Highly Efficient Heavy-Metal-Ion Removal from Shellfish Processing Liquid with Low Protein and Polysaccharide Loss by Hybrid Mesoporous Silica Diol-APDC-SBA15
QI Yanxia, SONG Yang, LIU Chang, QI Shizhe, WANG Haibo,CAO Jijuan, and ZHAO Qiancheng, 4), 5), *
Highly Efficient Heavy-Metal-Ion Removal from Shellfish Processing Liquid with Low Protein and Polysaccharide Loss by Hybrid Mesoporous Silica Diol-APDC-SBA15
QI Yanxia1), 2), 5), SONG Yang1), 2), LIU Chang1), QI Shizhe1), WANG Haibo1), 2), 5),CAO Jijuan3), and ZHAO Qiancheng1), 2), 4), 5), *
1),,116023,2),,116023,3),,116600,4),,116034,5),116034,
Heavy metal ions in shellfish products are harmful to human health, and their removal with low nutrient loss remains challenging. Herein, a new type of mesoporous silica (SBA15), modified internally with ammonium pyrrolidine dithiocarbamate(APDC) and externally with alkyl-diol groups, which was named as Diol-APDC-SBA15, was successfully developed and characterized by powder X-ray diffraction patterns, nitrogen adsorption, and Fourier transform infrared spectroscopy. The solutions with lead, chro-mium, cadmium, and copper were used to investigate the adsorption capacity of Diol-APDC-SBA15. Diol-APDC-SBA15 was adopted to remove heavy metals from cooking liquids of clams (), hydrolysate liquids of oysters (), and polysaccharide solution from the cooking liquid of. The efficiencies of removing heavy metal ions and the loss rates of proteins and polysaccharides were examined. The results showed that the adsorption capacities of Diol-APDC- SBA15 for Pb, Cr, Cd, and Cu in standard heavy-metal solutions were 161.4, 166.1, 29.6, and 60.2mgg?1, respectively. The removal efficiency of Diol-APDC-SBA15 for Pb in the three shellfish processing liquids ranged from 60.5% to 99.6%. The Cr removal effi- ciency was above 99.9% in the oyster hydrolysate liquid. Meanwhile, the percentages of polysaccharide loss were 5.5% and 3.7% in the cooking liquid of clam and polysaccharide solution, respectively, and the protein loss was 1.2% in the oyster hydrolysate liquid. Therefore, the Diol-APDC-SBA15 material exhibits a great potential application in the removal of heavy metals from shellfish pro- cessing liquids with low losses of proteins and polysaccharides.
heavy-metal removal; modified mesoporous silica; shellfish processing liquid; protein; polysaccharide
1 Introduction
The bioaccumulation of heavy metals in shellfish has be- come more serious in recent years due to seawater pollu- tion (Santos., 2013; Li., 2019). Some heavy me- tal ions, such as Pb, Cr, Cd, and Cu ions, are toxic and car- cinogenic and harmful to human health at low concentra- tions due to long-term bioaccumulation (Bogdanovic., 2014; Drewnowska., 2017; Wang., 2020). More attention has been focused on bivalve mollusks due to their bioaccumulation of toxic trace elements and relative posi- tion in the food chain (Tapia., 2010; Heidari., 2013; Bogdanovic., 2014; Barbosa., 2018). Therefore, the removal of heavy metals in shellfish is important in foodsafety control (Bille., 2015; Mirlean., 2019). Manytechniques, including precipitation, coagulation, ion ex- change, and adsorption have been developed for the treat- ment of aquatic products containing heavy metal ions; how- ever, most of these approaches are costly or ineffective (Ha- mi Dindar., 2015; Yang., 2019).
A number of adsorbents, including activated carbon (Za- caroni., 2015), chitosan and alginate nanocomposites (Gokila., 2017), hybrid materials based on silica par- ticles (Radi., 2016), magnetic nanoparticle-functiona- lized lactic acid bacteria cells (Li., 2020), montmo- rillonite (Liu., 2019),-coated multi-walled carbon nanotubes (Moallaei., 2020), and cellulose na- nofibrous mats (Zhang., 2020) have been applied to remove heavy metal ions from food and water. However, these materials usually exhibit inherent disadvantages, such as low adsorption capacity, low selectivity, long equilib- rium time, or mechanical and thermal instability, particular- ly when the initial concentrations of metal ions are relative- ly low. The preparation of silica-based mesoporous mate- rials has attracted considerable attention due to their uni- quely large surface area, regular pore structure, high me- chanical and thermal stability, and easily modified surface properties (Zhu., 2016; Jadhav., 2020). Surface- modified mesoporous silicas are promising adsorbents with high adsorption capacities for heavy metals. Mesoporous silicas functionalized with organic compounds by grafting organosiloxane precursors have been widely studied (Lee., 2016; Vunain., 2016). Mesoporous materials mo-dified with ligands containing nitrogen or sulfur exhibit high affinities for the adsorption of heavy metal ions, in- cluding Cu, Ni, Zn, Pb, Ag, Cr, and Co (Zhang and Li, 2013; Zhang., 2020). Ammonium pyrrolidine dithiocarba- mate (APDC), a compound containing nitrogen and sulfur, has been used to modify mesoporous materials due to itshigh affinity for heavy metals (Goubert-Renaudin., 2009).
The reduction of nonspecific adsorption is another chal- lenge in the removal of heavy metal ions from food, espe- cially with respect to unexpected losses of nutrients, such as proteins and polysaccharides (Deere., 2003; Yan., 2011). The extraction of heavy metals from protein solutions is more challenging due to the interaction of me- tal ions with proteins, and decreasing heavy metal ion con- centrations to regulatory standards is difficult. In addition, as proteins can be possibly adsorbed to sorbents, the extrac- tion of heavy metal ions is accompanied by decreasing pro- tein concentrations. Furthermore, the nonspecific adsorption of proteins occurs in the active sites involved in heavy-me-tal removal, resulting in the decreased efficiency of heavy- metal-ion removal (Howard., 2008; Zhang., 2008). Thus, to efficiently extract metals from protein solutions, scientists need to develop sorbents with strong binding af- finities toward metal ions and limited adsorption capabili- ties for proteins. However, the available studies are limited.Sulfonated polystyrene nanospheres have been reported to remove heavy metals from collagen solutions with adsorp- tion capacities of 50.7, 15.0, 8.7, and 39.0mgg?1for Pb2+, Mn2+, Cr3+, and Cd2+, respectively, and a protein loss per- centage of 15.2% at pH 4.5 (Peng., 2018). However, high removal rates of heavy metals and low losses of pro- teins are still expected. Therefore, increasing the removal rate of heavy metals while avoiding the nonspecific ad-sorption of other nutrients is an important topic in the study of heavy-metal removal. Our previous work demonstrated that alkyl-diol groups modified on the external surface of a mesoporous material can efficiently decrease the nonspe- cific adsorption of proteins (Qi., 2010).
In this study, we synthesized and characterized a newhybrid SBA15 mesoporous material modified with APDC on the inner surface and alkyl-diol groups on the outer sur-face, which was named as Diol-APDC-SBA15. The appli- cability of Diol-APDC-SBA15 for the removal of heavy metal ions, including Pb, Cr, Cd, and Cu, from shellfish pro- cessing liquids was systematically investigated.
2 Materials and Methods
2.1 Instruments and Equipment
Powder X-ray diffraction (XRD) patterns were determin- ed using an X’pert Pro vertical goniometer (PANalytical, Holland). Nitrogen adsorption measurements were perform- ed at 77 K with a NOVA 2200e surface area and pore size analyzer (Quantachrome , USA). Fourier transform infrared (FT-IR) spectroscopy was performed with a spectrum 100/ 100N system (PerkinElmer, USA) with a scan range of 4000–400cm?1at 25℃±3℃. Each protein or polysaccha- ride concentration was determined using an ultraviolet-vi- sible spectrometer (Lambda 35, PerkinElmer). The metal ion concentrations were measured by inductively coupled plasma atomic emission spectrometry (ICP-AES) on a Per- kinElmer Optima 8000 spectrometer (PerkinElmer, USA).
2.2 Materials and Reagents
TEOS and [3-(2,3-epoxypropoxy)propyl]trimethoxysilane were purchased from Meryer Chemical Technology Co. (Shenzhen, China). Potassium iodide and APDC were ob- tained from Solarbio Technology Co. (Beijing, China). PEG- PPG-PEG (P123) was purchased from Sigma-Aldrich Tra- ding Co., Ltd. (Shanghai, China). Acetic acid and sodium acetate were obtained from Damao Chemical Reagent Fac- tory (Tianjin, China). Standard stock solutions of Pb(II), Cr(III), Cd(II), and Cu(II) were obtained from Beijing Century Aoke Biological Technology Co. (Beijing, China). Bovine serum albumin (BSA) and trypsin were obtained from Sigma (St Louis, MO, USA). Deionized water (R>18.2MΩ) was purified by a Millipore (Billerica, MA, USA) purification system.
The cooking liquid of clams (was obtained from Liaoning Ande Food Co., Ltd., and the soluble solid content of the cooking liquid was adjusted to 10% in our laboratory before use. The oyster (Thunberg) hydrolysate liquid was prepared by hydro- lyzing oysters with trypsin followed by ultra-filtration witha 3000Da molecular weight cut-off. The low-molecular- weight components were collected and lyophilized. The powder was dissolved in water at a ratio of 1:50 (w/v)be- fore use. The polysaccharide solution was prepared by dis- solving the dry powder of crude polysaccharides extract- ed from the concentrated cooking liquid of clam in water at a ratio of 1:50 (w/v) and stirring until complete dissolu- tion without precipitation for use.
2.3 Mesoporous Material Preparation
2.3.1 Preparation of APDC-SBA15 mesoporous material
First, 72-mL water, 8.9-mL concentrated hydrochloric acid, and 3.56-g P123 were added to a round flask with stirring at 41℃. Then, after complete dissolution of P123, 8-mL TEOS was dropwise added slowly. After stirring the solution for 10min, the formed gel was transferred to a Tef- lon bottle and maintained at 40℃ for 24h. The resultant product was filtered, washed with ethanol and distilled wa- ter, and then dried at 100℃ for 5h. The surfactant (P123) in the materials was extracted by acidified ethanol, and 100mL 1.2molL?1aqueous HCl and 100-mL absolute alcohol were added to 1g material and refluxed for 24h, followed by drying at 100℃ for 5h (Zhao., 1998). The modi- fication of APDC on the mesoporous material was perform- ed as follows: 4mL 3-chloropropyltriethoxysilane and 4.0g potassium iodide were added to a round flask and stir- red at 40℃ for 2h. Then, 4.0g APDC was added, and the solution was stirred at 40℃ for 3h. Next, 80mL absolute ethanol and 2.0g SBA15 were added, and the solution wasrefluxed for 12h. The resultant product was filtered, wash- ed, and dried at 100℃ for 10h (Jiang., 2013). The re- sultant materials were designated as APDC-SBA15.
2.3.2 Preparation of Diol-APDC-SBA15 mesoporous material
The material Diol-APDC-SBA15 was prepared in accor-dance with the previous research (Qi., 2010) with some modifications. Briefly, 4.0g P123 was suspended in a solution of 85mL water and 10mL concentrated hydro- chloric acid at 41℃ and stirred until complete dissolution. Then, 10mL TEOS, 4.0mL 3-chloropropyltriethoxysilane, and 4.0g APDC were added to the above mixture succes- sively and stirred for 30min. The mixture was transferred to an autoclave and then maintained at 80℃ for 12h. The resultant product was filtered, washed with water, and then dried at 100℃ for 10h.
Next, 2g of the above sample was suspended in 80mL ethanol and added with 3mL [3-(2,3-epoxypropoxy)propyl] trimethoxysilane. After refluxing under nitrogen protection for 24h, the suspension was filtered, washed with ethanol, and dried at 100℃. The material was treated with acidified ethanol to remove the surfactant (P123) and to convert the (2,3-epoxypropoxy)propyl group to an alkyl-diol group as previously described (Qi., 2010). The resultant ma- terial was designated as Diol-APDC-SBA15.
2.4 Material Characterization
The Diol-APDC-SBA15 material was characterized bypowder XRD, nitrogen adsorption, and FT-IR analyses. XRD patterns were set for scanning in the 2θ range of 0.5?–8?. Nitrogen adsorption measurements were performed at 77 K with a NOVA 2200e surface area and pore size analyzer. FT-IR spectroscopy was performed with a spectrum 100/ 100N system (scan range, 4000–400cm?1; 25℃±3℃).
2.5 Heavy-Metal Adsorption from Standard Heavy- Metal Solutions by Diol-APDC-SBA15
A standard heavy-metal solution of Pb, Cr, Cd, and Cu metal ions was prepared, and the adsorption conditions were optimized. The solution pH is an important parameter for the heavy-metal removal efficiency. In the adsorption ex- periments, Pb, Cr, Cd, and Cu metal ion solutions were eachmixed at a concentration of 4mgL?1. Then, 30mL of the metal ion solution was treated with acetate-sodium acetate solution to adjust the pH to 4–8, followed by mixing with 30mg of Diol-APDC-SBA15 at 25℃±3℃. After stirring for 30min, the suspension was centrifuged, and the super- natant was collected. The metal ion concentrations of the initial and final solutions were measured by ICP-AES.
In the adsorption time investigation, Pb, Cr, Cd, and Cu metal ion solutions were each mixed at a concentration of 4mgL?1at optimum pH. Then, 30mL of the metal solution was mixed with 30mg Diol-APDC-SBA15 at 25℃±3℃. After stirring for 3, 5, 10, 30, 60, or 120min, the suspen- sion was centrifuged, and the supernatant was collected. Themetal ion concentrations of the initial and final solutions were also measured by ICP-AES.
In the adsorption concentration investigation, mixed me- tal ion solutions containing Pb, Cr, Cd, and Cu with con- centrations of 2, 4, 8, 10, 15, 20, 30, 50, 100, 150, 300, 400,600, and 800mgL?1were prepared first. Next, 30mL of themixed-metal solution at optimum pH in 0.2molL?1acetate-sodium acetate buffer solution was mixed with 30mg of Diol-APDC-SBA15 at 25℃±3℃. After stirring for 30min, the suspension was centrifuged, and the supernatant was collected. The metal ion concentrations of the initial and fi- nal solutions were measured by ICP-AES.
2.6 Practical Application of Diol-APDC-SBA15 for Removal of Heavy Metals from Shellfish Processing Liquids
2.6.1 Removal efficiency of heavy metals from shellfish processing liquids
The reliability and practicability of the proposed method were investigated by removing Pb, Cr, Cd, and Cu ions fromtwo shellfish product processing liquids. First, 30mL of the prepared sample solution was added to a centrifuge tube, in which 30mg of APDC-SBA15 or Diol-APDC-SBA15 was added. After mixing, the mixture in centrifuge tubes were shaken for 30min, and centrifuged for 5min at 1500rmin?1, and the supernatant was collected. The removal ef- ficiencies for heavy metal ions and the amounts of proteinsand polysaccharides in the samples before and after heavy- metal removal were measured, and the differences between the two mesoporous materials in the removal of aquatic pro- ducts were compared.
Then, 5mL of concentrated nitric acid was added to a 5mL liquid sample, and the mixture was heated on a hot plate(150℃) until the whole solution became colorless and tran- sparent. Then, the solution was filtered through a filter pa- per and finally diluted to a volume of 50mL for further ex-periments. The metal removal efficiency was calculated us- ing the following equation:
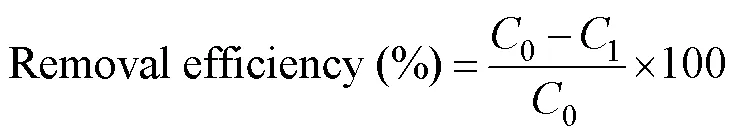
where0is the concentration of heavy metal ions before absorption (mgL?1), and1is the concentration of heavy metal ions after absorption (mgL?1).
To estimate the possible matrix effects on the low con- tents of heavy metal ions, the cooking liquid ofwas also spiked with four heavy metal ions at a concentration of 10mgL?1and analyzed using the proposedmethod. The contents of Pb, Cr, Cd, and Cu before and af-ter the removal of heavy metal ions in the samples were al- so determined by ICP-AES.
2.6.2 Losses of proteins and polysaccharides from shellfish processing liquids
In this experiment, the losses of proteins and polysaccha- rides were determined to investigate the losses of proteins and polysaccharides caused by the removal of heavy me- tal ions from the samples. The protein content was deter- mined by the biuret method using BSA as the standard (Lu- bran, 1978). The polysaccharide content was determined by the phenol-sulfuric acid method (A 490nm) using glu- cose as the standard (Dubois., 1956). The expression of loss percentage is shown in Eq. (2):

where0is the concentration of protein or polysaccharide before adsorption (mgL?1), and1is the concentration of protein or polysaccharide after adsorption (mgL?1).
All tests were performed in triplicate.
3 Results and Discussion
3.1 Preparation and Characterization of Diol-APDC-SBA15
Scheme 1 shows the heavy metal ion removed by the Diol-APDC-SBA15 material. The APDC group was anchor- ed to the mesoporous silica materiala co-condensationmethod, and alkyl-diol groups were grafted on the external surface of the mesoporous material by the post-graft method when the pore former was in the mesopores. The APDC groups of the Diol-APDC-SBA15 material were used for heavy metal ion removal. The alkyl-diol groups on the ex- ternal surface of the mesoporous material reduced the non- specific adsorption of proteins and polysaccharides.
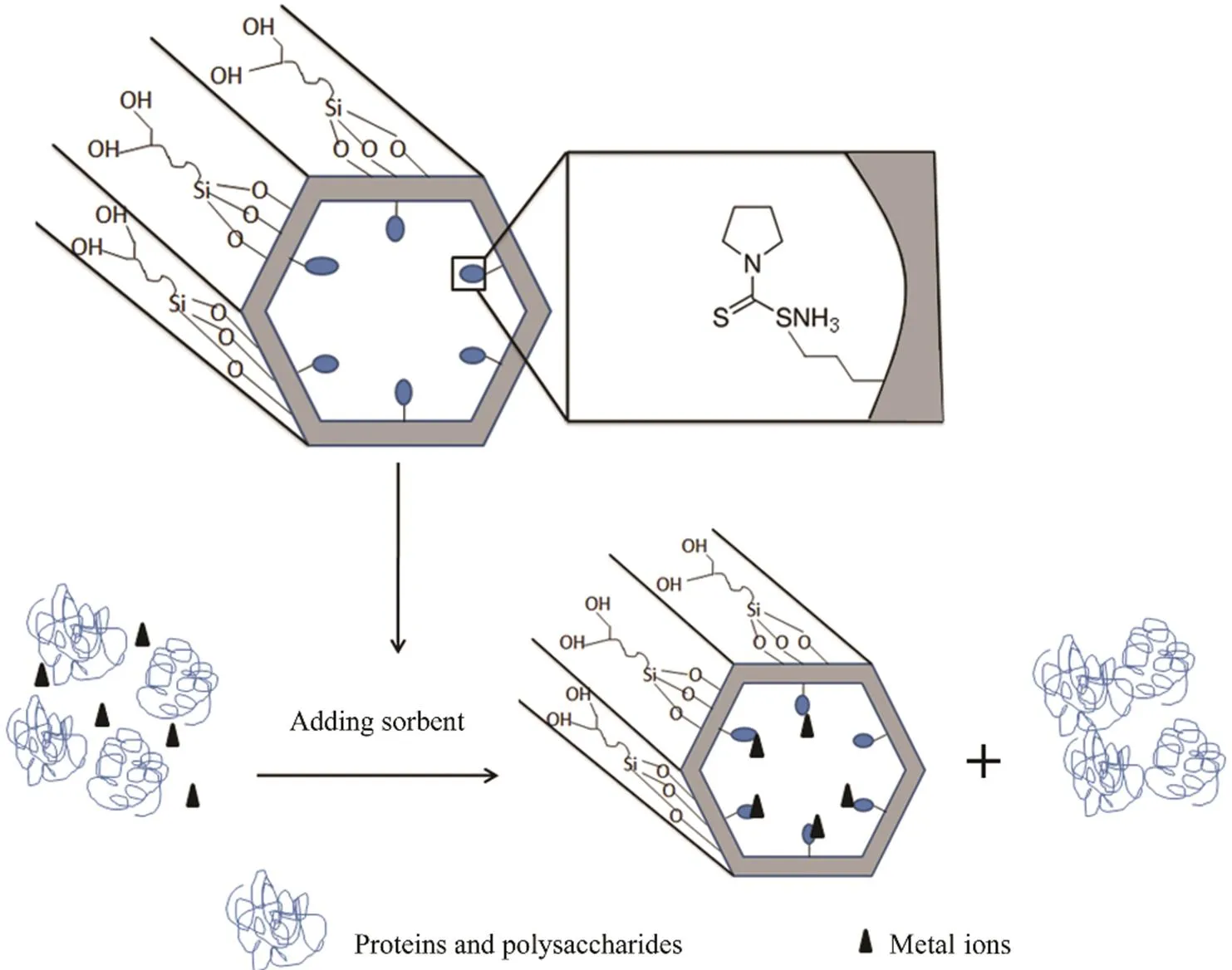
Scheme 1 Sorption schematic of heavy metal ions using Diol-APDC-SBA15.
Fig.1A shows the XRD patterns of the Diol-APDC-SBA- 15 mesoporous sorbent. The sorbent exhibited an intense (100) peak near 1.0?, indicating that the ordered hexagonal structure was not completely destroyed during the surface modification process. The pore diameter distributions of the samples were further studied by N2adsorption-desorption analyses. The pore diameter of the mesoporous materialwas 33.0?, and its distribution was calculated from the ad- sorption branch of the isotherm by the Barrett-Joyner-Ha- lenda method. The Brunauer-Emmett-Teller surface area was 133m2g?1, which was attributed to the SBA15 surface having undergone stepwise organic functionalization by APDC and alkyl-diol groups.
Fig.1B shows the FT-IR spectrum of the Diol-APDC- SBA15 material. The bands near 800 and 960cm?1wereassigned to the Si–O–Si stretching and bending modes of the mesoporous silica framework, respectively, and the wideband between 1200 and 1050cm?1was assigned to the C=Swagging vibration of the APDC group. The bands near 2900cm?1were assigned to C–H bonds. The bands near 3400cm?1corresponded to the O–H bonds of the alkyl-diol groups and the N–H stretching modes of the APDC group. These results indicated that alkyl-diol and APDC groups were successfully grafted onto the material surface.
3.2 Optimization of the Adsorption Conditions of Heavy Metal Ions with Standard Heavy-Metal Solution
The optimal pH of the standard heavy-metal solution for heavy-metal removal was pH 6 (Fig.2A). The removal ef- ficiency was low at pH 4, and it increased with the increaseof pH from pH 4 to 6. However, the removal efficiency de- creased with the increase of pH from pH 6 to 8. In the re- moval experiment, the electron-donating elements (N and S) of the mesoporous material supported complexation re- actions with metal ions. At the pH range of 4–8, the N and S atoms exhibited their chelating capabilities for heavy me- tal ions, and the empty orbit of the Pb ion chelated with N and S to form a stable ligand. Cr, Cd, and Cu ions with empty orbits followed the similar mechanisms. When the pH was low, the N and S atoms combined with H+ions, butthe two types of atoms could not coordinate the metal ions. At alkaline pH, metal ions may precipitate; thus, electron- donating atoms will not coordinate ions, and the removal efficiency decreases (Zhang., 2020). At pH 6.0, the N and S atoms exhibited their strong chelating capabilities, and the adsorption capacity for heavy metals was the high- est.
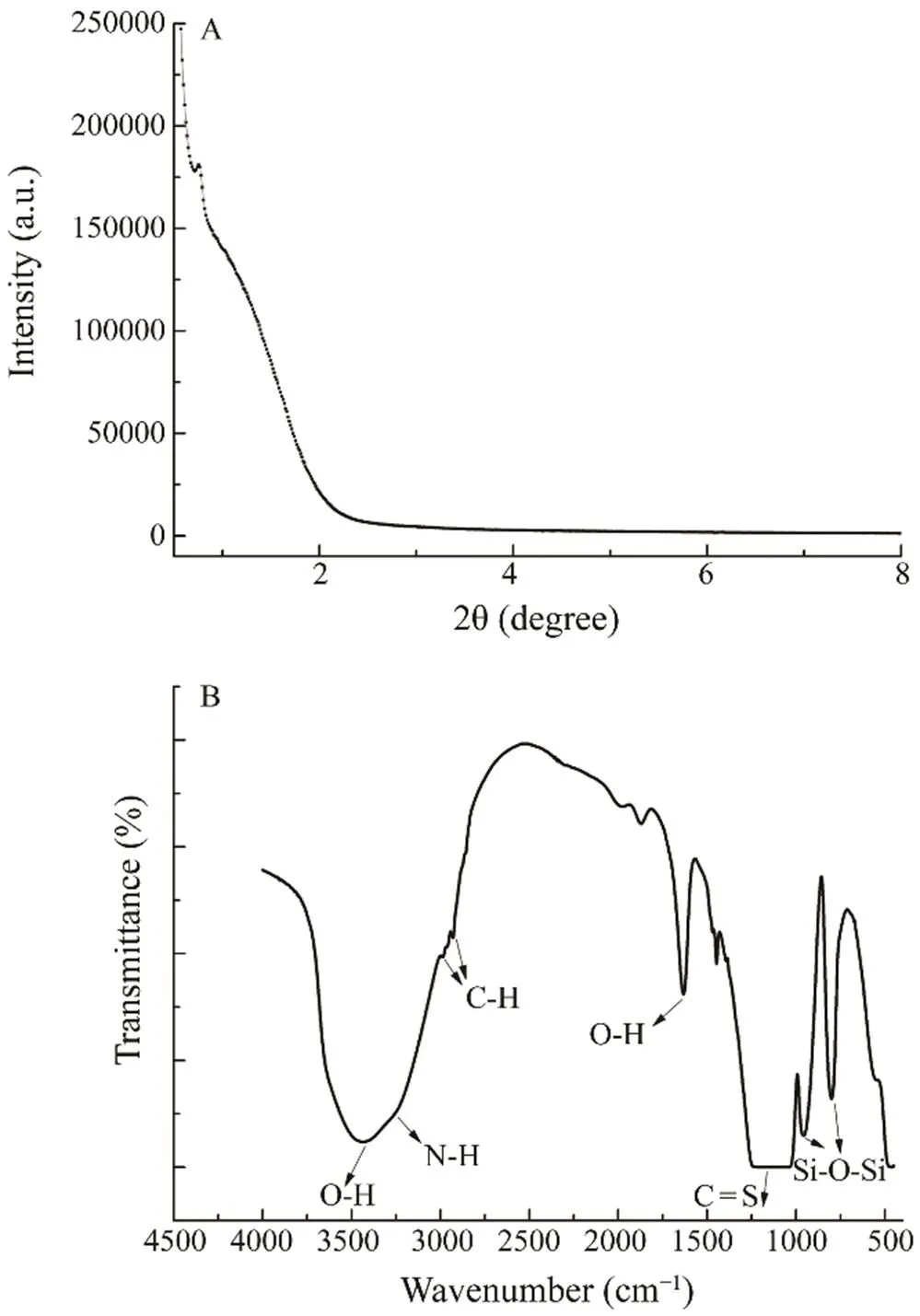
Fig.1 Powder XRD patterns (A) and FT-IR spectra (B) of Diol-APDC-SBA15.
The adsorption kinetics of the Diol-APDC-SBA15 ma- terial were investigated, and the removal efficiencies for Pb,Cr, Cu, and Cd metal ions at different times were determin- ed (Fig.2B). The equilibrium time was 10min, which was considerably shorter than those of other materials for re-moving all four metal ions. Equilibration times of 120min for clay and 360min for activated charcoal in the removal of Cu were previously reported (Zacaroni., 2015). Our material required a short equilibrium time because the or- dered pore channels of the Diol-APDC-SBA15 material al- lowed the heavy metal ions to easily reach the APDC groups.
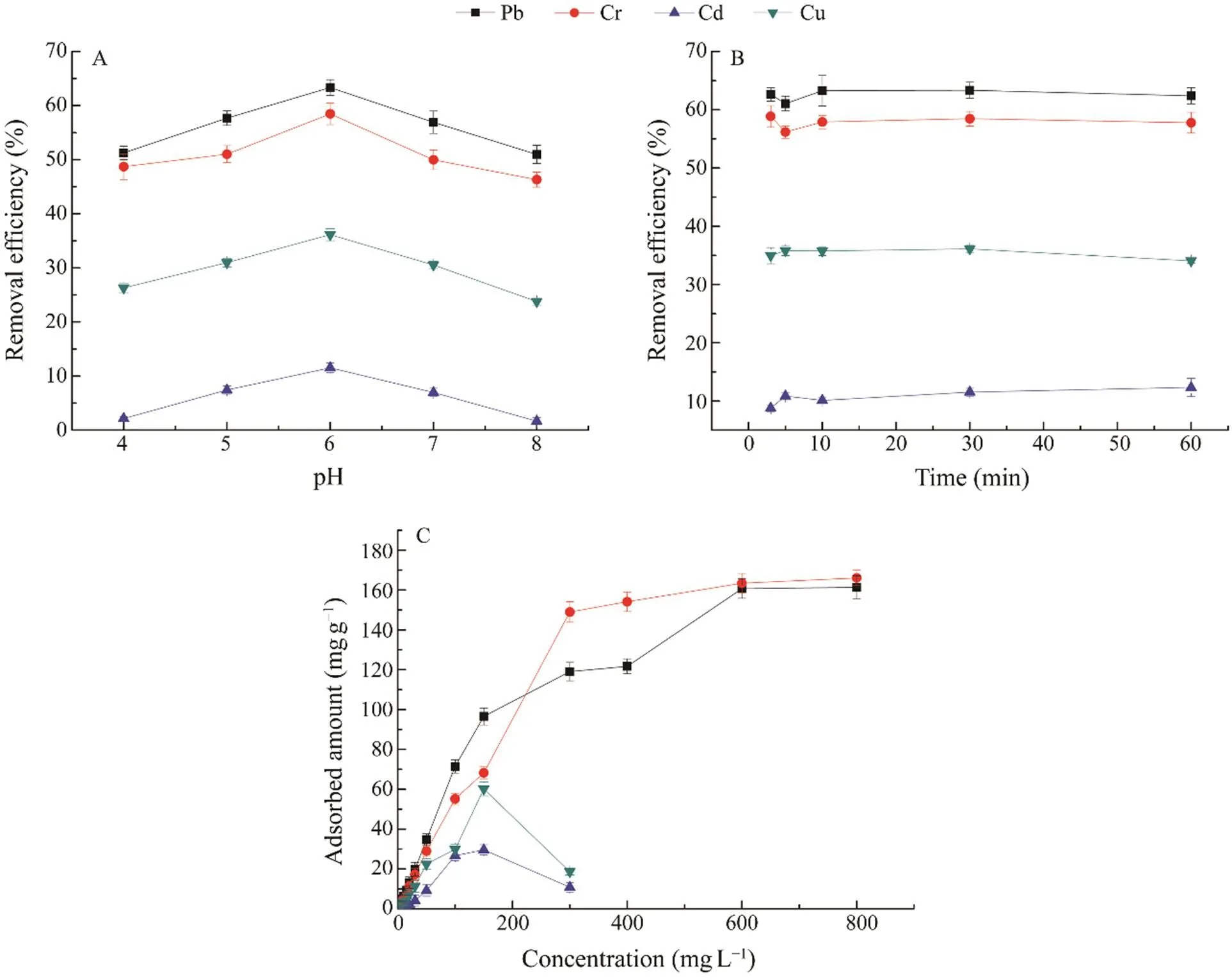
Fig.2 Investigation of (A) the efficiencies of removing heavy metal ions at different pH values; (B) adsorption kinetics of heavy metal ions; (C) amounts of heavy metal ions adsorbed at different concentrations.
The amounts of Pb and Cr ions adsorbed by the Diol- APDC-SBA15 material increased with the increasing me- tal ion concentrations from 2 to 800mgL?1, but the adsorb- ed amounts for Cd and Cu ions reached the highest values at the concentration of 150mgL?1. This result might have been due to the precipitation of Cd and Cu ions at high con- centrations. The equilibrium adsorption amounts of four heavy metal ions, including Pb, Cr, Cd, and Cu, were 161.4, 166.1, 29.6, and 60.2mgg?1, respectively, and the order of adsorption amounts was Pb>Cr>Cu>Cd (Fig.2C). The equilibrium adsorption amounts for Diol-APDC-SBA15were slightly higher than those of the ethylenediaminepro-pyl-2-pyridylimine-modified mesoporous material (106.62mgg?1for Pb and 48.26mgg?1for Cu) (Zhang., 2020),and considerably higher than those of clay-, charcoal-, andmagnetic nanoparticle-functionalized lactic acid bacteria cells. The maximum amounts of Pb and Cd adsorbed bymagnetic nanoparticle-functionalized lactic acid bacteriacells were 0.17 and 0.57mgg?1, respectively (Li., 2020); the maximum capacity of clay for absorbing Cu was 10.8mgg?1, and that of charcoal was 5.9mgg?1(Zacaroni.,2015). The amounts of Pb and Cd adsorbed by Diol-APDC-SBA15 were 948 and 51 times greater than those by thefunctionalized lactic acid bacteria cell adsorbent, respective- ly. The amounts of Cu adsorbed by Diol-APDC-SBA15 were 5 and 9 times higher than those by clay and charcoal, respectively. The high surface area of the Diol-APDC-SBA15 material and high metal ion affinity of the APDCgroup might have contributed to the high adsorbed amounts of metal ions.
3.3 Removal of Heavy Metals from Shellfish Pro-cessing Liquids Using Mesoporous Materials
To evaluate the heavy-metal-ion removal efficiency from shellfish processing liquids, we removed the heavy metals in the cooking liquid of clams (, hydro- lysate liquid of oysters (Thunberg), and poly- saccharides solution from the cooking liquid ofusing Diol-APDC-SBA15 and APDC-SBA15 ma- terials. The results are summarized in Table 1. The remo- val efficiencies of Diol-APDC-SBA15 and APDC-SBA15 materials for Pb in the cooking liquid were prominent and above 99.6%. The removal efficiencies of the APDC- SBA15 material for Pb and Cr ions from oyster hydrolysate liquid were 62.3% and above 99.9%, respectively. The re- moval efficiencies of the Diol-APDC-SBA15 material for Pb and Cr were 77.0% and above 99.9%, respectively. Theremoval efficiencies of Diol-APDC-SBA15 and APDC- SBA15 materials for Pb from the polysaccharide solution were 60.5% and 84.5%, respectively. The two mesoporous materials had high removal efficiencies for Pb and Cr. Diol- APDC-SBA15 showed a higher removal efficiency for Pb from oyster hydrolysate liquid than APDC-SBA15, but APDC-SBA15 had a higher removal efficiency for Pb from the polysaccharide solution than Diol-APDC-SBA15. How- ever, the removal efficiencies of the two materials for Cd and Cu were below 3%, which might be due to uncertain- ties in the low concentrations of Cd and Cu in shellfish pro- cessing liquids.
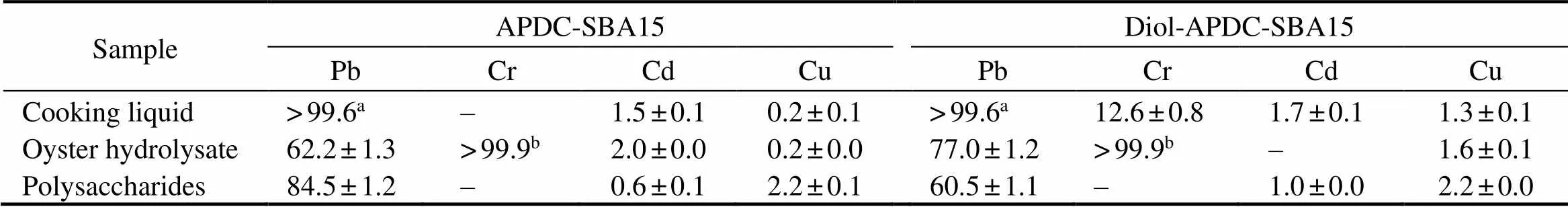
Table 1 Efficiencies of removing heavy metal ions from shellfish processing liquid (%)
Notes:aThe concentration of Pb after absorption was below the detection limit of 1.35μgL?1of the ICP-AES instrument; when the concentration of Pb after absorption was 1.35μgL?1, the removal efficiency for Pb was 99.6%.bThe concentration of Cr after absorption was below the detection limit of 0.13μgL?1of the ICP-AES instrument; when the concentration of Cr after absorption was 0.13μgL?1, the removal efficiency for Cr was 99.9%. ‘–’ not available (undetected).
To further investigate the efficiencies of removing the four heavy metal ions from the cooking liquid of clams (), we added a standard heavy-metal-ion solu- tion with a concentration of 10mgL?1for each heavy me- tal ion to the cooking liquid of clams. Table 2 shows the ef- ficiencies of removing metal ions from this cooking liquid after the addition of standard heavy metal ions. The remo- val efficiencies of APDC-SBA15 for Pb, Cr, Cd, and Cu were 73.2%, 77.6%, 44.8%, and 33.3%, respectively. The corresponding removal efficiencies of Diol-APDC-SBA15
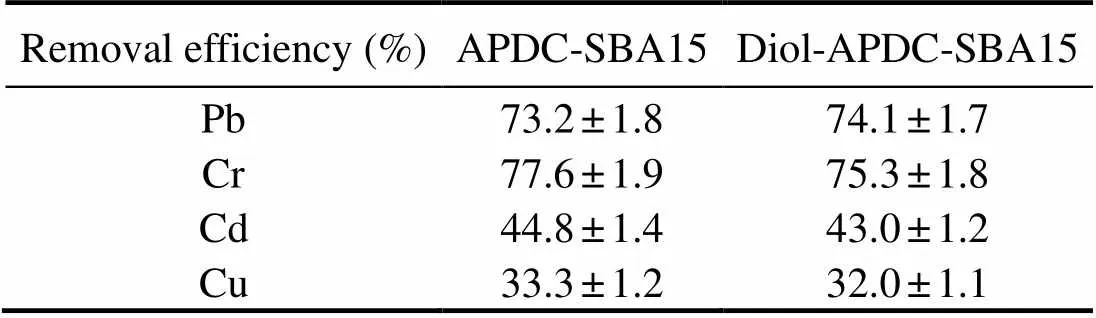
Table 2 Efficiencies of removing heavy metal ions in spiked cooking liquid of clam
were 74.1% (Pb), 75.3% (Cr), 43.0% (Cd), and 32.0% (Cu). The two mesoporous materials exhibited nearly the same removal efficiencies for the four heavy metal ions, indica- ting that the external modification with alkyl-diol groups did not influence the adsorption of heavy metal ions.
The losses of polysaccharides and proteins from the shell-fish processing liquids after heavy metal ion removal were also determined. The results are shown in Table 3. The loss- es of polysaccharides reduced from 7.3% to 5.5% when theabsorbent was changed from APDC-SBA15 to Diol-APDC- SBA15. Given the low concentration of proteins and ma- trix interference of the cooking liquid of clams, the losses of proteins were undetected for the two materials. The loss- es of proteins from oyster (Thunberg) hydro-lysate liquids were 14.6% and 1.2% for APDC-SBA15 and Diol-APDC-SBA15, respectively. The lower loss of pro- tein with Diol-APDC-SBA15 was due to the repulsion of proteins of the alkyl-diol groups, which has been previous- ly reported (Qi., 2010). The polysaccharide loss by Diol-APDC-SBA15 from the polysaccharide solution was 3.7%. This result indicated that the external modification of alkyl-diol groups can decrease the losses of protein and polysaccharides during heavy metal ion removal. In sum- mary, the Diol-APDC-SBA15 material can efficiently re- move heavy metal ions from shellfish processing liquids with low losses of proteins and polysaccharides.
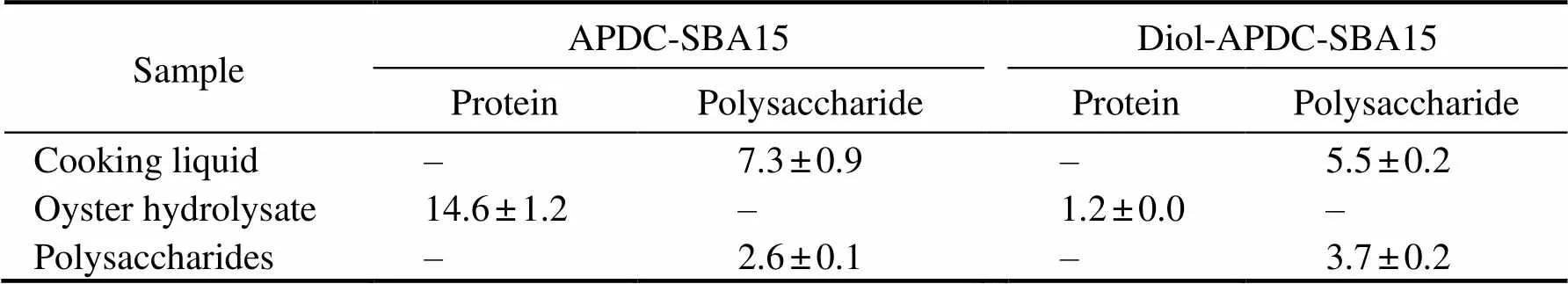
Table 3 Loss rates of protein and polysaccharide in shellfish processing liquid (%)
Note: ‘–’ not available (undetected).
4 Conclusions
Mesoporous silica modified internally with APDC and externally with alkyl-diol groups, which was named as Diol- APDC-SBA15, was successfully prepared and applied to remove heavy metal ions (Pb, Cr, Cd, and Cu) from shellfishprocessing liquids. The results showed that the Diol-APDC- SBA15 material can efficiently remove metal ions from standard heavy metal ion solutions and shellfish process- ing liquids. In addition, alkyl-diol group modification of the external surface of the mesoporous material efficiently de- creased the losses of proteins and polysaccharides during the removal process. Therefore, Diol-APDC-SBA15 is a promising sorbent material for the removal of metal ions from complex shellfish processing liquids.
Acknowledgements
This work was supported by the National Key R&D Pro- gram of China (No. 2018YFD0901004), the National NaturalScience Foundation of China (No. 31601538), the Key Science and Technology Program of Liaoning Province (No.2020JH1/10200001), the Fundamental Research Founda-tion of Education Department of Liaoning Province (No.JL202008), and the Science & Technology Innovation Foun- dation of Dalian (No. 2019J12SN61).
Barbosa, I. S., Brito, G. B., Santos, G. L., Santos, L. N., Teixei- ra, L. S. G., Araujo, R. G. O.,., 2018. Multivariate data ana- lysis of trace elements in bivalve molluscs: Characterization andfood safety evaluation.,273: 64-70, https://doi. org/10.1016/j.foodchem.2018.02.063.
Bille, L., Binato, G., Cappa, V., Toson, M., Pozza, M. D., Arcan- geli, G.,., 2015. Lead, mercury and cadmium levels in edi-ble marine molluscs and echinoderms from the Veneto Region (north-western Adriatic Sea–Italy).,50: 362- 370, https://doi.org/10.1016/j.foodcont.2014.09.018.
Bogdanovic, T., Ujevic, I., Sedak, M., Listes, E., Simat, V., Pe- tricevic, S.,., 2014. As, Cd, Hg and Pb in four edible shell- fish species from breeding and harvesting areas along the eas- tern Adriatic Coast, Croatia.,146: 197-203, https://doi.org/10.1016/j.foodchem.2013.09.045.
Deere, J., Magner, E., Wall, J. G., and Hodnett, B. K., 2003. Ad-sorption and activity of proteins onto mesoporous silica.,85: 19-23, https://doi.org/10.1023/A:102215640 5117.
Drewnowska, M., Falandysz, J., Chudzińska, M., Han?, A., Saba, M., and Bara?kiewicz, D., 2017. Leaching of arsenic and six- teen metallic elements frommushrooms after food processing.–,84: 861- 866, https://doi.org/10.1016/j.lwt.2017.04.066.
Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A., and Smith, F., 1956. Colorimetric method for determination of sugars andrelated substances.,28: 350-356, https://doi. org/10.1021/ac60111a017.
Gokila, S.,Gomathi, T., Sudha, P. N., and Anil, S., 2017. Removalof the heavy metal ion chromiuim(VI) using chitosan and algi- nate nanocomposites.,104: 1459-1468, https://doi.org/10.1016/j.ijbiomac. 2017.05.117.
Goubert-Renaudin, S., Gaslain, F., Marichal, C., Lebeau, B., Sch- neider, R., and Walcarius, A., 2009. Synthesis of dithiocarba- mate-functionalized mesoporous silica-based materials: Interest of one-step grafting.,33: 528-537, https://doi.org/10.1039/B811780B.
Hami Dindar, M., Yaftian, M. R., Pilehvari, M., and Rostamnia, S., 2015. SBA-15 mesoporous materials decorated with orga- nic ligands: Use as adsorbents for heavy metal ions.,12: 561-572, https://doi.org/10. 1007/s13738-014-0513-8.
Heidari, B., Bakhtiari, A. R., and Shirneshan, G., 2013. Concen- trations of Cd, Cu, Pb and Zn in soft tissue of oyster () collected from the Lengeh Port Coast, Persian Gulf, Iran: A comparison with the permissible limits for pub- lic health.,141: 3014-3019, https://doi.org/10. 1016/j.foodchem.2013.06.002.
Howard, M. D., Jay, M., Dziubla, T. D., and Lu, X., 2008. PEgylation of nanocarrier drug delivery systems: State of the art.,4: 133-148, https://doi. org/10.1166/jbn.2008.021.
Jadhav, S. A., Patil, V. S., Shinde, P. S., Thoravat, S. S., and Patil, P. S., 2020. A short review on recent progress in mesoporous silicas for the removal of metal ions from water.,74: 4143-4157, https://doi.org/10.1007/s11696-020-01255-6.
Jiang, F. M., Pu, Q. M., Ren, F. L., Huang, H. Q., Cao, F. Y., Li, Y.,., 2013. Green graft process for preparation of func- tional mesoporous silica materials.,17: 122-128, https://doi.org/10.1179/1433075X12Y.0000 000041.
Lee, J. Y., Chen, C. H., Cheng, S., and Li, H. Y., 2016. Adsorp- tion of Pb(II) and Cu(II) metal ions on functionalized large-pore mesoporous silica.,13: 65-76, https://doi.org/10.1007/s13762- 015-0841-y.
Li, P., Pan, Y., Fang, Y., Du, M., Pei, F., Shen, F.,.,2019. Concentrations and health risks of inorganic arsenic and me- thylmercury in shellfish from typical coastal cities in China: A simultaneous analytical method study.,278: 587-592, https://doi.org/10.1016/j.foodchem.2018.11.085.
Li, X., Ming, Q., Cai, R., Yue, T., Yuan, Y., Gao, Z.,., 2020. Biosorption of Cd2+and Pb2+from apple juice by the magne- tic nanoparticles functionalized lactic acid bacteria cells.,109: 106916, https://doi.org/10.1016/j.foodcont.2019. 106916.
Liu, Y., Luan, J., Zhang, C., Ke, X., and Zhang, H., 2019. The ad- sorption behavior of multiple contaminants like heavy metal ions and p-nitrophenol on organic-modified montmorillonite.,26: 10387- 10397, https://doi.org/10.1007/s11356-019-04459-w.
Lubran, M. M., 1978. The measurement of total serum proteins by the Biuret method.,8: 106.
Mirlean, N., Ferraz, A. H., Seus-Arrache, E. R., Andrade, C. F., Costa, L. P., and Johannesson, K. H., 2019. Mercury and se- lenium in the Brazilian subtropical marine products: Food com- position and safety.,84: 103310, https://doi.org/10.1016/j.jfca.2019.103310.
Moallaei, H., Bouchara, J. P., Rad, A., Singh, P., Raizada, P., Tran, H. N.,., 2020. Application ofsp. immobilized on multi-walled carbon nanotubes for solid-phase extraction and trace analysis of heavy metal cations.,322: 126757, https://doi.org/10.1016/j.foodchem.2020.126757.
Peng, Y., Shen, Y., Ge, M., Pan, Z., Chen, W., and Gong, B., 2018. Efficient extraction of heavy metals from collagens by sulfo- nated polystyrene nanospheres,,275: 377-384, https://doi.org/10.1016/j.foodchem.2018.09.111.
Pereira Santos, L. F., Sitonio Trigueiro, I. N., Lemos, V. A., Nó- brega Furtunato, D. M., and Cássia Vieira Cardoso, R., 2013. Assessment of cadmium and lead in commercially important seafood from S?o Francisco do Conde, Bahia, Brazil.,33: 193-199, https://doi.org/10.1016/j.foodcont.2013. 02.024.
Qi, Y., Wei, J., Wang, H., Zhang, Y., Xu, J., Qian, X.,., 2010. Improved selection of LMW over HMW proteins from human plasma by mesoporous silica particles with external modification.,80: 703-709, https://doi.org/10.1016/j.talanta.2009.07.050.
Qi, Y., Wu, D., Wei, J., Ding, K., Wang, H., Zhang, Y.,.,2010. Selective extraction of low molecular weight proteins bymesoporous silica particles with modified internal and external surfaces., 398: 1715- 1722, https://doi.org/10.1007/s00216-010-4081-1.
Radi, S., Toubi, Y., El-Massaoudi, M., Bacquet, M., Degoutin, S., and Mabkhot, Y. N., 2016. Efficient extraction of heavy metals from aqueous solution by novel hybrid material based on sili- ca particles bearing new Schiff base receptor.,223: 112-118, https://doi.org/10.1016/j.molliq. 2016.08.024.
Tapia, J., Vargas-Chacoff, L., Bertrán, C., Carrasco, G., Torres, F., Pinto, R.,., 2010. Study of the content of cadmium, chromium and lead in bivalve molluscs of the Pacific Ocean (Maule Region, Chile).,121: 666-671, https:// doi.org/10.1016/j.foodchem.2009.12.091.
Vunain, E., Mishra, A. K., and Mamba, B. B., 2016. Dendrimers, mesoporous silicas and chitosan-based nanosorbents for the re-moval of heavy-metal ions: A review., 86: 570-586, https://doi.org/10. 1016/j.ijbiomac.2016.02.005.
Wang, X., Wu, J., Yu, B., Dong, K. F., and Zhang, C., 2020. Heavy metals in aquatic products and the health risk assess- ment to population in China.,27: 22708-22719, https://doi.org/10.1007/ s11356-020-08685-5.
Yan, F., Sun, L., Li, F., Zhuang, J., Wang, H., and Yang, W., 2011.Mesoporous silica-coated superparamagnetic particles prepared by pseudomorphic transformation and their application in pu- rification of plasmid DNA.,13: 6613-6620, https://doi.org/10.1007/s11051-011-0569-7.
Yang, H., Hu, Y., Wang, X., Fu, W., Tian, H., and Alam, E., 2019.Investigation on synthesis of ion-imprinted mesoporous adsor-bents by using ultrasound- and microwave-assisted preparation and their dynamic adsorption properties on heavy metals.,26: 10987-10999, https://doi.org/10.1007/s11356-019-04436-3.
Zacaroni, L. M., Magriotis, Z. M., Gra?as Cardoso, M., Santiago, W. D., Mendon?a, J. G., Vieira, S. S.,., 2015. Natural clay and commercial activated charcoal: Properties and application for the removal of copper from cacha?a.,47: 536-544, https://doi.org/10.1016/j.foodcont.2014.07.035.
Zhang, D., and Li, J. H., 2013. Ordered SBA-15 mesoporous si- lica with high amino-functionalization for adsorption of heavy metal ions.,58: 879-883, https://doi. org/10.1007/s11434-012-5594-0.
Zhang, D., Xu, W., Cai, J., Cheng, S. Y., and Ding, W. P., 2020. Citric acid-incorporated cellulose nanofibrous mats as food ma- terials-based biosorbent for removal of hexavalent chromium from aqueous solutions.,149: 459-466, https://doi.org/10.1016/j.ijbiomac. 2020.01.199.
Zhang, L., Ma, Z. C., and Liu, J., 2008. Influence of bovine serum albumin on the adsorption equilibrium between Cu(2+) and δ- MnO2.,27: 756-761, https://doi.org/ CNKI:SUN:STXZ.0.2008-05-014.
Zhang, Y., Cao, X., Sun, J., Wu, G., Wang, J., and Zhang, D., 2020. Synthesis of pyridyl Schiff base functionalized SBA-15 mesoporous silica for the removal of Cu(II) and Pb(II) from aqueous solution.,94: 658-670, https://doi.org/10.1007/s10971-019-05205-x.
Zhao, D. Y., Huo, Q. S., Feng, J. L., Chmelka, B. F., and Stucky, G. D., 1998. Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered, hydrother- mally stable, mesoporous silica structures.,120: 6024-6036, https://doi.org/10. 1021/ja506344k.
Zhu, J. Y., Zhu, X. Y., Gu, J. L., Zhao, L. M., Jiang, L. H., and Qiu, Y. J., 2016. Effective adsorption and concentration of carnosine by nickel species within mesoporous silica.–, 74: 211-218, https://doi.org/10.1016/j.lwt. 2016.07.016.
(September 16, 2021; revised October 27, 2021; accepted June 28, 2022)
? Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2023
Corresponding author. Tel: 0086-411-84763508
E-mail: qczhao@dlou.edu.cn
(Edited by Qiu Yantao)
 Journal of Ocean University of China2023年1期
Journal of Ocean University of China2023年1期
- Journal of Ocean University of China的其它文章
- Quality Changes and Safety Evaluation of Ready-to-Eat Roasted Antarctic Krill (Euphausia superba) During Storage at Room Temperature (25℃)
- The Influence of Sea Sprays on Drag Coefficient at High Wind Speed
- Ship Weather Routing Based on Hybrid Genetic Algorithm Under Complicated Sea Conditions
- L-Band Analysis of the Effects of Oil Slicks on Sea Wave Characteristics
- A Method for Reducing Ocean Wave-Induced Magnetic Noises in Shallow-Water MT Data Using a Complex Adaptive Filter
- Wave Force on the Crown Wall of Rubble Mound Breakwaters at Intermediate Depths
