Carbon isotope ratios of n-alkanoic acids:new organic proxies for paleo-productivity in Antarctic ponds
CHEN Xin, JIN Jing, NIE Yaguang, ZHANG Jifeng, DONG Liang, HUANG Xianyu, Steven D. EMSLIE & LIU Xiaodong*
Article
Carbon isotope ratios of-alkanoic acids:new organic proxies for paleo-productivity in Antarctic ponds
CHEN Xin1,2,3*, JIN Jing1, NIE Yaguang4, ZHANG Jifeng5, DONG Liang2,3,6, HUANG Xianyu7, Steven D. EMSLIE8& LIU Xiaodong1*
1Anhui Province Key Laboratory of Polar Environment and Global Change, School of Earth and Space Sciences, University of Science and Technology of China, Hefei 230026, China;2School of Oceanography, Shanghai Jiao Tong University, Shanghai 200030, China;3Key Laboratory for Polar Ecosystem and Climate Change of Ministry of Education, School of Oceanography, Shanghai Jiao Tong University, Shanghai 200030, China;4Information Materials and Intelligent Sensing Laboratory of Anhui Province, Institutes of Physical Science and Information Technology, Anhui University, Hefei 230601, China;5Research Center for Ecology, College of Science, Tibet University, Lhasa 850000, China;6Key Laboratory of Polar Science, MNR, Polar Research Institute of China, Shanghai 200136, China;7Hubei Key Laboratory of Critical Zone Evolution, School of Geography and Information Engineering, China University of Geosciences, Wuhan 430078, China;8Department of Biology and Marine Biology, University of North Carolina, Wilmington, NC 28403, USA
Primary productivity in the Antarctic aquatic environment with simple ecosystems is sensitive to climate and environmental fluctuations. We investigated13C values for-alkanoic acids derived from phototrophic organisms in a lacustrine sediment core (IIL3) to indicate primary productivity in ponds on Inexpressible Island in the western Ross Sea, Antarctica. Short-chain-alkanoic acids (C14–C18) were abundant in the IIL3 sediment profile. The carbon isotope ratios of short-chain-alkanoic acids in the sediment samples and floating microbial mats were similar, indicating that the short-chain-alkanoic acids in the IIL3 sediment profile predominantly originated from phototrophic organisms. The13C values for the short-chain-alkanoic acids varied widely through the sediment profile, and13C-enrichment of-alkanoic acids was most likely related to high productivity due to carbon-limited conditions caused by enhanced photosynthetic efficiency. The13C values for the-alkanoic acids changed over the past 3200 years in similar ways to organic proxies for aquatic productivity (-alkanoic acid and sterol sedimentary fluxes). C16-alkanoic acid was enriched in13C in periods of high aquatic productivity ~750–1650 and 3000–3200 a BP but depleted in13C in periods of relatively low productivity ~150–600 and 2500–3000 a BP. The results indicated that carbon isotope ratios of lipids from phototrophic organisms could be used as new proxies to reconstruct paleo-productivity in Antarctic lakes and ponds and therefore improve our understanding of past climate changes.
East Antarctica, lipid biomarkers, carbon isotopic ratios, pond primary productivity, climate change
1 Introduction
Antarctica is an ideal place for investigating past climate and environmental changes because of its high latitude, uncomplicated ecosystems, and sensitivity to global climate fluctuations (Lee et al., 2017; Stenni et al., 2017; Giralt et al., 2020). There are numerous and widely distributed lakes and ponds in ice-free regions of Antarctica, and such water bodies can preserve terrestrial and biological information at a relatively high resolution in an easily datable form (Sun et al., 2000; Giralt et al., 2020). Lacustrine sediment has already been found to be suitable for improving our understanding of local and regional paleo-ecology and paleo-climate changes in Antarctica (Sun et al., 2000; Jin et al., 2021; Strugnell et al., 2022).
Aquatic ecosystem structures and primary productivity can quickly respond to regional climate and environmental changes, and various proxies have been used to investigate past changes in lake and pond productivity in Antarctica (Hodgson et al., 2005; Borghini et al., 2016; Mahesh et al., 2019). For example, the carbon isotope composition of bulk organic matter (13COM) has been used widely to determine changes in aquatic primary productivity (Meyers, 1997),13C-enriched organic matter indicating more productivity (Bird et al., 1991; Wei et al., 2016; Jin et al., 2021). However, organic matter in Antarctic lacustrine sediment generally has complex sources (Carrizo et al., 2019; Chen et al., 2020, 2021), including animal feces, terrestrial plant matter, and aquatic algae and bacteria, which have different carbon isotope compositions. Casta?eda and Schouten (2011) found that various environmental factors (e.g., the growth rate, nitrogen supply, pH, salinity, and temperature) strongly affect the13C values of organic matter in phototrophic organisms. Chlorophyll(Chl), which is produced by phototrophs, has been used many times as an organic proxy for aquatic primary productivity in the past half century (Leavitt and Hodgson, 2001; Chen et al., 2015). However, post-deposition degradation can lead to bias when Chlis used to reconstruct historical changes in primary productivity in lakes and ponds in some environments. For example, it has been found that intact Chlcould be partly degraded in sediment in “shallow” lakes with low accumulation rates and oxidizing conditions in Antarctica (Squier et al., 2004; Hodgson et al., 2005; Roberts et al., 2006). It can be appreciated from the information presented above that using13COMand Chlas proxies to allow aquatic primary productivity records for Antarctica to be reconstructed is complicated. Confounding effects of various factors in certain sedimentary environments need to be eliminated. Other relatively reliable proxies for aquatic primary productivity in lakes and ponds in Antarctica should be developed to allow historical primary productivity to be reliably reconstructed.
Lipid biomarkers (e.g.,-alkanoic acids) are ubiquitous in lacustrine sediments, and compound-specific carbon and hydrogen isotopes have been used as accurate proxies in paleo-climate and paleo-ecological studies for several decades (Sachse et al., 2012; Sessions, 2016; Huang and Meyers, 2019; Chen et al., 2022, 2023).-Alkyl lipids with different chain lengths have more specific source organisms than bulk organic matter, and-alkyl lipids in sediment are more resistant than bulk organic matter to biological and abiotic degradation processes. The13C values of-alkyl lipids are not markedly changed by even moderate degradation (Huang et al., 1997; Li et al., 2017). The concentrations and carbon isotope compositions of-alkyl lipids derived from phototrophic algae may therefore be used as indicators to investigate historical changes in primary productivity in lakes (Aichner et al., 2010a; Casta?eda and Schouten, 2011). The underlying principle is that photosynthesis by algae uses12CO2in preference to13CO2. However, algae will assimilate more13C-enriched dissolved CO2as the photosynthetic efficiency increases, resulting in lipids with relatively high13C values being synthesized (Bird et al., 1991; Wei et al., 2016; Mahesh et al., 2019; Ashley et al., 2021). The13C values of mid-chain-length-alkanes derived from aquatic macrophytes have been successfully used as indicators of carbon limitation to assess historic primary productivity in lakes on the Qinghai-Xizang Plateau (Aichner et al., 2010a, 2010b). Using compound-specific-alkane carbon isotope data to assess paleo-productivity offers the advantages that the sources of lipids can be traced to particular organisms and-alkanes are preserved exceptionally well in sediment. However, no study of whether lipid carbon isotopes can be used to investigate historic primary productivity in Antarctic lakes and ponds has been performed.
Vascular plants are absent from the western Ross Sea region in Antarctica, and moss and lichen are only found in slightly wet areas. Abundant microbial mats (containing algae and cyanobacteria as the primary photoautotrophic organisms) have been found in lakes and ponds on Inexpressible Island in North Victoria Land in the western Ross Sea (Fumanti et al., 1997). In previous studies we found that short-chain-alkanoic acids in sediment from surface lakes and ponds on Inexpressible Island are predominantly derived from phototrophic organisms (Chen et al., 2019, 2021). Antarctic lakes and ponds with abundant microbial mats are therefore ideal for determining carbon isotope signals of-alkanoic acids in sediment profiles. In the study described here, we analyzed the distributions and compound-specific carbon isotope ratios of-alkanoic acids in a lacustrine sediment profile (IIL3) from Inexpressible Island. We assessed the possibility of using the13C values of short-chain-alkanoic acids in the lacustrine sediment profile to reconstruct historical changes in primary productivity. We believe that the results indicate that short-chain-alkanoic acids can be used as new organic indicators to investigate historic primary productivity in lakes and ponds and therefore to investigate climate change in Antarctica.
2 Materials and methods
2.1 Study site and sampling
The study site was described in previous publications (Chen et al., 2019, 2021). Briefly, the IIL3 sediment core was collected from a small pond on Inexpressible Island, North Victoria Land, western Ross Sea, Antarctica. The location of the sampling site is shown in Figure 1. Inexpressible Island has an extreme climate with strong katabatic winds, low air temperatures, and little precipitation because of air masses coming from the Ross Sea, Ross Ice Shelf, and Victoria Land (Monaghan et al., 2005). Data from meteorological stations on Inexpressible Island indicate that the mean annual temperature is ?16.1 °C, the maximum temperature in the austral summer is 5.4 °C, and the minimum temperature in the austral winter is ?39.3 °C. The mean relative humidity is 42.1% (Ding et al., 2015). All of the lakes and ponds on Inexpressible Island are completely covered with ice in winter but most were ice free when the core was collected in summer. No visible outlets were observed for most of lakes and ponds in the study area during our field investigations, and water is lost mainly through evaporation (Michaud et al., 2012). We previously found that lakes and ponds with no ice cover when the core was collected had relatively high water isotope values and salinities because of strong evaporation (Chen et al., 2021). Abundant floating microbial mats containing cyanobacteria and algae are the primary phototrophic organisms in the lakes and ponds. Vascular plants are absent from Inexpressible Island, moss and lichen are also very rare arounding the study area.
Figure 1 Map showing the study area. Location of Inexpressible Island, Ross Sea, Antarctica (a and b). c, Location of the pond on Inexpressible Island that the lacustrine sediment profile IIL3 was collected from (redrawn from Jin et al. (2021)).
The IIL3 sediment core was collected using a poly(vinyl chloride) pipe ~12 cm in diameter that was hammered into the sediment. The IIL3 core was 54 cm long. Subsamples were collected at 0.5 cm intervals along the core. The samples were stored at ?20 °C until they were analyzed. Field observations and geochemical analyses indicated that the IIL3 sediment core did not contain appreciable amounts of animal feces (Wei et al., 2016; Jin et al., 2021). The IIL3 sediment profile chronology was determined by accelerator mass spectrometry14C dating of bulk organic matter, and the results were reported in a previous publication (Jin et al. 2021). An age–depth model based on the SHCal13 Southern Hemisphere terrestrial source calibration was established, and the inferred age of the bottom section of the IIL3 profile was 3179 a BP (Jin et al., 2021).
2.2 Sample analysis
The procedure used to analyze n-alkanoic acids and sterols was described in detail by Chen et al. (2019). Briefly, total lipids were extracted from a ~1 g aliquot of a sediment sample by accelerated solvent extraction (using an Dionex ASE 200 instrument; Thermo Fisher Scientific, Waltham, MA, USA) using a 9∶1 (volume ratio) mixture of dichloromethane and methanol. The extract was passed through an aminopropylsilyl gel column and then an acid fraction was eluted using 4% acetic acid in ether and a neutral fraction was eluted using a 2∶1 (volume ratio) mixture of dichloromethane and isopropanol. The neutral fraction was then passed through a silica gel column, which was eluted with hexane (fraction N1), dichloromethane (fraction N2), a 3∶1 (volume ratio) mixture of hexane and ethyl acetate (fraction N3), and methanol (fraction N4). Fraction N3 was silylated using,-bis (trimethylsilyl) trifluoroacetamide before instrumental analysis. Fatty acids were methylated using anhydrous 5% HCl in methanol at 60 °C overnight. The methyl esters of carboxylic acids were purified by passing the extract through a silica gel flash column, which was eluted using dichloromethane. Fatty acid methyl esters and sterols were determined using an Agilent 6890+ gas chromatograph with flame ionization detection (Agilent Technologies, Santa Clara, CA, USA) with a DB-1 column (30 m long, 320 μm inner diameter, 0.17 μm film thickness; Agilent Technologies). The compounds of interest were identified using an Agilent 6890 gas chromatograph with an Agilent 5973N quadrupole mass spectrometer (Agilent Technologies). Hexamethylbenzene and 5α-cholestane were added to the fatty acid methyl ester and sterol fractions to allow the concentrations to be calculated from the proportional relationships between the peak areas of the biomarkers. Sedimentary lipid fluxes were calculated using the equation sedimentary lipid flux (μg·cm?2·a?1) = concentration (μg·g?1) × sedimentation rate (cm·a?1) × dry bulk density (g·cm?3). The sedimentation rate and dry bulk density were taken from a previous publication (Jin et al., 2021).
The carbon isotope compositions of methylated-alkanoic acids were determined using an HP 6890 gas chromatograph (Agilent Technologies, Santa Clara, CA, USA) connected through a combustion reactor to a Thermo Finnigan Delta V plus isotope ratio mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) (Thomas et al., 2014). External standards (C16, C18, C22, C24, and C28fatty acid methyl esters) with predetermined carbon isotope ratios were analyzed after every sixth sample injection to monitor instrument stability (Gao et al., 2011; Chen et al., 2019). The13C values for the individualalkanoic acids were corrected for the carbon isotopes in the methyl group added during methylation (Tierney et al., 2010). Carbon isotope values were expressed in units of per mil (‰) relative to Vienna Pee Dee Belemnite. The mean standard deviation for duplicate analyses of the extracts was <0.4‰.
Organic carbon isotope analysis of the samples treated with 1 mol?L?1HCl was performed using the sealed tube combustion method (Wei et al., 2016). The analytical precision (standard deviation) of the organic carbon isotope measurements was better than ±0.1‰. The13CTOCvalues for the IIL3 sediment core were reported previously by Jin et al. (2021).
3 Results
3.1 Lipid distribution patterns and concentrations in the sediment profile
The-alkanoic acid distribution in the surface sediment (the top 6 cm) in the IIL3 core was described previously (Chen et al., 2019). The-alkanoic acids were dominated by C16and C24, and compound-specific carbon and hydrogen isotope analyses indicated that they were predominately derived from phototrophs and heterotrophs, respectively (Chen et al., 2019, 2021). We mainly focused on-alkanoic acids with carbon chain lengths of C14–C18derived from phototrophic organisms and therefore did not consider mid-and long-chain-alkanoic acids (C22–C30) derived from heterotrophic microbes. The short-chain-alkanoic acid concentrations varied markedly through the IIL3 sediment profile (Table S1). The total short-chain (C14–C18)-alkanoic acid concentrations in the IIL3 core were 8.46–63.44 μg·g?1(Table S1).
Three sterols (cholesterol, sitosterol, and stigmasterol) were found in the IIL3 sediment profile (Table S2). Cholesterol was the most abundant sterol, and was found at concentrations of 5.40–27.97 μg·g?1. The sitosterol concentrations were 4.28–14.94 μg·g?1and the stigmasterol concentrations were 2.42–9.01 μg·g?1.
3.2 Compound-specific carbon isotopes in the sediment profile
We measured the13C values of-alkanoic acids with even numbers of carbon atoms in the chains. The concentrations of-alkanoic acids with odd numbers of carbon atoms in the chains were too low for accurate carbon isotope measurements to be made. The mean13C values of C18-alkanoic acid (around ?26‰) was slightly lower than C14and C16-alkanoic acids (Table 1 and Figure 2). The13C values for the-alkanoic acids in the IIL3 sediment profile varied (Figure 3). The-alkanoic acid13C values were high for sediment 54–50, 38–20, and 15–8 cm deep and low for sediment 50–40, 20–18, and 10–5 cm deep.
4 Discussion
4.1 Potential sources of the lipids in the sediment
Short-chain-alkanoic acids are usually found in phototrophic organisms such as algae and cyanobacteria (Ficken et al., 2000; Liu and Liu, 2017). Short-chain-alkanoic acids were abundant throughout the IIL3 sediment profile, indicating that these compounds may have been mainly derived from phototrophic organisms (e.g., algae and cyanobacteria) (Chen et al., 2019, 2021). Compound-specific carbon isotope analysis can allow the sources of organic matter in complex sedimentary environments to be identified. The mean13C value for C16-alkanoic acid in the IIL3 sediment core was ?23.5‰, and the mean13C value for the microbial mat samples was similar (Figure 2). Taking the very low concentrations of-alkanoic acids found in soils into consideration (Chen et al., 2019), these results suggest that short-chain- alkanoicacids in the IIL3 sediment profile primarily originated in phototrophic organisms growing in microbial mats. The13C values for the short-chain-alkanoic acids decreased as the carbon chain length increased (Figure 2), suggesting that the chain elongation process resulted in13C depletion (Hayes, 2001; Chikaraishi and Naraoka, 2007).

Table 1 δ13C values for short-chain n-alkanoic acids and total organic matter (TOC) in the IIL3 sediment profile
Note: The13CTOCvalues and radiocarbon dates for the IIL3 sediment samples were taken from Jin et al. (2021).

Figure 2 Mean13C values for short-chain-alkanoic acids in the IIL3 sediment samples. Each error bar indicates the standard deviation for all of the relevant data. The microbial mat, moss, and soil data were taken from Chen et al. (2019).
Figure 3 Variations in13C values for short-chain-alkanoic acids in the IIL3 sediment profile.
Algae in the lakes and ponds on Inexpressible Island have previously been systematically investigated (Fumanti et al., 1997). Bacillariophyta, Chlorophyta, and Cyanophyta were found to be the most important algae in the lakes and ponds.,, andwere the most abundant Cyanophyta, andandwere the most abundant Bacillariophyta. Microalgae contain a wide range of sterols, and Bacillariophyta, Chrysophyta, and Cryptophtya often contain epi-brassicasterol, stigmasterol, and cholesterol, with cholesterol the dominant sterol in chlorophytes (Rampen et al., 2010; Volkman, 2018). Cholesterol, stigmasterol and sitosterol were found throughout the IIL3 sediment profile (Table S2). Very similar results were previously found for phototrophic organisms in microbial mats in lakes and ponds on Inexpressible Island (Fumanti et al., 1997). Short-chain (e.g., C16and C18)-alkanoic acids are often found in phototrophic organisms such as cyanobacteria and microalgae (Grimalt et al., 1991; Dalsgaard et al., 2003). Jungblut et al. (2009) systematically characterized-alkanoic acids in cyanobacteria-dominated microbial mats from the McMurdo Ice Shelf in Antarctica. C16-alkanoic acid was found to have primarily originated in cyanobacteria and microalgae, which dominated the microbial mats in the study area (Jungblut et al., 2009). Our results led us to conclude that short-chain-alkanoic acids in the IIL3 sediment profile predominantly originated in phototrophic organisms, including Bacillariophyta, Chlorophyta, and Cyanophyta.
4.2 Factors affecting the δ13C values of n-alkanoic acids in the IIL3 sediment profile
The13C values for-alkanoic acids in phototrophic algae and cyanobacteria are mainly controlled by the carbon isotope ratios of dissolved inorganic carbon (DIC), post-deposition degradation, and changes in photosynthetic fractionation (p) (Aichner et al., 2010a, 2010b; Casta?eda and Schouten, 2011; Ashley et al., 2021). Here, we will discuss these potential drivers of variations in the13C values of-alkanoic acids in the IIL3 sediment profile separately.
4.2.1 Isotope composition of DIC
The carbon isotope composition of DIC in lakes and ponds assimilated by phototrophic organisms strongly affects the13C values of the lipids produced by the phototrophic organisms. In mainland lakes with sufficient dissolved CO2, phototrophic algae preferentially use12CO2, resulting in the residual CO2becoming enriched in13C. However, CO2in the atmosphere cannot exchange with CO2in the water in Antarctic lakes and ponds at times when the lakes and ponds are covered with ice, and continuing photosynthesis can cause CO2in the lake water to become enriched in13C (Bird et al., 1991; Wei et al., 2016). The IIL3 sediment core was collected from a pond no more than 0.5 m deep (Chen et al., 2019). No ice cover was present during our field study in the austral summer because the air temperature had quickly increased above freezing point (Chen et al., 2021). CO2in the atmosphere can exchange freely with CO2in lake and pond water during the period algae and cyanobacteria grow. The relatively high temperatures and solar radiation levels during the austral summer promote blooms of phototrophic organisms. Increased photosynthesis causes a CO2-diffusion-limited environment to develop because dissolved CO2is rapidly consumed (Bird et al., 1991; Aichner et al., 2010b; Wei et al., 2016; Ashley et al., 2021). Algae and cyanobacteria therefore assimilate a larger proportion of13CO2for photosynthesis, leading to higher13C value of-alkanoic acids.
Bedrock erosion and meltwater can also supply DIC (e.g., bicarbonate and carbonate) to lakes and ponds (Neumann et al., 2004). Bicarbonate assimilated by phototrophs may also cause13C-enrichment in lipids because the13C value is generally higher for bicarbonate than CO2in the atmosphere (Aichner et al., 2010b). Lakes and ponds on Inexpressible Island are mostly at ~pH 8.5 (Fumanti et al., 1997), at which bicarbonate preferentially forms. In a previous study, relatively low bicarbonate concentrations of 0.23–1.58 mEq·L?1were found in lakes and ponds on Inexpressible Island that had not been affected by animal feces (Porcino et al., 2020).13C values of ?0.86‰ to ?0.04‰ have been found for bicarbonate supplied by bedrock and meltwater in the McMurdo Dry Valleys in Antarctica (Neumann et al., 2004). However, the small carbon isotope composition range for bicarbonate cannot have caused the relatively large variations (~6‰) in the13C values of the-alkanoic acids in the IIL3 sediment profile (Figure 3), suggesting that assimilation of bedrock-supplied bicarbonate by phototrophic organisms could be ignored. We therefore concluded that phototrophic organisms in the studied pond all used dissolved CO2supplied by the atmosphere and that bicarbonate supplied by meltwater was not likely to be used by the organisms. According to Antarctic ice core records,13C values for CO2in the atmosphere have varied over time by <0.5‰ (Indermühle et al., 1999), meaning such variations could not have contributed to the relatively large variations in the13C values for the-alkanoic acids in the IIL3 sediment profile. The carbon isotope compositions of DIC and CO2in the atmosphere were therefore not the main factors driving the variations in the13C values for the-alkanoic acids in the sediment profile.
4.2.2 Effects of post-deposition degradation on the13C values of-alkanoic acids
Microbial degradation strongly affects the carbon isotope compositions of-alkanoic acids in sediment. For example, the13C value for C16-alkanoic acid supplied by algae in oxic and anoxic seawater increased by ~4.6‰ during 100 d of incubation (Sun et al., 2004). The-alkanoic acid concentration decreased markedly in two–three months. However, such changes occurred relatively rapidly in sediment incubated at 15 °C but did not match the changes of-alkanoic acid concentration and13C value that we found in the IIL3 sediment profile. For example, the C16-alkanoic acid13C values were higher for sediment from 38–20 and 15–8 cm deep than the top sediment from the core (Figure 3). Similar patterns were found for the concentrations of-alkanoic acids with different chain lengths, indicating that microbial degradation was not the main factor causing the changes in the-alkanoic acid concentrations in the core (Table S1). It has been found in previous studies that decomposition in the field did not markedly affect the carbon isotope compositions of-alkyl lipids (Huang et al., 1997; Li et al., 2017; Yan et al., 2021). The extremely low temperatures on Inexpressible Island are suitable for preserving lipids in lacustrine sediment. We concluded that degradation had negligible effects on the-alkanoic acid13C values.
4.2.3 Changes in species in the past 3200 years
Changes in the biota species present over the past 3200 years could have affected the-alkanoic acid13C values because of different13C/12C fractionations between different species (Crosta et al., 2005). For example, different degrees of carbon isotope fractionation occur in diatoms such as, which are dominant in the open ocean, and species such asthat prefer to occupy sea ice (Popp et al., 1998). We investigated the different types of sterols produced by a small range of phototrophs in the core. Similar changes in the sedimentary fluxes of cholesterol, stigmasterol , and sitosterol were found from the IIL3 sediment profile data (Table S2 and Figure 4), indicating that the abundances of the three dominant phototrophs Bacillariophyta, Chlorophyta, and Cyanophyta changed in synchrony over the past 3200 years. Importantly, the short-chain-alkanoic acid13C difference between Bacillariophyta and Chlorophyta determined in laboratory experiments were <2‰ (Schouten et al., 1998). The13C values for individual short-chain-alkanoic acids in the sediment profile exhibited similar change patterns (Figure 3). The results suggested that the effects of species changes on-alkanoic acid13C values could also be neglected.
4.2.4 Factors controllingp
We assumed thatpwas the main driver of variations inthe-alkanoic acid13C values in the IIL3 sediment profile, considering the information presented above. Thepis controlled by the dissolved CO2concentration in surface water, with small carbon fractionation occurring at low than high dissolved CO2concentrations (Aichner et al., 2010b; Wei et al., 2016; Ashley et al., 2021). The dissolved CO2concentrations in Antarctic lakes and ponds are controlled by various factors including the CO2concentration in the atmosphere, the extent of ice cover (Neumann et al., 2004), and primary productivity (Bird et al., 1991).
As mentioned above, CO2can fully exchange between the atmosphere and the water in ponds on Inexpressible Island in summer because of the lack of ice. Dissolved CO2and CO2in the atmosphere will therefore reach equilibrium quickly. Antarctic ice core records indicate that the CO2concentration in the atmosphere has varied by <10 mg·m–3in the past 3000 years except after the Industrial Revolution (Monnin et al., 2004). The relatively small changes in the CO2concentration in the atmosphere are unlikely to have been the dominant drivers of the large changes in the-alkanoic acid13C values in the sediment core (Ashley et al., 2021). The most likely driver of variations in the-alkanoic acid13C values in the sediment core was primary productivity in the pond.
The carbon isotope compositions of organic matter produced by phototrophic algae and cyanobacteria strongly correlate with the primary productivities of lakes and ponds (Hodgson et al., 2005; Aichner et al., 2010a; Borghini et al., 2016; Mahesh et al., 2019). Phototrophic organisms preferentially assimilate12CO2for photosynthesis, leaving the residual CO2in the water enriched in13C. However, phototrophic organisms assimilate more13CO2under high primary productivity conditions than otherwise, leading to the13C values of the lipids produced by the phototrophic organisms leading to higher13C values in the lipids produced by the phototrophic organisms (Aichner et al., 2010a; Ashley et al., 2021). We previously found that sections of the IIL3 sediment profile containing13C-enriched organic matter matched periods of high primary productivity in the pond because of carbon-limited conditions caused by enhanced photosynthetic activity (Wei et al., 2016; Jin et al., 2021). We therefore suggest that the variations in the C16-alkanoic acid13C values in the IIL3 sediment profile were most likely associated with changes in the dissolved CO2concentrations in the water, driven by primary productivity in the pond.
It has also been concluded that the13C values of lipids in sediment derived from phototrophic organisms can be used to assess primary productivity in high productive lakes and oceans (Aichner et al., 2010a; Casta?eda and Schouten, 2011; Ashley et al., 2021). In a study in which fatty acids derived from phytoplankton were labeled with carbon isotopes it was found that the13C values of fatty acids strongly positively correlated with primary production (Dijkman et al., 2009), suggesting that the13C values of fatty acids can be used to indicate primary aquatic productivity. The13C values of C23-alkanes derived from aquatic macrophytes have been found to be excellent proxies for paleo-productivity in Lake Koucha in the eastern part of the Qinghai-Xizang Plateau (Aichner et al., 2010a). Using13C values of C23-alkanes derived from aquatic macrophytes is particularly effective for very productive lakes and ponds with stable and single phototrophic organism sources of organic matter, such as lakes and ponds in Qinghai-Xizang Plateau and Antarctica.
Figure 4 Primary productivity data for Inexpressible Island in the past 3200 years and air temperature changes determined from an ice core. The air temperature changes were determined from theD values for an ice core from the Talos Dome (10 point running mean) (Mezgec et al., 2017).
4.3 Using n-alkanoic acid δ13C values as proxies for paleo-productivity
Comparison of down core variations in compound- specific carbon isotopes with other proxies also can be used to interpret our records. The carbon isotope compositions of organic matter, and fatty acids and sterols concentration, have been successfully used to reconstruct historic aquatic primary productivity (Meyers, 2003; Hodgson et al., 2005; Casta?eda and Schouten, 2011). In previous studies, we found that the total organic matter13C values for a sediment core strongly correlated with primary productivity for a pond on Inexpressible Island, with13C enrichment indicating high productivity (Wei et al., 2016; Jin et al., 2021). Short-chain-alkanoic acids are biomarkers of aquatic phototrophic organisms, and short-chain-alkanoic acid concentrations can be used to indicate primary productivity of a lake (Huang et al., 1999; Hodgson et al., 2005). Sterols such as cholesterol, sitosterol, and stigmasterol have been found in various types of phytoplankton, including chlorophytes and diatoms (Volkman, 2018). The sediment fluxes of such compounds can also be used to indicate the primary productivity of a lake or pond (Casta?eda and Schouten, 2011). The sediment fluxes of short-chain-alkanoic acids and sterols strongly positively correlated with the C16-alkanoic acid carbon isotope ratios for the sediment profile (Figure 4). C16-alkanoic acid had higher13C values for the period ~750–1650 and 3000–3200 a BP, corresponding to higher sediment fluxes of sterols and short-chain-alkanoic acids, than for the other periods (Figure 4). Under carbon-limited conditions driven by high productivity conditions, dissolved12CO2will quickly be used up by phototrophic organisms, so more13CO2will be assimilated and the13C16values will be higher. In contrast, the C16-alkanoic acid13C values were relatively low for the periods ~150–600 and 2500–3000 a BP. The lower sediment fluxes of sterols and short-chain-alkanoic acids indicated lower productivity during these periods. We assumed that the changes in the C16-alkanoic acid13C values were closely associated with changes in the CO2concentration in the surface water, which is driven by the primary productivity of the lake or pond. The results indicated that the13C values of C16-alkanoic acids associated with phototrophic organisms can be used as new proxies for reconstructing the historic primary productivities of lakes and ponds in Antarctica.
4.4 Potential implications for the paleoclimate of Antarctica
We found that the13C values of-alkanoic acids derived from phototrophic organisms can be used to indicate primary productivity of lakes and ponds in Antarctica. It has been found in a number of previous studies (Bird et al., 1991; B?ttger et al., 1993; Mahesh et al., 2019) that aquatic primary productivity in Antarctica is primarily related to the CO2concentration in the atmosphere, physical diffusion barriers (ice cover), nutrition, and the water temperature. A higher CO2concentration in the atmosphere can promote the growth of algae, which will increase primary productivity in lakes and ponds (Jansson et al., 2012). The pond from which we collected the core is not covered with ice during the austral summer because the air temperature is relatively high (Chen et al., 2021). This means that CO2in the atmosphere can mix readily with the pond water. The small variations in the CO2concentration in the atmosphere reconstructed from Antarctic ice cores (< 10 mg·m–3over the past 3200 years (Monnin et al., 2004)) indicate that such variations were not the main drivers of variations in primary productivity in the pond from which our core was collected. Our field observations and geochemical analyses indicated that the pond from where the IIL3 core was collected has not been affected by animal feces (Wei et al., 2016; Chen et al., 2019; Jin et al., 2021). We hypothesized that the air temperature may be the most important factor driving changes in productivity in Antarctica over the past 3200 years.
We compared the aquatic productivity indicated by compound-specific carbon isotope data and sediment fluxes of short-chain-alkanoic acids and sterols with the air temperatures determined from an ice core collected from the Talos Dome in the western Ross Sea (Figure 4). The aquatic primary productivity was higher in the relatively warm periods ~750–1650 and 3000–3200 a BP than the colder periods ~150–600 and 2500–3000 a BP. It is a reasonable conclusion that a warmer climate (meaning that there would be more solar radiation and less ice cover during the austral summer than in periods of a cooler climate) would promote blooms of algae and cyanobacteria in lakes and ponds in Antarctica (Bird et al., 1991; Wei et al., 2016). Our results are supported by previous findings that the air temperature strongly affects primary productivity in lakes and ponds in Antarctica (Borghini et al., 2016; Mahesh et al., 2019). For example, the algal biomass indicated by pigments in lakes and ponds decreases as the latitude increases in Victoria Land, East Antarctica (Borghini et al., 2016). Recent global warming has caused productivity to increase rapidly in east Antarctic lakes because lakes and ponds are ice-free for longer during the austral summer (Roberts et al., 2006). We suggest that the13C values of-alkanoic acids derived from phototrophic organisms can be used as new organic proxies to reconstruct paleo-productivity and therefore indicate regional climate changes in ice-free regions of Antarctica.
5 Conclusions
We found that the13C values of-alkanoic acids produced by phototrophic organisms can be used as new proxies for the productivities of Antarctic lakes and ponds. The short-chain-alkanoic acids in sediment had similar13C values to floating microbial mats, indicating that short-chain-alkanoic acids in the sediment were primarily derived from phototrophic organisms. The carbon isotope compositions of short-chain-alkanoic acids in the IIL3 sediment profile were mainly related to primary productivity in the studied pond. At a high photosynthesis efficiency, dissolved CO2would have been rapidly consumed, resulting in CO2-diffusion-limited growth and more13CO2being assimilated into lipids in the aquatic microbial mats. The aquatic productivities predicted from the-alkanoic acid13C values were compared with the aquatic productivities predicted using various proxies (13CTOCand sedimentary fluxes of-alkanoic acids and sterols) in the same core. The C16-alkanoic acid13C values positively correlated with aquatic productivity, with13C enrichment indicating higher productivity. The results indicated that the13C values of-alkanoic acids derived from phototrophic organisms can be used as new reliable proxies for reconstructing historic changes in the primary productivities of lakes and ponds in Antarctica and then to indicate regional climate changes.
This study was supported by the National Natural Science Foundation of China (Grant nos. 42276240, 42206243, 41776188), the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant no. XDB40000000), the Shanghai Sailing Program (Grant no. 22YF1418800), the China Postdoctoral Science Foundation (Grant no. 2022M712038), and theShanghai Frontiers Science Center of Polar Science (SCOPS). We are grateful to the Chinese Arctic and Antarctic Administration (CAA) for supporting the project, and we thank the United States Antarctic Program (USAP), Antarctic Support Contract, and Italian Mario Zucchelli Station for logistical support. We also thank R. Murray and A. McKenzie for providing valuable field assistance on Inexpressible Island, Xueying Wang for preparing the samples, and Prof. Yongsong Huang for the help on performing analyses. We appreciate two anonymous reviewers and Associate Editor for constructive comments that helped us improve the manuscript.
Aichner B, Wilkes H, Herzschuh U, et al. 2010a. Biomarker and compound-specific δ13C evidence for changing environmental conditions and carbon limitation at Lake Koucha, eastern Tibetan Plateau. J Paleolimnol, 43(4): 873-899, doi:10.1007/s10933-009- 9375-y.
Aichner B, Herzschuh U, Wilkes H. 2010b. Influence of aquatic macrophytes on the stable carbon isotopic signatures of sedimentary organic matter in lakes on the Tibetan Plateau. Org Geochem, 41(7): 706-718, doi:10.1016/j.orggeochem.2010.02.002.
Ashley K E, Crosta X, Etourneau J, et al. 2021. Exploring the use of compound-specific carbon isotopes as a palaeoproductivity proxy off the coast of Adélie Land, East Antarctica. Biogeosciences, 18(19): 5555-5571, doi:10.5194/bg-18-5555-2021.
Bird M I, Chivas A R, Radnell C J, et al. 1991. Sedimentological and stable-isotope evolution of lakes in the Vestfold Hills, Antarctica. Palaeogeogr Palaeoclimatol Palaeoecol, 84(1-4): 109-130, doi:10. 1016/0031-0182(91)90039-T.
Borghini F, Colacevich A, Caruso T, et al. 2016. Algal biomass and pigments along a latitudinal gradient in Victoria Land lakes, East Antarctica. Polar Res, 35(1): 20703, doi:10.3402/polar.v35.20703.
B?ttger T, Schidlowski M, Wand U. 1993. Stable carbon isotope fractionation in lower plants from the Schirmacher and Untersee oases (central Dronning Maud Land, East Antarctica). Isot Environ Health Stud, 29(1/2): 21-25, doi:10.1080/10256019308046131.
Carrizo D, Sánchez-García L, Menes R J, et al. 2019. Discriminating sources and preservation of organic matter in surface sediments from five Antarctic Lakes in the Fildes Peninsula (King George Island) by lipid biomarkers and compound-specific isotopic analysis. Sci Total Environ, 672: 657-668, doi:10.1016/j.scitotenv.2019.03.459.
Casta?eda I S, Schouten S, 2011. A review of molecular organic proxies for examining modern and ancient lacustrine environments. Quat Sci Rev, 30(21/22): 2851-2891, doi:10.1016/j.quascirev.2011.07.009.
Chen Q Q, Nie Y G, Liu X D, et al. 2015. An 800-year ultraviolet radiation record inferred from sedimentary pigments in the Ross Sea area, East Antarctica. Boreas, 44(4): 693-705, doi:10.1111/bor.12130.
Chen X, Dong L, Zhao W, et al. 2022. The effects of metabolism and temperature on carbon isotope composition of lipids in marine bacteriumWP3. Chem Geol, 606: 120963, doi:10.1016/j.chemgeo.2022.120963.
Chen X, Liu X D, Jia H Z, et al. 2021. Inverse hydrogen isotope fractionation indicates heterotrophic microbial production of long-chain-alkyl lipids in desolate Antarctic ponds. Geobiology, 19(4): 394-404, doi:10.1111/gbi.12441.
Chen X, Liu X, Wei Y, et al. 2019. Production of long-chain-alkyl lipids by heterotrophic microbes: new evidence from Antarctic lakes. Org Geochem, 138: 103909, doi:10.1016/j.orggeochem.2019.103909.
Chen X, Wei Y Y, Nie Y G, et al. 2020. Carbon isotopes of-alkanoic acids in Antarctic ornithogenic sediments as indicators of sedimentary lipid sources and paleocological change. Sci Total Environ, 709: 135926, doi:10.1016/j.scitotenv.2019.135926.
Chen X, Zhao W S, Dong L, et al. 2023. Impact of metabolism and temperature on2H/1H fractionation in lipids of the marine bacteriumWP3. Biogeosciences, 20(7): 1491-1504, doi:10.5194/bg-20-1491-2023.
Chikaraishi Y, Naraoka H. 2007.13C andD relationships among three-alkyl compound classes (-alkanoic acid,-alkane and-alkanol) of terrestrial higher plants. Org Geochem, 38(2): 198-215, doi:10.1016/j. orggeochem.2006.10.003.
Crosta X, Crespin J, Billy I, et al. 2005. Major factors controlling Holocene δ13Corgchanges in a seasonal sea-ice environment, Adélie Land, East Antarctica. Global Biogeochem Cycles, 19(4): GB4029, doi:10.1029/2004gb002426.
Dalsgaard J, St John M, Kattner G, et al. 2003. Fatty acid trophic markers in the pelagic marine environment. Adv Mar Biol, 46: 225-340, doi:10.1016/s0065-2881(03)46005-7.
Dijkman N A, Boschker H T S, Middelburg J J, et al. 2009. Group-specific primary production based on stable-isotope labeling of phospholipid- derived fatty acids. Limnol Oceanogr Methods, 7(8): 612-625, doi:10.4319/lom.2009.7.612.
Ding M H, Bian L G, Zhang L, et al. 2015. Meteorological characteristics of Inexpressible Island, Antarctica. Chin J Polar Res, 27(4): 344-350, doi: 10.13679/j.jdyj.2015.4.344 (in Chinese with English abstract).
Ficken K J, Li B, Swain D L, et al. 2000. An n-alkane proxy for the sedimentary input of submerged/floating freshwater aquatic macrophytes. Org Geochem, 31(7/8): 745-749, doi:10.1016/S0146- 6380(00)00081-4.
Fumanti B, Cavacini P, Alfinito S. 1997. Benthic algal mats of some lakes of Inexpressible Island (northern Victoria Land, Antarctica). Polar Biol, 17(2): 25-30, doi:10.1007/s003000050101.
Gao L, Hou J, Toney J, et al. 2011. Mathematical modeling of the aquatic macrophyte inputs of mid-chain-alkyl lipids to lake sediments: implications for interpreting compound specific hydrogen isotopic records. Geochimica Cosmochimica Acta, 75(13): 3781-3791, doi:10. 1016/j.gca.2011.04.008.
Giralt S, Hernández A, Pla-Rabes S, et al. 2020. Holocene environmental changes inferred from Antarctic lake sediments//Oliva M, Fernández J R (eds.). Past Antarctica: paleoclimatology and climate change. Amsterdam: Elsevier, 51-66, doi:10.1016/b978-0-12-817925-3.00003-3.
Grimalt J O, Yruela I, Saiz-Jimenez C, et al. 1991. Sedimentary lipid biogeochemistry of an hypereutrophic alkaline lagoon. Geochimica Cosmochimica Acta, 55(9): 2555-2577, doi:10.1016/0016-7037(91) 90373-d.
Hayes J M. 2001. Fractionation of carbon and hydrogen isotopes in biosynthetic processes. Rev Mineral Geochem, 43(1): 225-277, doi:10.2138/gsrmg.43.1.225.
Hodgson D A, Verleyen E, Sabbe K, et al. 2005. Late Quaternary climate-driven environmental change in the Larsemann Hills, East Antarctica, multi-proxy evidence from a lake sediment core. Quat Res, 64(1): 83-99, doi:10.1016/j.yqres.2005.04.002.
Huang X, Meyers P A. 2019. Assessing paleohydrologic controls on the hydrogen isotope compositions of leaf wax-alkanes in Chinese peat deposits. Palaeogeogr Palaeoclimatol Palaeoecol, 516: 354-363, doi:10.1016/j.palaeo.2018.12.017.
Huang Y, Eglinton G, Ineson P, et al. 1997. Absence of carbon isotope fractionation of individual-alkanes in a 23-year field decomposition experiment with. Org Geochem, 26(7/8): 497-501, doi:10.1016/s0146-6380(97)00027-2.
Huang Y S, Alayne Street-Perrott F, Alan Perrott R, et al. 1999. Glacial-interglacial environmental changes inferred from molecular and compound-specific δ13C analyses of sediments from Sacred Lake, Mt. Kenya. Geochimica Cosmochimica Acta, 63(9): 1383-1404, doi:10.1016/s0016-7037(99)00074-5.
Indermühle A, Stocker T F, Joos F, et al. 1999. Holocene carbon-cycle dynamics based on CO2trapped in ice at Taylor Dome, Antarctica. Nature, 398(6723): 121-126, doi:10.1038/18158.
Jansson M, Karlsson J, Jonsson A. 2012. Carbon dioxide supersaturation promotes primary production in lakes. Ecol Lett, 15(6): 527-532, doi:10.1111/j.1461-0248.2012.01762.x.
Jin J, Chen X, Xu L, et al. 2021. Chronology and paleoclimatic implications of lacustrine sediments at Inexpressible Island, Ross Sea, Antarctica. Palaeogeogr Palaeoclimatol Palaeoecol, 576: 110497, doi:10.1016/j.palaeo.2021.110497.
Jungblut A D, Allen M A, Burns B P, et al. 2009. Lipid biomarker analysis of cyanobacteria-dominated microbial mats in meltwater ponds on the McMurdo Ice Shelf, Antarctica. Org Geochem, 40(2): 258-269, doi:10.1016/j.orggeochem.2008.10.002.
Leavitt P R, Hodgson D A. 2001. Sedimentary pigments//Smol J P, Birks H J B, Last W M (eds.). Tracking environmental change using lake sediments. Volume 3: terrestrial, algal, and siliceous indicators. Dordrecht, The Netherlands: Kluwer Academic Publishers, 1-32.
Lee J R, Raymond B, Bracegirdle T J, et al. 2017. Climate change drives expansion of Antarctic ice-free habitat. Nature, 547(7661): 49-54, doi:10.1038/nature22996.
Li R, Fan J, Xue J, et al. 2017. Effects of early diagenesis on molecular distributions and carbon isotopic compositions of leaf wax long chain biomarker-alkanes: comparison of two one-year-long burial experiments. Org Geochem, 104: 8-18, doi:10.1016/j.orggeochem. 2016.11.006.
Liu H, Liu W. 2017. Concentration and distributions of fatty acids in algae, submerged plants and terrestrial plants from the northeastern Tibetan Plateau. Org Geochem, 113: 17-26, doi:10.1016/j.orggeochem.2017. 08.008.
Mahesh B S, Warrier A K, Mohan R. 2019. Impact of Antarctic climate during the Late Quaternary: records from Zub Lake sedimentary archives from Schirmacher Hills, East Antarctica. Palaeogeogr Palaeoclimatol Palaeoecol, 514: 398-406, doi:10.1016/j.palaeo.2018. 10.029.
Meyers P A. 1997. Organic geochemical proxies of paleoceanographic, paleolimnologic, and paleoclimatic processes. Org Geochem, 27(5/6): 213-250, doi:10.1016/S0146-6380(97)00049-1.
Meyers P A. 2003. Applications of organic geochemistry to paleolimnological reconstructions: a summary of examples from the Laurentian Great Lakes. Org Geochem, 34(2): 261-289, doi:10.1016/S0146-6380(02) 00168-7.
Mezgec K, Stenni B, Crosta X, et al. 2017. Holocene sea ice variability driven by wind and polynya efficiency in the Ross Sea. Nat Commun, 8: 1334, doi:10.1038/s41467-017-01455-x.
Michaud L, Caruso C, Mangano S, et al. 2012. Predominance of,, andwithin the prokaryotic community of freshwater shallow lakes in the northern Victoria Land, East Antarctica. FEMS Microbiol Ecol, 82(2): 391-404, doi:10.1111/j.1574-6941.2012.01394.x.
Monaghan A J, Bromwich D H, Powers J G, et al. 2005. The climate of the McMurdo, Antarctica, region as represented by one year of forecasts from the Antarctic mesoscale prediction system. J Clim, 18(8): 1174-1189, doi:10.1175/jcli3336.1.
Monnin E, Steig E J, Siegenthaler U, et al. 2004. Evidence for substantial accumulation rate variability in Antarctica during the Holocene, through synchronization of CO2in the Taylor Dome, Dome C and DML ice cores. Earth Planet Sci Lett, 224(1/2): 45-54, doi:10.1016/j. epsl.2004.05.007.
Neumann K, Lyons W B, Priscu J C, et al. 2004. The carbon isotopic composition of dissolved inorganic carbon in perennially ice-covered Antarctic lakes: searching for a biogenic signature. Ann Glaciol, 39: 518-524, doi:10.3189/172756404781814465.
Popp B N, Laws E A, Bidigare R R, et al. 1998. Effect of phytoplankton cell geometry on carbon isotopic fractionation. Geochimica Cosmochimica Acta, 62(1): 69-77, doi:10.1016/s0016-7037(97)00333-5.
Porcino N, Cosenza A, Azzaro M. 2020. A review on the geochemistry of lakes in Victoria Land (Antarctica). Chemosphere, 251: 126229, doi:10.1016/j.chemosphere.2020.126229.
Rampen S W, Abbas B A, Schouten S, et al. 2010. A comprehensive study of sterols in marine diatoms (Bacillariophyta): implications for their use as tracers for diatom productivity. Limnol Oceanogr, 55(1): 91-105, doi:10.4319/lo.2010.55.1.0091.
Roberts D, Hodgson D A, McMinn A, et al. 2006. Recent rapid salinity rise in three East Antarctic lakes. J Paleolimnol, 36(4): 385-406, doi:10.1007/s10933-006-9010-0.
Sachse D, Billault I, Bowen G J, et al. 2012. Molecular paleohydrology: interpreting the hydrogen-isotopic composition of lipid biomarkers from photosynthesizing organisms. Annu Rev Earth Planet Sci, 40: 221-249, doi:10.1146/annurev-earth-042711-105535.
Schouten S, Klein Breteler W C M, Blokker P, et al. 1998. Biosynthetic effects on the stable carbon isotopic compositions of algal lipids: implications for deciphering the carbon isotopic biomarker record. Geochimica Cosmochimica Acta, 62(8): 1397-1406, doi:10.1016/ s0016-7037(98)00076-3.
Sessions A L. 2016. Factors controlling the deuterium contents of sedimentary hydrocarbons. Org Geochem, 96: 43-64, doi:10.1016/j. orggeochem.2016.02.012.
Squier A H, Airs R L, Hodgson D A, et al. 2004. Atmospheric pressure chemical ionisation liquid chromatography/mass spectrometry of the ultraviolet screening pigment scytonemin: characteristic fragmentations. Rapid Commun Mass Spectrom, 18(23): 2934-2938, doi:10.1002/rcm. 1714.
Stenni B, Curran M A J, Abram N J, et al. 2017. Antarctic climate variability on regional and continental scales over the last 2000 years. Clim Past, 13(11): 1609-1634, doi:10.5194/cp-13-1609-2017.
Strugnell J M, McGregor H V, Wilson N G, et al. 2022. Emerging biological archives can reveal ecological and climatic change in Antarctica. Glob Change Biol, 28(22): 6483-6508, doi:10.1111/gcb. 16356.
Sun L G, Xie Z Q, Zhao J L. 2000. A 3, 000-year record of penguin populations. Nature, 407(6806): 858, doi:10.1038/35038163.
Sun M Y, Zou L, Dai J, et al. 2004. Molecular carbon isotopic fractionation of algal lipids during decomposition in natural oxic and anoxic seawaters. Org Geochem, 35(8): 895-908, doi:10.1016/j. orggeochem.2004.04.001.
Thomas E K, Huang Y S, Morrill C, et al. 2014. Abundant C4plants on the Tibetan Plateau during the late glacial and early Holocene. Quat Sci Rev, 87: 24-33, doi:10.1016/j.quascirev.2013.12.014.
Tierney J E, Russell J M, Huang Y. 2010. A molecular perspective on Late Quaternary climate and vegetation change in the Lake Tanganyika Basin, East Africa. Quat Sci Rev, 29(5/6): 787-800, doi:10.1016/j. quascirev.2009.11.030.
Volkman J K. 2018. Lipids of geochemical interest in microalgae//Wilkes H (eds.). Hydrocarbons, oils and lipids: diversity, origin, chemistry and fate. Handbook of hydrocarbon and lipid microbiology. Cham: Springer International Publishing, 1-34, doi:10.1007/978-3-319- 54529-5_10-1.
Wei Y Y, Jin J, Nie Y G, et al. 2016. Sources of organic matter and paleo-environmental implications inferred from carbon isotope compositions of lacustrine sediments at Inexpressible Island, Ross Sea, Antarctica. Adv Polar Sci, 27(4): 233-244, doi:10.13679/j.advps.2016. 4.00233.
Yan C Y, Zhang Y F, Zheng M, et al. 2021. Effects of redox conditions and temperature on the degradation of Sphagnumn-alkanes. Chem Geol, 561: 119927, doi:10.1016/j.chemgeo.2020.119927.
Table S1 Concentrations of short-chain-alkanoic acids in the IIL3 sediment profile

Depth/cmC14/(μg·g?1)C15/(μg·g?1)C16/(μg·g?1)C17/(μg·g?1)C18/(μg·g?1) 0.56.832.9340.661.1811.83 23.561.4025.940.9110.59 42.470.9422.750.6712.43 61.530.6511.160.394.30 82.881.0424.660.749.27 101.800.5016.340.476.28 122.300.7318.430.539.53 142.631.0321.890.7613.41 160.860.369.390.274.68 181.570.4912.050.497.62 201.440.4712.040.518.17 221.090.4010.040.354.89 241.380.4411.780.469.43 261.380.4712.380.5610.60 281.160.4111.200.418.03 301.480.5612.540.366.49 320.800.317.470.253.45 340.980.3710.960.274.31 362.971.0424.060.687.30 380.660.276.250.192.64 400.780.317.410.343.06 420.730.336.920.192.84 441.000.428.630.284.10 460.840.378.240.213.69 480.770.306.950.252.87 500.700.297.160.203.23 520.550.185.410.152.16 541.220.4010.140.367.08
Table S2 Concentrations of sterols in the IIL3 sediment profile
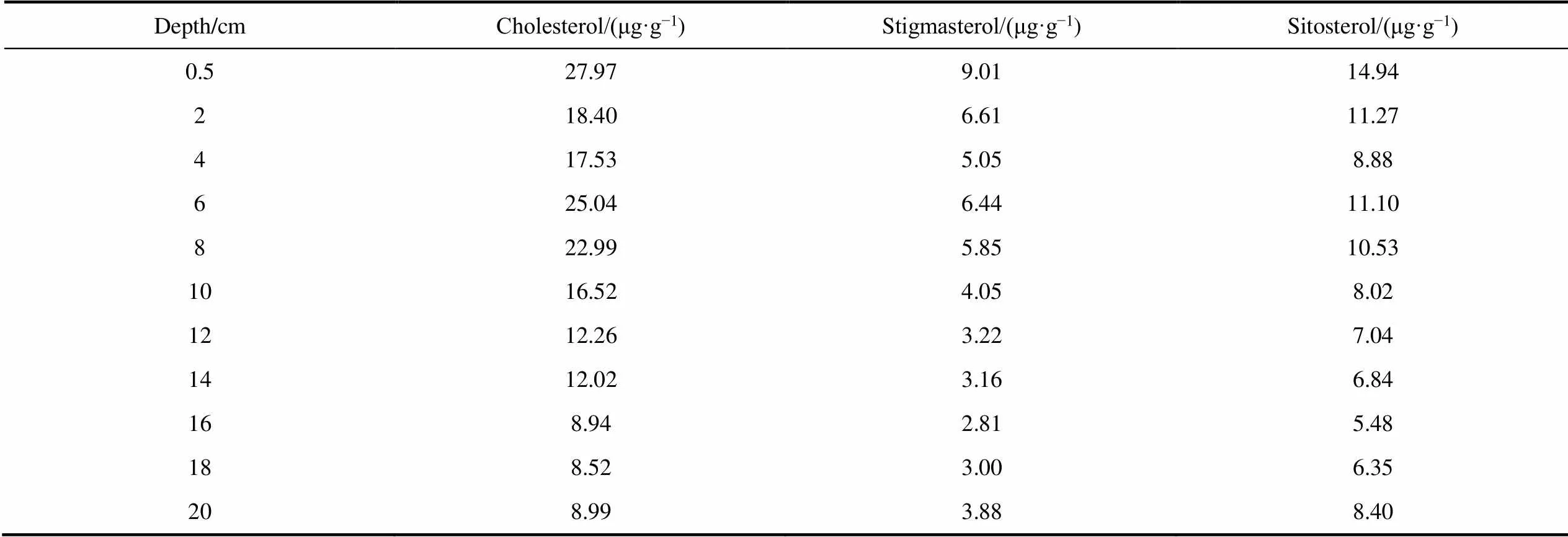
Depth/cmCholesterol/(μg·g?1)Stigmasterol/(μg·g?1)Sitosterol/(μg·g?1) 0.527.979.0114.94 218.406.6111.27 417.535.058.88 625.046.4411.10 822.995.8510.53 1016.524.058.02 1212.263.227.04 1412.023.166.84 168.942.815.48 188.523.006.35 208.993.888.40
Continued

Depth/cmCholesterol/(μg·g?1)Stigmasterol/(μg·g?1)Sitosterol/(μg·g?1) 2213.154.819.52 2410.523.587.17 2611.225.2410.25 286.763.717.38 3011.784.578.90 326.762.776.15 3411.644.558.88 3614.376.0811.63 3815.966.6311.98 4011.734.067.35 4219.255.088.55 4413.904.688.48 4615.655.8710.63 4811.554.267.40 5018.386.4211.10 525.402.424.28 548.913.615.66
: Chen X, Jin J, Nie Y G, et al. Carbon isotope ratios of-alkanoic acids: new organic proxies for paleo-productivity in Antarctic ponds. Adv Polar Sci, 2023, 34(4): 304-317,doi: 10.12429/j.advps.2023.0004
, ORCID: 0000-0001-8560-8127, E-mail: xinchen1991@sjtu.edu.cn (Chen X); ORCID: 0000-0002-4333- 8197, E-mail: ycx@ustc.edu.cn (Liu X D)
10.12429/j.advps.2023.0004
2 June 2023;
25 September 2023;
30 December 2023
 Advances in Polar Science2023年4期
Advances in Polar Science2023年4期
- Advances in Polar Science的其它文章
- Advances in Polar Science CONTENTS(Volume 34,2023)
- Eleven “Opinion Editorials” were published
- Call for papers: Special Issue on “Novel technologies for sustainable monitoring of polar environment upscaling from in situ observations to aerial and space-borne remote sensing”
- Distributions of dissolved oxygen and apparent oxygen utilization in the Cosmonaut Sea and Amundsen Sea in austral summer 2021
- Chlorella across latitudes:investigating biochemical composition and antioxidant activities for biotechnological applications
- Current state of research on microplastics in the marine-atmosphere environment of the Arctic region
