Effects of Temperature on the Preparation of Magnesium Carbonate Hydrates by Reaction of MgCl2 with Na2CO3*
CHENG Wenting (程文婷), LI Zhibao (李志寶),** and George P. Demopoulos
?
Effects of Temperature on the Preparation of Magnesium Carbonate Hydrates by Reaction of MgCl2with Na2CO3*
CHENG Wenting (程文婷)1, LI Zhibao (李志寶)1,**and George P. Demopoulos2
1Key Laboratory of Green Process and Engineering, Institute of Process Engineering, National Engineering Laboratory for Hydrometallurgical Cleaner Production Technology, Chinese Academy of Sciences, Beijing 100190, China2Department of Mining, Materials Engineering, McGill University, 3610 University Street, Montreal, Quebec, Canada
Homogeneous (unseeded) precipitation of magnesium carbonate hydrates by the reaction of MgCl2with Na2CO3in supersaturated solutions between 273 and 363 K was investigated. The compositions, morphologies and filtration characteristics of the precipitates were studied in detail. The magnesium carbonate hydrates obtained at 313 K and in the range of 343-363 K showed good morphologies and filtration characteristics. Magnesium oxides (MgO) with high purity (97.6%-99.4%) were obtained by calcining magnesium carbonate hydrates at 1073 K.
homogeneous precipitation, magnesium carbonate hydrates, magnesium oxide, filtration characteristic
1 INTRODUCTION
The Qinghai salt lakes are well known for their huge reserves of potassium chloride (KCl) and magnesium chloride (MgCl2) in China [1]. In recent years, potassium fertilizer with 1.1 Mt·a-1has been produced and a large amount of magnesium chloride has been left as the by-product or even waste in salt lakes of Qinghai. This has caused not only the waste of magnesium resources, but also the environmental pollution [2]. Therefore, it is imperative to develop an effective utilization of MgCl2by extraction of magnesium from brine. Magnesium is recovered from seawater and brines on a large scale worldwide by the precipitation of its compounds [3]. In these processes, it is necessary to select proper temperatures and target precipitates that have excellent filtration characteristics.
Solid magnesium carbonate hydrates can exist in several modifications. Some of the compounds are widely used because of their technological importance in various industrial applications [4, 5]. A large number of chemical methods have been developed to generate various morphologies of them. For example, Kloprogge. [6] fabricated two different morphologies of MgCO3·3H2O, a conglomerate consisted of very thin sheetlets and a well-formed needle, at 298 K. Mitsuhashi. [7] developed a procedure to generate needle-like MgCO3·3H2O and microtube Mg5(CO3)4(OH)2·4H2O by the carbonation of an aqueous suspension of magnesium hydroxide with carbon dioxide in the temperature range between 308 and 343 K. Wang. [8] prepared needle-like MgCO3·3H2O by the reaction of MgCl2with (NH4)2CO3in supersaturated solutions. More recently, ATR-FTIR and Raman spectroscopy were employed to monitor the precipitation by mixing Na2CO3solutions in equilibrium with a CO2atmosphere with MgCl2solutions, and MgCO3was obtained in autoclave [9]. It seems that the investigations in the effect of temperature in a wide range on the morphology, size and characteristic of magnesium carbonate hydrates are very limited.
In this study, homogeneous (unseeded) precipitation by the reaction of MgCl2with Na2CO3in supersaturated solutions is investigated in the range of 273-363 K. The temperatures are chosen in such a way that it favors the operation and energy saving. The composition, morphology and filtration characteristics of the obtained magnesium carbonate hydrates are studied in detail. The aim of the present work is to select proper temperatures and target precipitates with excellent filtration characteristics for extracting magnesium from the brine, and prepare magnesium carbonate hydrates and magnesium oxide with high purity.
2 EXPERIMENTAL
2.1 Materials
All chemical reagents, MgCl2·6H2O, Na2CO3and KCl, used in the experiments were analytical grade without further purification. The water used in all experimental work for solution preparation, dilution, crystal washing,. was double distilled water (conductivity<0.1 μS·cm-1).
2.2 Experimental procedures
2.2.1
The experiments were performed in a 1-liter double-jacketed glass reactor connected with a water circulator as shown in Fig. 1. A standard volume (400 ml) of MgCl2(0.5 mol·L-1) located in the reactor was brought to a desired temperature with the aid of the circular water. Upon attainment of the temperature, addition of Na2CO3solution (400 ml, 0.5 mol·L-1) was started with simultaneous initiation of stirring at 300 r·min-1and the addition speed was 3 ml·min-1. When the addition procedure was completed, stirring continued for 2 h.
At the end of each cycle the slurry was divided into two parts. One part was filtered and then washed with distilled water for 3 times, to remove any possible ionic remnants, and finally dried in an oven at 323 K for 10 h. A small sample from the dried magnesium carbonate hydrates was subjected to solid analysis. The other part was used for the measurement of filtration characteristics. A small sample from the slurry was used to determine the particle size distribution.

Figure 1 Experimental setup used in the precipitation process 1—reactor; 2—circular water; 3—thermometer; 4—pH meter; 5—motor-drive; 6—2-blade radial impeller; 7—4 baffles; 8—burette
2.2.2
Magnesium oxide (MgO) is an important material for its wide applications in catalyst, toxic waste remediation, refractory material, superconductor products [10], and so on. Many techniques have been established to prepare MgO in various forms, including chemical-vapor-deposition [11], sol-gel processing [12],. However, MgO can be obtained more easily by calcinating their corresponding precursors (magnesium carbonate hydrates) in muffle stove at 1073 K for 4 h [13, 14]. We prepared MgO samples in this way.
2.2.3
Filtration characteristic of the precipitate was measured immediately after the crystallization to avoid any change of properties due to the change of temperature. The filtration was carried out using a press filter under 80 kPa and three pieces of filter paper (10-20mm) of 9 cm diameter. A certain volume (100 ml) slurry was placed in the filter and sealed tightly immediately. Upon bringing the pressure to 80 kPa in the filter and the liquid discharging, the timer started. When the flow of the liquid stopped, the filtration time was recorded.
2.2.4Measurement of the uptake of K and Na in magnesium carbonate hydrates
The uptake of K+and Na+in magnesium carbonate hydrates was investigated by the reaction in a mixture of 98% MgCl2and 2% KCl (the total volume was 400 ml, and the total concentration was 0.5 mol·L-1) and Na2CO3(400 ml, 0.5 mol·L-1) at 313 K and 353 K. After the slurry was finally dried in an oven at 323 K for 10 h, a sample from the dried magnesium carbonate hydrates was subjected to solid analysis.
2.3 Characterization
The structure and morphology of the synthesized samples were examined using X-ray powder diffraction and scanning electron microscopy. X-ray powder diffraction (XRD, X’Pert PRO MPD, PANalytical, Netherlands) patterns were recorded on a diffractometer (using Cu Kαradiation) operating at 40 kV/30 mA. A scanning rate of 0.02(°)·s-1was applied to record the patterns in the 2angle range from 5° to 90°. The morphology and particle size of the samples were examined by a scanning electron microscopy (SEM, JEOL-JSM-6700F). Particle size distribution was examined by a laser diffraction particle size analyzer (LS-13-320). The uptake of K+and Na+was examined by a flam photometer (FP640). The concentration of magnesium ion in the solution was determined by titration method using standard EDTA solution.
3 RESULTS AND DISCUSSION
3.1 XRD observation
It is generally recognized that the properties of crystals are profoundly influenced by the temperature during their preparation, and at higher temperatures crystal growth occurs with a corresponding change in the properties [15]. The magnesium carbonate solids in an equilibrium solution may transform to other phases at different temperatures [16]. For example, MgCO3·3H2O will easily change to Mg5(CO3)4(OH)2·4H2O at 333K [8]. In this investigation the compositions of magnesium carbonate hydrates prepared between 273 and 363 K were measured by means of X-rays.
At lower temperature (273-313K), all peaks in the XRD pattern of the sample are in good agreement with the MgCO3·3H2O reference data [17] (JCPDS 70-1433, a-d in Fig. 2). It is evident from Figs. 3 and 4 that 323 K results in the formation of Mg5(CO3)4(OH)2·5H2O, while 333-363 K leads to the formation of hydromagnesite [Mg5(CO3)4(OH)2·4H2O].
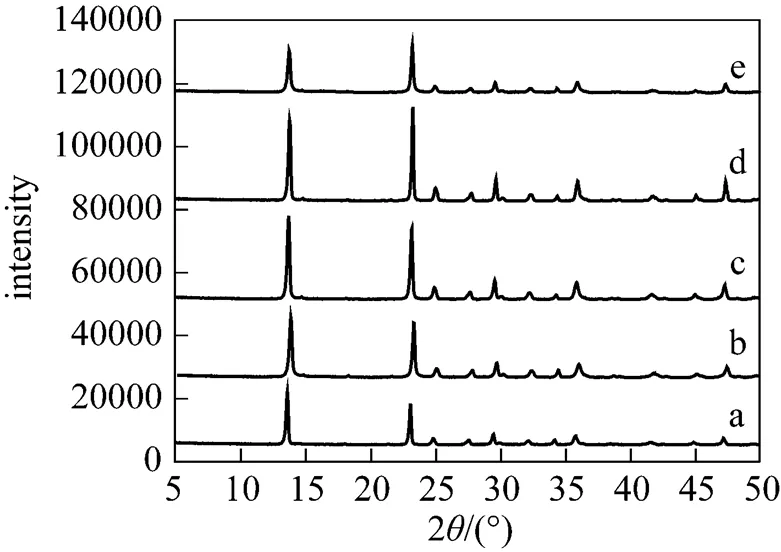
Figure 2 Influence of temperature on XRD pattern of precipitates (a) 273 K; (b) 283 K; (c) 293 K; (d) 303 K; (e) 313 K
Figure 3 XRD pattern of precipitate at 323K
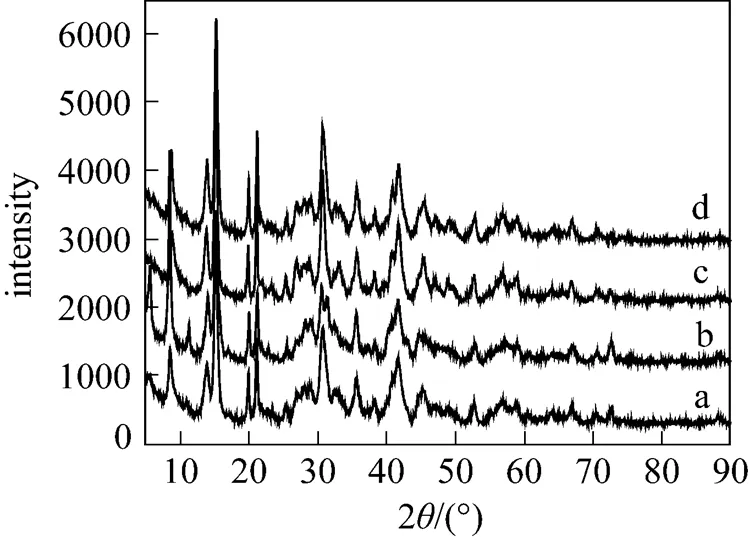
Figure 4 Influence of temperature on XRD pattern of precipitates (a) 333 K; (b) 343 K; (c) 353 K; (d) 363 K
Figure 5 displays the typical XRD pattern of the MgO samples. The diffraction peaks of (111), (200), (220), (331) and (222) are in good agreement with the values in the literature (JCPDS 87-0651). No peaks for other impurities are observed, indicating that the MgO samples are of high purity.

Figure 5 XRD patterns of MgO by calcination of corresponding precursors prepared at different temperatures (a) 273 K; (b) 283 K; (c) 293 K; (d) 303 K; (e) 313 K; (f) 323 K; (g) 333 K; (h) 343 K; (i) 353 K; (j) 363 K
3.2 Effect of reaction temperature on morphology
By carefully adjusting the reaction parameters precipitates can be produced with either the well-formed, relatively large crystallites or, at the other extreme, amorphous or even gel-like products [18]. Therefore, it is important to investigate the effect of reaction temperature on the size and morphology of magnesium carbonate hydrates prepared.
Figure 6 provides a set of typical SEM images corresponding to the magnesium carbonate hydrates prepared at different temperatures. These morphologies are drastically changed with the variation of reaction temperature. At 273 K, the sample exhibits poor morphology and the surface is covered by many small grain-like crystals [Figs. 6 (a) and (b)]. In the temperature range from 283 to 313 K, needle-like particles are produced and the sizes vary with the reaction temperature [Figs. 6 (c)-(f)]. In the range of 283-293 K, the sizes are non-uniform, the length is in the range of 5-20mm and the axis diameter is in the range of 4-6mm. With the increase of reaction temperature to 303 K, the axis diameter of some particles decreases to 1-2mm. However, further increasing the temperature to 313 K, the length and the axis diameter increase slightly, to 30-50mm, and 3-6mm, respectively. The reason may be that at lower reaction temperature (such as 273-293 K), the nuclei has a lower diffusion rate due to the higher viscosity of initial solution, which greatly hinders their coalescence and self-assembly into needle-like particles. As a result, the growth rate of the nuclei is higher than the nucleation rate, and the particle grows to a larger one. With the increase of temperature (such as 303 K), the viscosity of the solution gradually decreases, which accelerates the collision rate of the nuclei. A higher collision rate brings about more nucleated particles, so it is prone to produce smaller particles. When the temperature reaches 313 K, the higher collision rate may also contribute to an increase in the probability of coalescence, and the particle size increases slightly [19].
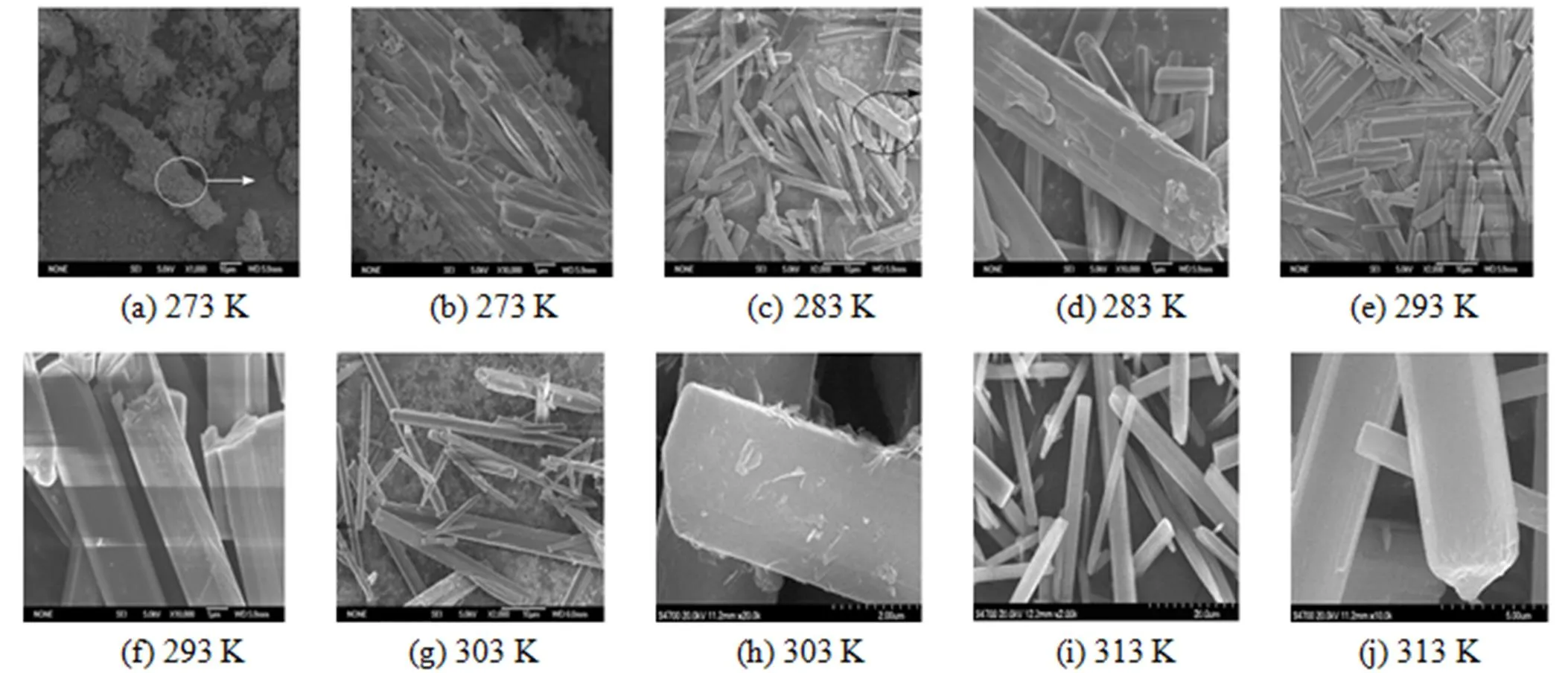
Figure 6 Typical SEM morphologies for the particles of magnesium carbonate hydrates prepared at different temperatures [(b), (d), (f), (h) and (j) are the magnification image of (a), (c), (e), (g) and (i), respectively]
Figure 7 Typical SEM morphologies for the particles of magnesium carbonate hydrates prepared at different temperatures [(b), (d), (f), (h) and (j) are the magnification image of (a), (c), (e), (g) and (i), respectively]
The needle-like particles shown in Fig. 6 indicate that the surface structures of crystals change with the reaction temperature. The surface of the needle-like particles obtained at lower temperature (283 K) is covered by some small rod-like particles. At the reaction temperature of 293 and 303 K, the surfaces are still covered by small lamellar shape crystals. However, the needle-like particles exhibit smooth surfaces at 313 K.
At the temperature range of 323 to 363 K, the morphologies of the particles change greatly. As can be seen in Figs. 7 (a)-(d), the needle-like particles transform to micro-tubes and consist of many sheet-like particles at 323 K, while they transfer to amorphous at 333 K. Above 343 K, the irregular spherical particles are obtained [Figs. 7 (e)-(j)]. They consist of rosette-like microstructure of irregular- shaped pores with crystalline walls interconnecting to each other. The disperse degree and average size of the spherical particles increase with reaction temperature. It is well known that the morphologies of crystals are determined by the anisotropy of growth rates in different crystallographic directions [20]. Various surface structures in Figs. 6 and 7 illustrate that the reaction temperature has a significant influence on the anisotropy of growth rates, so the particles display different macroscopic shapes with the variation of reaction temperature.
3.3 Effect of reaction temperature on the filtration characteristics
Solid-liquid separation by precipitates (crystal, coagulation and flocculation) is an important part in many technical processes [21]. The filtration technique is the most common process used in solid-liquid slurries or mixtures. In this study, the solid and liquid are separated by filtration. The method is stated in Section 2.2.3. For this process to be successful the magnesium carbonate hydrates should have good filtration properties to facilitate removal of the mother liquor.
It is well know that the forms and properties of particles are closely related. When the form of particle changes, the properties are also altered even for the same substance [22]. Therefore, it is important to measure the filtration characteristics of magnesium carbonate hydrates obtained at different temperatures.
Figure 8 displays the filtration time of the precipitates obtained in the range of 273-363 K. It is found that the filtration characteristics of crystals are dependent on their size and surface smoothness. It is easier to filter larger crystals than small ones and to filter smooth crystals than coarse ones. At lower temperature (273 K), the slurry is very thick and adheres to the reactor wall. It is more difficult to filter the samples of this slurry although the crystal size is large. From 273 to 283 K, the filtration time has a drastic decrease. The reason is that the transformation of crystal from poor morphology to needle-like one reduces the filtration resistance. In the range of 283-303 K, the sizes of crystals are non-uniform and the surfaces are rough, so that the filtration characteristics of crystals are still not good though the crystals are needle-like. However, at 313 K, the crystal has its well-defined needle-like structure (large size and smooth surface) and the filtration is satisfactory.
At 323 and 333 K, the slurries become a little sluggish and difficult to filter. Crystals with micro-tube or amorphous morphologies exhibit bad filtration characteristics. Above 343 K, the filtration time is short and decreases with the increase of crystal size. This indicates that crystals present as spherical particles also have good filtration characteristics.
Temperature has an important influence on the size and morphology of crystals, and it indirectly affects the filtration characteristics of the crystal. Compared of Fig. 8 with Figs. 6 and 7, it can be found that crystals with good morphologies and regular size distribution exhibit good filtration characteristics. It suggests that magnesium carbonate hydrates prepared at 313 K and in the range of 343-363 K are well suited to filtration.
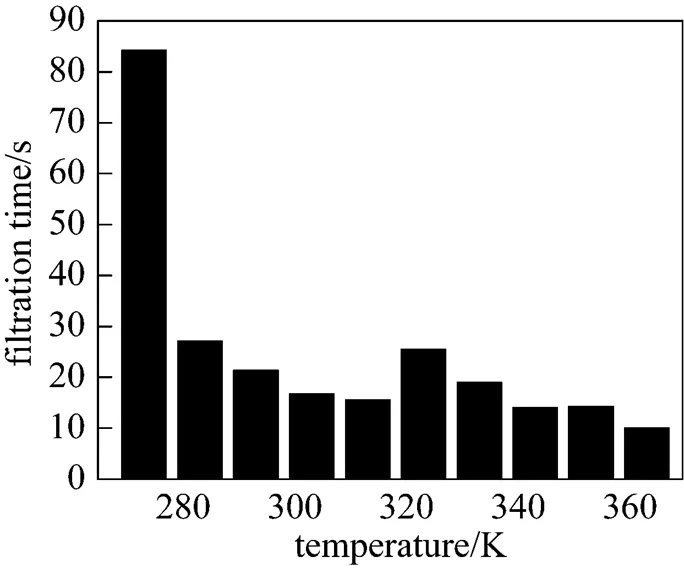
Figure 8 Influence of reaction temperature on the filtration time of magnesium carbonate hydrates
3.4 Purity of MgO
A certain mount of MgO sample is dissolved in slightly excessive hydrochloric acid of low concentration. The concentration of magnesium ion in the solution is determined by titration method using standard EDTA solution. Table 1 provides the purity of MgO by calcination of the corresponding magnesium carbonate hydrates precursors obtained at different reaction temperatures. The purities of MgO are high.
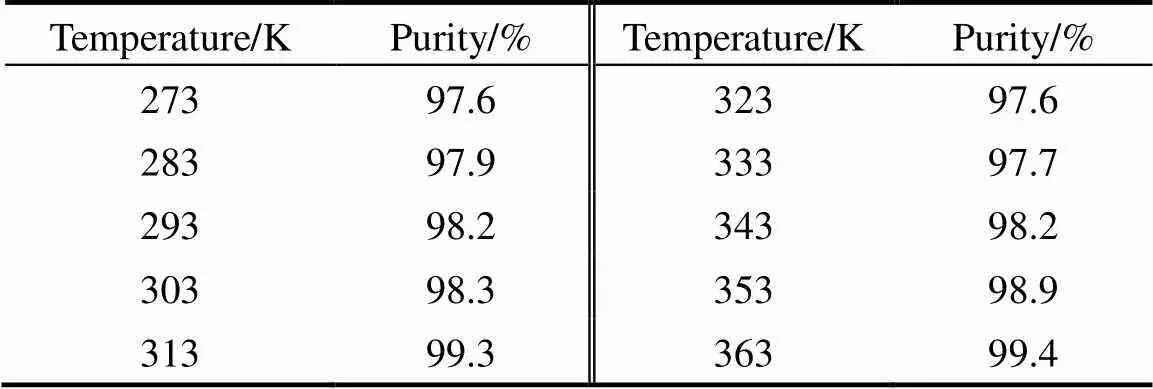
Table 1 The purities of MgO samples prepared at different reaction temperatures

Table 2 Uptake of K+ and Na+ in magnesium carbonate hydrates
3.5 Uptake of impurities (K+ and Na+) in magnesium carbonate hydrates
Extraction of magnesium from the brine relies upon the physical difference between the magnesium carbonate hydrates and the impurities to facilitate separation. However, when the impurity is bound into the structure of the magnesium carbonate hydrates crystal, such as K+existed in the brine and Na+imputed to the precipitate Na2CO3, the separation can only be achieved by chemical means. Therefore, it is necessary to investigate the uptake of impurities in magnesium carbonate hydrates.
Table 2 provides the content of K+(based on 100 g magnesium carbonate hydrates) and Na+(based on 100 g magnesium carbonate hydrates) in the magnesium carbonate hydrates prepared at 313 K and 353 K. The uptake of K+and Na+by magnesium carbonate hydrates is slight and can be neglected. Obviously, the ionic impurities can be removed from the magnesium carbonate hydrates by washing with distilled water without further disposal.
4 CONCLUSIONS
Magnesium carbonate hydrates were prepared usinghomogeneous precipitation process at different reaction temperatures. In the range of 273-313K, nesquehonite (MgCO3·3H2O) with needle-like morphology was obtained. Mg5(CO3)4(OH)2·5H2O was formed at 323 K, while hydromagnesite [Mg5(CO3)4(OH)2·4H2O] was formed over the range of 333-363 K. The morphology of the magnesium carbonate hydrates changed from micro-tube to amorphous, and eventually to spherical-like particles with the increase of reaction temperature. The magnesium carbonate hydrates obtained at 313 K and in the range of 343-363 K showed good morphologies and filtration characteristics. The ionic impurities (K+and Na+) could be removed from the magnesium carbonate hydrates by washing with distilled water without further disposal and their uptake in magnesium carbonate hydrates could be neglected. MgO with high purity was obtained by calcining magnesium carbonate hydrates at 1073 K.
It seems that 313 K and 343-363 K were the appropriate reaction temperatures for extracting magnesium from brine. By the method in this study, magnesium can be effectively extracted from the brine for utilization of MgCl2, and magnesium carbonate hydrates with various morphologies and magnesium oxide with high purity are produced.
1 Zhang, P.X., Zhang, B.Z., Tang, Y., Yang, C.D., Huang, S.Q., Wu, J.Q., Saline Lake Resources of China and Its Exploitation, Science Press, Beijing, 99-107 (1999). (in Chinese)
2 Ma, P., “Comprehensive utilization of salt lake resources”,..., 15, 365-375 (2000).
3 Mullin, J.W., Crystallization, 4th edition, Butterworth-Heinemann, Woburn, MA (2001).
4 Freitag, F., Kleinebudde, P., “How do roll compaction/dry granulation affect the tableting behaviour of inorganic materials? Comparison of four magnesium carbonates”,...., 19, 281-289 (2003).
5 Botha, A., Strydom, C.A., “Preparation of a magnesium hydroxy carbonate from magnesium hydroxide”,, 62, 175-183 (2001).
6 Kloprogge, J.T., Martens, W.N., Nothdurft, L., Duong, L.V., Webb, G.E., “Low temperature synthesis and characterisation of nesquehonite”,...., 22, 825-829 (2003).
7 Mitsuhashi, K., Tagami, N., Tanabe, K., Ohkubo, T., Sakai, H., Koishi, M., Abe, M., “Synthesis of microtubes with a surface of ‘house of cards’ structureneedlelike particles and control of their pore size”,, 21, 3659-3663 (2005).
8 Wang, Y., Li, Z.B., Demopoulos, G.P., “Controlled precipitation of nesquehonite by the reaction of MgCl2with (NH4)2CO3at 303 K”,..., 310, 1220-1227 (2007).
9 Hachen, M., Prigiobbe, V., Baciocchi, R., Mazzotti, M., “Precipitation in the Mg-carbonate system-effects of temperature and CO2pressure”,..., 63, 1012-1028 (2008).
10 Zhao, Y.N., Zhu, G.C., “Synthesis of MgO microspheres with nanosheets in a mechanical force reactor and its optical property”,..., 142, 93-97 (2007).
11 Tokita, S., Ohshio, H., Saitoh, H., “Large-area film structure consisted by aggregation of zinc oxide micro-whiskers”,....., 749, 349-354 (2003).
12 Kordas, G., “Sol-gel preparation of MgO fibers”,..., 10, 1157-1160 (2000).
13 Lanas, J., Alvarez, J.I., “Dolomitic lime: thermal decomposition of nesquehonite”,.., 421, 123-132 (2004).
14 Yan, C.L., Xue, D.F., “Novel self-assembled MgO nanosheet and its precursors”,..., 109, 12358-12361 (2005).
15 Boswell, M.C., Iler, R.K., “Nickel catalysts (I) The effect of the temperature of preparation on the crystal size and composition of nickel oxide”,...., 58, 924-928 (1936).
16 Li, Z.B., Demopoulos, G.P., “Model-based construction of calcium sulfate phase-transition diagrams in the HCl-CaCl2-H2O system between 0 and 100oC”,...., 45, 4517-4524 (2006).
17 Giester, G., Lengauer, C.L., Rieck, B., “The crystal structure of nesquehonite, MgCO3·3H2O, from Lavrion, Greece”,.., 70, 153-163 (2000).
18 Waltion, A.G., The Formation and Properties of Precipitates, Interscience Publishers, New York (1967).
19 Zhang, Z.P., Zheng, Y.J., Ni, Y.W., Liu, Z.M., Chen, J.P., Liang, X.M., “Temperature- and pH-dependent morphology and FT-IR analysis of magnesium carbonate hydrates”,..., 110, 12969-12973 (2006).
20 Liang, J.M., Ma, Y., Zheng, Y., Davis, H.T., “Solvent-induced crystal morphology transformation in a ternary soap system: sodium stearate crystalline fibers and platelets”,, 17, 6447-6454 (2001).
21 Schwarz, S., Jaeger, W., Paulke, B.R., “Cationic flocculants carrying hydrophobic functionalities: Applications for solid/liquid separation”,..., 111, 8649-8654 (2007).
22 Ohkubo, T., Suzuki, S., Mitsuhashi, K., “Preparation of petaloid microspheres of basic magnesium carbonate”,, 23, 5872-5874 (2007).
2008-11-28,
2009-02-28.
the National Natural Science Foundation of China (20876161) and the National Basic Research Program of China (2007CB613501, 2009CB219904).
** To whom correspondence should be addressed. E-mail: zhibaoli@home.ipe.ac.cn
 Chinese Journal of Chemical Engineering2009年4期
Chinese Journal of Chemical Engineering2009年4期
- Chinese Journal of Chemical Engineering的其它文章
- Removal of Uranium (VI) by Fixed Bed Ion-exchange Column Using Natural Zeolite Coated with Manganese Oxide*
- Phase Equilibrium of Isobutanol in Supercritical CO2
- Conversion of Methane by Steam Reforming Using Dielectric-barrier Discharge*
- Permeability and Selectivity of Sulfur Dioxide and Carbon Dioxide in Supported Ionic Liquid Membranes*
- Hydroxyapatite Coatings on Titanium Prepared by Electrodeposition in a Modified Simulated Body Fluid*
- Model Study on a Submerged Catalysis/Membrane Filtration System for Phenol Hydroxylation Catalyzed by TS-1*
