data.
The activation energy of the systems follows the sequence: Ea(secondary)<Ea(tertiary)<Ea(primary),exhibiting the effect of IL structure in the absorption reaction.The calculated results with liquid model demonstrate hydroxyalkyl ammonium ILs should be the good candidate for absorbing and recycling SO2. The study may provide a good method in designing novel IL system for regenerable absorbing SO2.
Supporting Information available: The detailed results of standard Gibbs free energy change calculation,geometry optimization,thermodynamic properties,and transition state calculation have been included.This information is available free of charge via the internet at http://www.whxb.pku.edu.cn.
1 Lin,Q.;Fu,H.Y.;Yuan,M.L.;Chen,H.;Li,X.J.Acta Phys.-Chim.Sin.,2006,22:1272 [林 棋,付海燕,袁茂林,陳 華,李賢均.物理化學(xué)學(xué)報(bào),2006,22:1272]
2 Gao,L.X.;Wang,L.N.;Qi,T.;Li,Y.P.;Chu,J.L.;Qu,J.K.Acta Phys.-Chim.Sin.,2008,24:939 [高麗霞,王麗娜,齊 濤,李玉平,初景龍,曲景奎.物理化學(xué)學(xué)報(bào),2008,24:939]
3 Zhang,X.Z.;Jiao,K.Acta Phys.-Chim.Sin.,2008,24:1439 [張旭志,焦 奎.物理化學(xué)學(xué)報(bào),2008,24:1439]
4 Izgorodina,E.I.;Forsyth,M.;MacFarlane,D.R.Physical Chemistry Chemical Physics,2009,11:2452
5 Imanishi,A.;Tamura,M.;Kuwabata,S.Chem.Commun.,2009: 1775
6 Wasserscheid,P.;Welton,T.Ionic liquids in synthesis.Weinheim: Wiley-VCH,2003:1-355
7 Sheldon,R.Chem.Commun.,2001:2399
8 Wasserscheid,P.;Keim,W.Angewandte Chemie-International Edition,2000,39:3772
9 Sun,G.H.;Li,K.X.;Fan,H.;Gu,J.Y.;Li,Q.;Liu,Y.Acta Phys.-Chim.Sin.,2008,24:103 [孫國(guó)華,李開喜,范 慧,谷建宇,李 強(qiáng),劉 越.物理化學(xué)學(xué)報(bào),2008,24:103]
10 Yang,P.X.;An,M.Z.;Su,C.N.;Wang,F.P.Acta Phys.-Chim. Sin.,2008,24:2032 [楊培霞,安茂忠,蘇彩娜,王福平.物理化學(xué)學(xué)報(bào),2008,24:2032]
11 Wu,W.Z.;Han,B.X.;Gao,H.X.;Liu,Z.M.;Jiang,T.;Huang,J. Angewandte Chemie-International Edition,2004,43:2415
12 Huang,J.;Riisager,A.;Berg,R.W.;Fehrmann,R.Journal of Molecular Catalysis A:Chemical,2008,279:170
13 Barrosse-Antlle,L.E.;Hardacre,C.;Compton,R.G.Journal of Physical Chemistry B,2009,113:1007
14 Huang,J.;Riisager,A.;Wasserscheid,P.;Fehrmann,R.Chem. Commun.,2006:4027
15 Anderson,J.L.;Dixon,J.K.;Maginn,E.J.;Brennecke,J.F. Journal of Physical Chemistry B,2006,110:15059
16 Yuan,X.L.;Zhang,S.J.;Lu,X.M.Journal of Chemical and Engineering Data,2007,52:596
17 Ando,R.A.;Siqueira,L.J.A.;Bazito,F.C.;Torresi,R.M.; Santos,P.S.Journal of Physical Chemistry B,2007,111:8717
18 An,D.;Wu,L.B.;Li,B.G.;Zhu,S.P.Macromolecules,2007,40: 3388
19 Jiang,Y.Y.;Zhou,Z.;Jiao,Z.;Li,L.;Wu,Y.T.;Zhang,Z.B. Journal of Physical Chemistry B,2007,111:5058
20 Zhang,Z.M.;Wu,L.B.;Dong,J.;Li,B.G.;Zhu,S.P.Industrial &Engineering Chemistry Research,2009,48:2142
21 Zhou,C.G.;Yao,S.J.;Wu,J.P.;Forrey,R.C.;Chen,L.; Tachibana,A.;Cheng,H.S.Physical Chemistry Chemical Physics, 2008,10:5445
22 Liu,J.X.;Wei,X.;Zhang,X.G.;Wang,G.X.;Han,E.S.;Wang, J.G.Acta Phys.-Chim.Sin.,2009,25:91 [劉潔翔,魏 賢,張曉光,王桂香,韓恩山,王建國(guó).物理化學(xué)學(xué)報(bào),2009,25:91]
23 Zhou,D.H.;Wang,Y.Q.;He,N.;Yang,G.Acta Phys.-Chim.Sin., 2006,22:542 [周丹紅,王玉清,賀 寧,楊 剛.物理化學(xué)學(xué)報(bào),2006,22:542]
24 Jiang,S.Y.;Teng,B.T.;Yuan,J.H.;Guo,X.W.;Luo,M.F.Acta Phys.-Chim.Sin.,2009,25:1629 [蔣仕宇,滕波濤,袁金煥,郭曉偉,羅孟飛.物理化學(xué)學(xué)報(bào),2009,25:1629]
25 Siqueira,L.J.A.;Ando,R.A.;Bazito,F.F.C.;Torresi,R.M.; Santos,P.S.;Ribeiro,M.C.C.Journal of Physical Chemistry B, 2008,112:6430
26 Wang,Y.;Pan,H.;Li,H.;Wang,C.Journal of Physical Chemistry B,2007,111:10461
27 Wang,Y.;Wang,C.M.;Zhang,L.Q.;Li,H.R.Physical Chemistry Chemical Physics,2008,10:5976
28 Delley,B.Journal of Physical Chemistry,1996,100:6107
29 Delley,B.Journal of Chemical Physics,2000,113:7756
30 Perdew,J.P.;Wang,Y.Physical Review B,1992,45:13244
31 Perdew,J.P.;Chevary,J.A.;Vosko,S.H.;Jackson,K.A.; Pederson,M.R.;Singh,D.J.;Fiolhais,C.Physical Review B, 1992,46:6671
32 Halgren,T.A.;Lipscomb,W.N.Chemical Physics Letters,1977, 49:225
33 Hirshfeld,F.L.Theoretica Chimica Acta,1977,44:129
34 Perdew,J.P.;Burke,K.;Ernzerhof,M.Physical Review Letters, 1996,77:3865
35 Hirano,T.A note on thermochemistry//MOPAC Manual.7th ed. Stewart,J.J.P.Ed.Stewart Computatinal Chemistry,Colrado Springs,1993:77-81.http://openmopac.net/

