Synthesis,Structure,and Properties of Rare-Earth Complexes Based on Pyridine Dicarboxylic Acid Ligands
WANG Sheng HUANG Rui-Qin WANG Shao-Cong LIU Zheng TANG Qun
(Guangxi Key Laboratory of Electrochemical and Magneto-chemical Functional Materials,College of Chemical and Biological Engineering,Guilin University of Technology,Guilin,Guangxi 541004,China)
Abstract:The 2,6-lutidine-3,5-dicarboxylic acid(H2L1)was used as the main ligand,1,10-phenanthroline(L2)as the auxiliary ligand,and the complexes[Dy2(L1)3(L2)2]n(1),{[Tb2(L1)3(L2)2]·5H2O}n(2),and{[Eu2(L1)3(L2)2]·5H2O}n(3)were synthesized by solvothermal reaction with dysprosium nitrate pentahydrate,terbium nitrate hexahydrate,and europium nitrate hexahydrate,respectively.The structures and properties of the complexes were characterized by single-crystal X-ray diffraction,IR,fluorescence spectroscopy,and thermogravimetric analysis.The results show that complexes 1-3 all use rare-earth ions as metal nodes to connect with ligands L12-and L2to form an infinitely extending 1D chain structure.CCDC:2107136,1;2109709,2;2109716,3.
Keywords:rare-earth complex;dipicolinate;1,10-phenanthroline;crystal structure
0 Introduction
Coordination polymers(CPs)are multifunctional materials formed by the self-assembly of metal salts and organic ligands.Complexes have shown excellent performance in catalysis[1-2],adsorption[3-4],loading[5-6],detection[7-8],sensing[9-10],anti-corrosion[11-12]and attracted more and more attention of scientific researchers.The complexes with tunable pore size and function can be obtained by changing factors such as metal elements,organic ligands,and reaction conditions.Polycarboxylic acid ligands are widely used to construct 1D,2D,and 3D supramolecular CPs with novel structures and unique properties due to their various flexible bonding methods.Pyridine carboxylic acid ligands with special configurations have a strong coordination ability with metals and pore size and polarity can be adjusted by uncoordinated nitrogen atoms to obtain porous materials with special structures and properties.Such complexes synthesized by pyridine carboxylic acid ligands have high stability and generally have relatively high melting points.In recent years,the superiority of nitrogenous heterocyclic ligands has greatly promoted the development of such complexes[13-15].Abdolmaleki[16]used picolinic acid as the main ligand and 2-methylpyrazole as the auxiliary ligand,and combined calcium,cobalt,and zirconium to synthesize four complexes.The research showed that the synthesized complexes had antibacterial properties.Zhao[17]took europium as the metal center,combined with dipicolinic acid to synthesize complexes with good fluorescence properties,and alkaline phosphatase can be rapidly detected by the photo-sensing effect of the complexes.
In this work, three kinds of complexes[Dy2(L1)3(L2)2]n(1),{[Tb2(L1)3(L2)2]·5H2O}n(2),and{[Eu2(L1)3(L2)2]·5H2O}n(3)were synthesized by solvothermal reaction with the main ligand 2,6-lutidine-3,5-dicarboxylic acid(H2L1),the auxiliary ligand 1,10-phenanthroline(L2),and rare-earth metal ions.
1 Experimental
1.1 Materials and measurements
Dysprosium nitrate pentahydrate(Dy(NO3)3·5H2O),terbium nitrate hexahydrate(Tb(NO3)3·6H2O),europium nitrate hexahydrate(Eu(NO3)3·6H2O),N,N-dimethylformamide(DMF),H2L1,L2,etc.were all analytically pure reagents,purchased from Shanghai MacLean Reagent Company,and not purified when used.
A Perkin-Elmer 240Q elemental analyzer(American)was used for the determination of C,N,and H elements.IR spectra were determined in a range of 4 000-400 cm-1by using Shimadzu FTIR-8400 Fourier transform infrared spectrometer(Japan)and KBr tablet.UV-Vis spectra were measured by a UV-5500PC dualbeam UV-Vis spectrophotometer(China)at room temperature.The fluorescence spectra were measured by an EDXRF fluorescence spectrometer(China)at room temperature.The samples were dissolved in reference solvent DMF(10 μmol·L-1)in UV-Vis and fluorescence measurements. Thermogravimetric analyses(TGA)were performed on an SDT-Q600 synchronous thermogravimetric/differential thermogravimetric analyzer(American).
1.2 Synthesis of the complexes
Complex 1:a mixture of H2L1(0.039 0 g,0.20 mmol),L2(0.036 0 g,0.20 mmol),Dy(NO3)3·5H2O(0.087 7 g,0.20 mmol),DMF(6 mL),and distilled water(4 mL)was added to the beaker and stirred at room temperature until the mixed solution was clear.The solution was stirred for 30 min before the solution was transferred to the Teflon-lined reaction kettle.Finally,the solution in the reaction kettle was heated at 90℃ for 72 h and then cooled at a rate of 5℃·h-1until it dropped to room temperature.The reaction kettle was taken out and light green transparent needle crystals were obtained.Yield:53.36%(based on Dy).Anal.Calcd.for C51H37Dy2N7O12(%):C 48.43,N 7.75,H 2.95;Found(%):C 48.38,N 7.68,H 2.98.
Complex 2:except for Tb(NO3)3·6H2O(0.130 6 g,0.20 mmol),the synthesis method and steps of complex 2 were the same as those of complex 1.After the reaction kettle had cooled to room temperature,light yellow transparent needle-shaped crystals were isolated.Yield:54.6%(based on Tb).Anal.Calcd.for C51H47N7O17Tb2(%):C 45.45,N 7.27,H 3.51;Found(%):C 45.41,N 7.32,H 3.57.
Complex 3:except for Eu(NO3)3·6H2O(0.089 2 g,0.20 mmol),the synthesis method and steps of complex 3 were the same as those of complex 1.After the reaction kettle had cooled to room temperature,light yellow transparent needle-shaped crystals were obtained.Yield:54.6% (based on Eu).Anal.Calcd.for C51H47Eu2N7O17(%):C 45.92,N 7.35,H 3.55;Found(%):C 45.83,N 7.32,H,3.59.
1.3 Crystal structure determination
The crystals with dimensions of 0.21 mm×0.20 mm×0.16 mm(1),0.19 mm×0.17 mm×0.14 mm(2),and 0.18 mm×0.15 mm×0.12 mm(3)were selected to be measured with an Agilent Technologies G8910A single crystal diffractometer at 293 K with MoKαradiation(λ=0.071 073 nm)andφ-ωscanning was used to collect the diffraction point data of the crystal.The orig-inal data were restored using the CryAlis Pro program,and all data were corrected for Lp factor and empirical absorption[18].The rough structure was solved by the direct method in SHELXS-2014,and the full-matrix least-squares method was used to refine the coordinates of non-hydrogen atoms and their anisotropic temperature factors by using the SHELXL-2014 program.All hydrogen atom coordinates were obtained by theoretical hydrogenation.The free water molecules of complexes 2 and 3 were highly disordered and could not be modeled properly,thus the SQUEEZE routine of PLATON was applied to remove the contributions to the scattering from the solvent molecules.The relevant crystallographic data are listed in Table 1,and the principal bond lengths and bond angles are listed in Table 2.
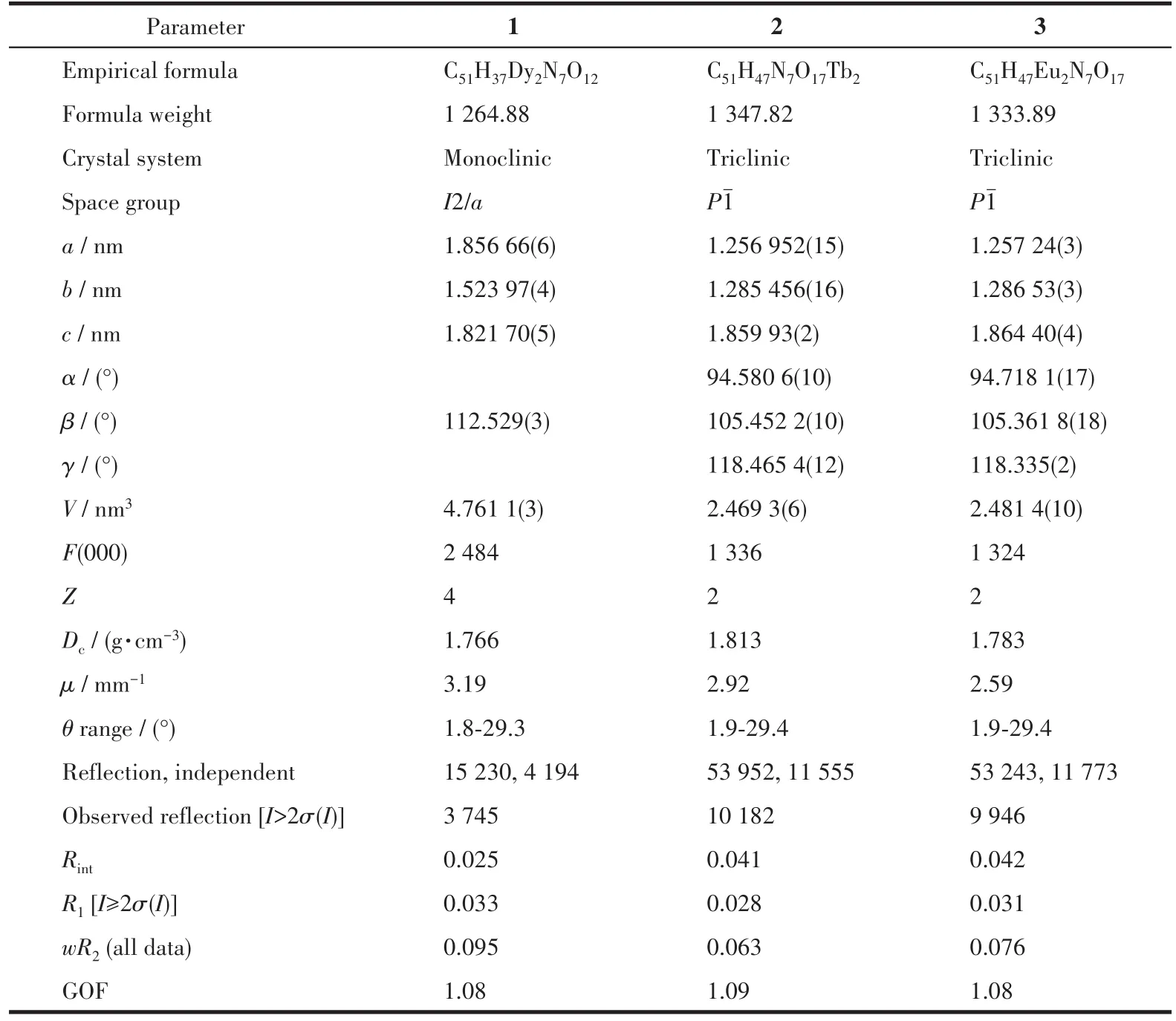
Table 1 Crystallographic data for complexes 1-3
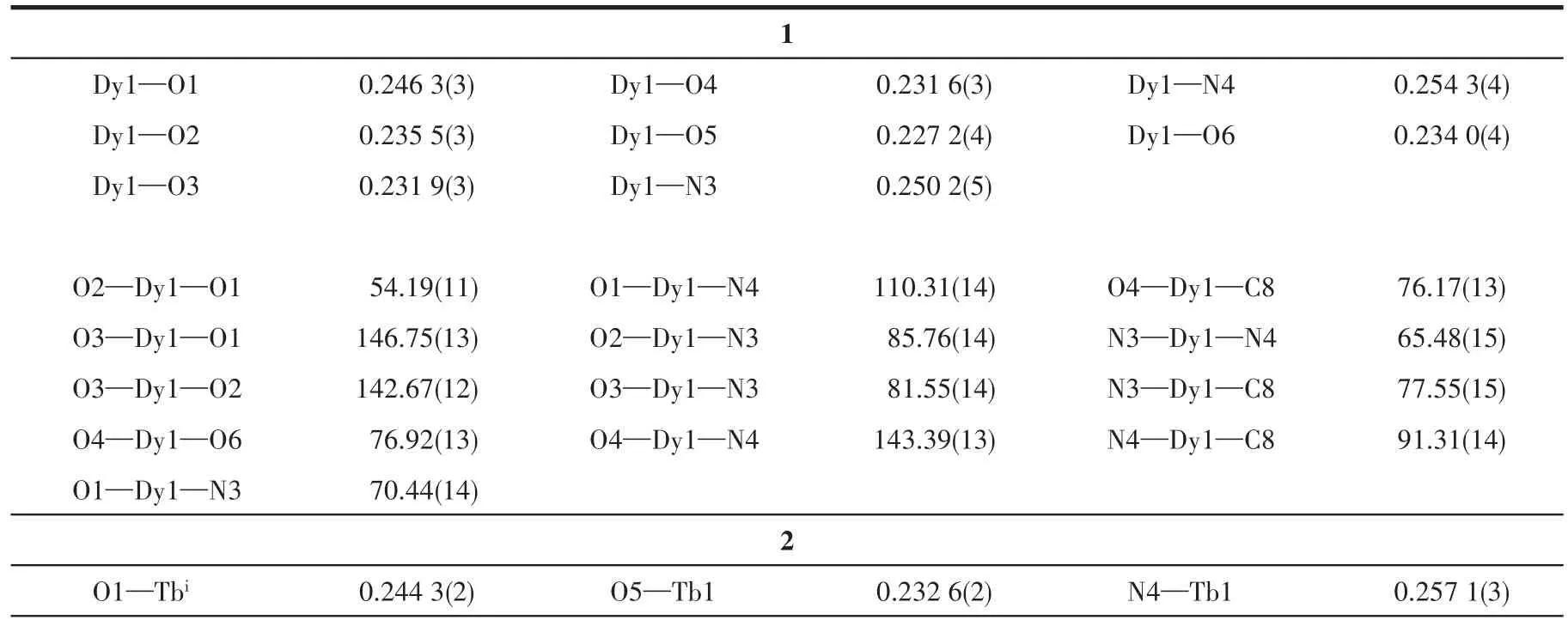
Table 2 Selected bond lengths(nm)and angles(°)for complexes 1-3
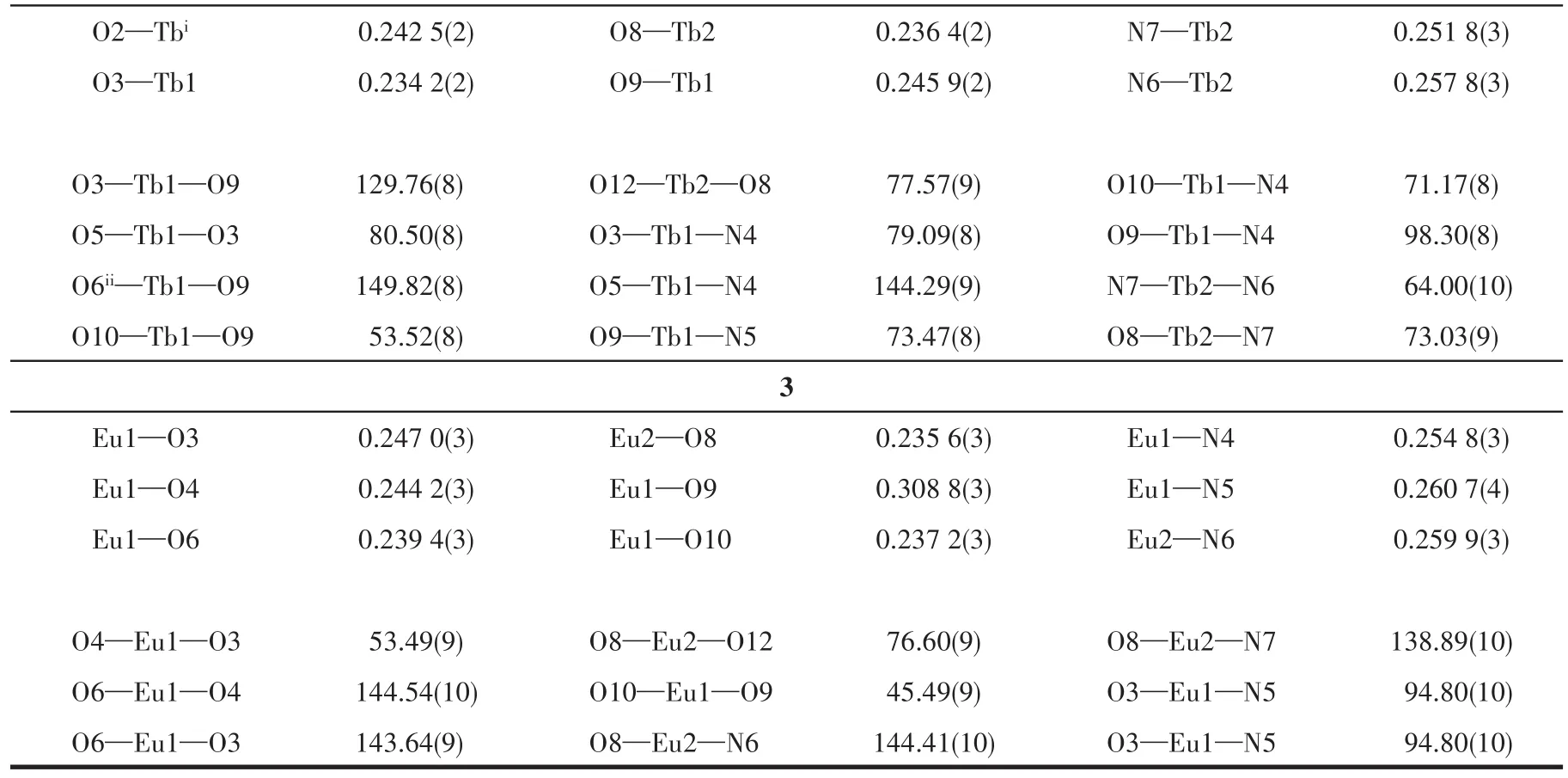
Continued Table 2
CCDC:2107136,1;2109709,2;2109716,3.
2 Results and discussion
2.1 Crystal structural analysis
2.1.1 Crystal structure of complex 1
The basic structure of complex 1 contains two Dy(Ⅲ)central ions,three main L12-ligands,and two auxiliary L2ligands and the asymmetric unit structure is shown in Fig.1a.In this complex,the central Dy(Ⅲ)ion is coordinated with six carboxyl oxygen atoms(O1,O2,O3,O4,O5,O6)from L12-and two nitrogen atoms(N3,N4)from L2respectively,forming an eight-coordinate structure.Among the atoms participating in the coordination,the sum of the bond angles formed by the four atoms of O3,O6,O4,and O5 is 359.989°,which is close to the ideal 360°,indicating that the four coordinating atoms form a plane and there is a good flatness.The Dy(Ⅲ)ion is in the center of the twisted triangular prism composed of O3,O6,O4,O5,O2,and N3.The coordinated atoms O1 and N4 on the ligand are on the periphery of the triangular prism,forming a twist double-capped triangular prism with the six atoms that make up the triangular prism(Fig.1b).The Dy(Ⅲ)ion was used as the metal node to connect with L12-and L2to form an infinitely extending 1D chain structure in complex 1(Fig.1c).
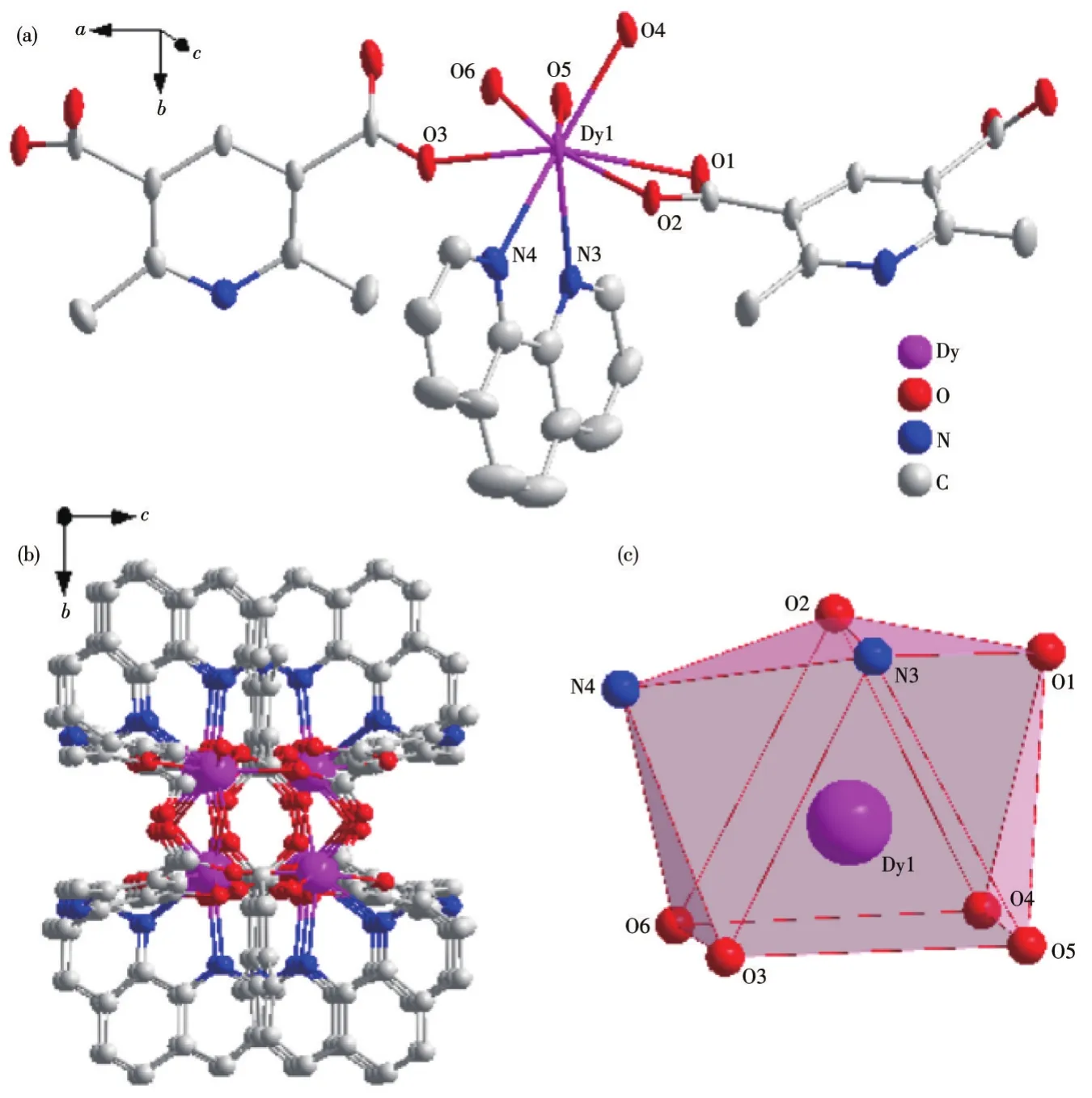
Fig.1 Crystal structure of complex 1:(a)ellipsoid diagram with 40%probability level;(b)polyhedral diagram;(c)3D stacking diagram along the a-axis
The Dy—O bond lengths are between 0.227 2 and 0.246 3 nm and the Dy—N bond lengths are between 0.250 2 and 0.254 3 nm.The O—Dy—X(X=O,N)bond angles are between 54.19(11)°and 146.75(13)°.These bond lengths and bond angles are all within the normal range.
2.1.2 Crystal structure of complexes 2 and 3
Single crystal X-ray crystallographic analysis reveals that complexes 2 and 3 are isomorphous.Therefore,only the structure of complex 2 will be described in detail.There are two central Tb(Ⅲ)ions,three mainligands,and two auxiliary L2ligands in the basic structure of complex 2 and the asymmetric unit structure is shown in Fig.2a.Similar to the Dy(Ⅲ)ion of complex 1,the central Tb(Ⅲ))ion is coordinated with six carboxyl oxygen atoms of L12-and two nitrogen atoms of L2respectively to form an eight-coordinate structure in complex 2.Like complex 1,four coordinated atoms N4,N5,O9,and O10 form a plane with good planarity in complex 2,and eight atoms coordinating with the Tb(Ⅲ)ions also form a twisted double-capped triangular prism(Fig.2b).The Tb(Ⅲ)ion was used as the metal node to connect with L12-and L2to form an infinitely extending 1D chain structure in complex 2(Fig.2c).
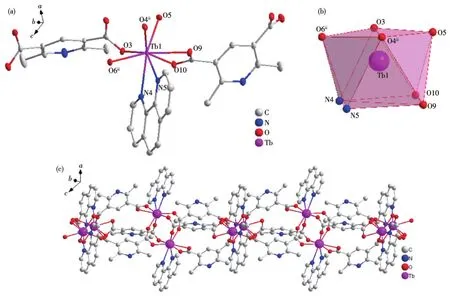
Fig.2 Crystal structure of complex 2:(a)ellipsoid diagram with 50%probability level;(b)polyhedral diagram;(c)1D chain structure
The Tb—O bond lengths are between 0.232 6 and 0.245 9 nm and the Tb—N bond lengths are between 0.251 8 and 0.257 8 nm.The O—Tb—X(X=O,N)bond angles are between 53.52(8)°and 149.82(8)°.These bond lengths and bond angles are all within the normal range.
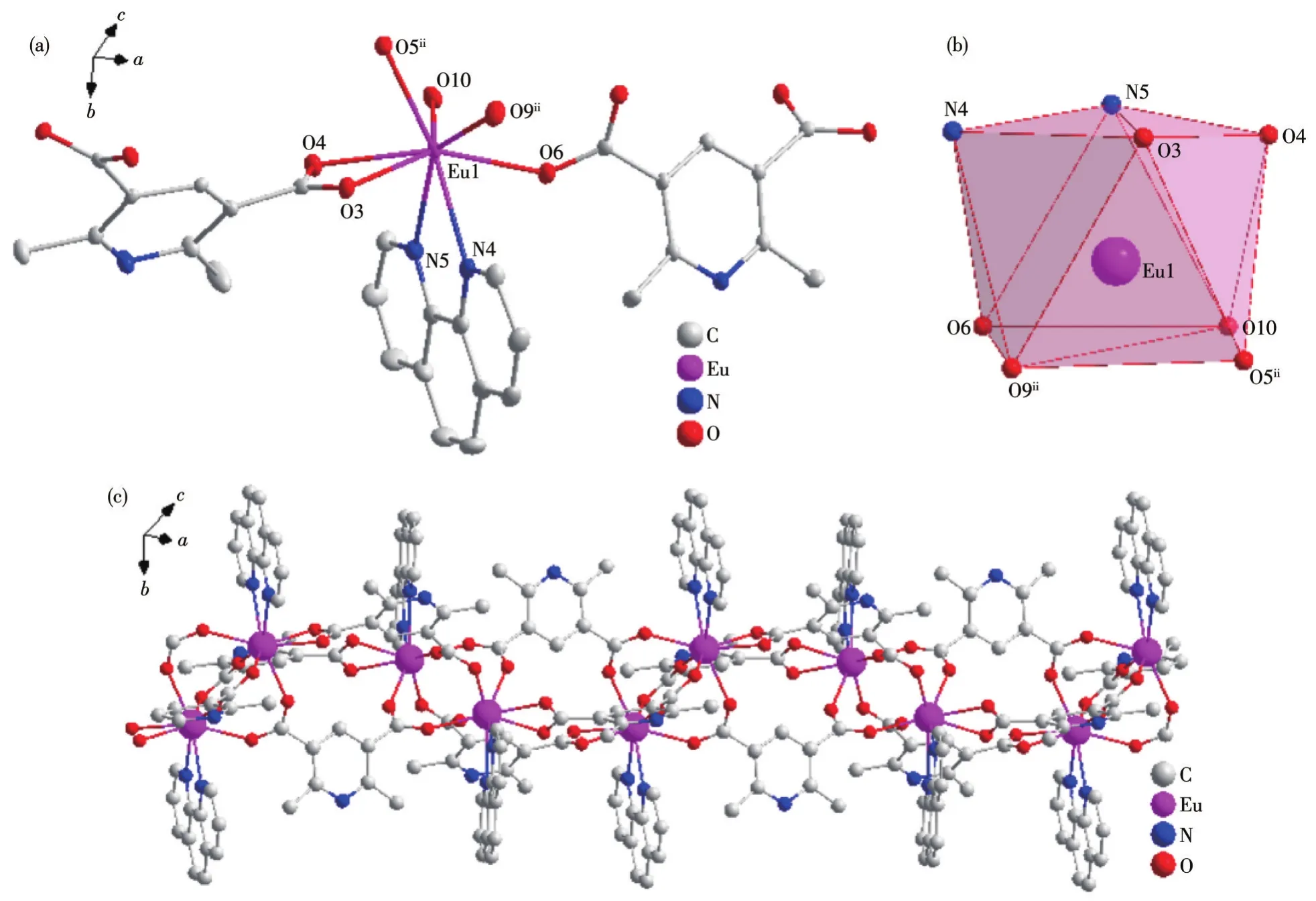
Fig.3 Crystal structure of complex 3:(a)ellipsoid diagram with 40%probability level;(b)polyhedral diagram;(c)1D chain structure
2.2 IR spectroscopic analysis of complexes 1-3
The IR spectra of ligands H2L1,L2,and complexes 1-3 are shown in Fig.4.The N—H stretching vibration absorption peaks at 3 395 cm-1for L2and 3 411 cm-1for H2L1,and this peak shifted to 3 416,3 420,and 3 420 cm-1for complexes 1-3,respectively.The carboxyl group O—H stretching vibration absorption peak appeared at 3 547 cm-1for H2L1but it did not appear for complexes 1-3,indicating that the carboxyl group is involved in the coordination reaction.The C=O stretching vibration absorption peak for H2L1appeared at 1 718 cm-1and this peak appeared at 1 625,1 626,and 1 631 cm-1for complexes 1-3,indicating that carbonyl oxygen shifts after forming coordinate bonds with metals.The C—H stretching vibration absorption peak for H2L1appeared at 1 385 cm-1while this peak appeared at 1 386,1 390,and 1 390 cm-1for complexes 1-3,respectively.These results indicate that both ligands participate in the coordination reaction.
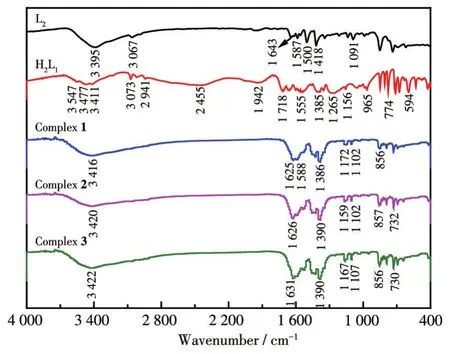
Fig.4 IR spectra of complexes 1-3 and the ligands
2.3 UV-Vis spectrum analysis of complexes 1-3
The UV-Vis spectra of ligands 1-3 and ligands H2L1and L2are shown in Fig.5.The ligands and complexes 1-3 all had an absorption peak near 270 nm.According to the molecular structure analysis of the ligands,this peak corresponds to the absorption wavelengths generated by theπ-π*transition of the unsaturated bond,which can be assigned to the B absorption band on the aromatic ring.Compared with H2L1,the absorption peaks of complexes 1-3 were red-shifted because the conjugation system in the ligand molecule increases,and the degree of conjugation increases after the ligand is coordinated.Compared with H2L1,the absorption wavelengths of complexes 1-3 had a certain degree of redshift,which may be because the HOMO energy level of the metal complex increases,and the energy level difference between HOMO and LUMO decreases after the ligand is coordinated with metal ions[19].
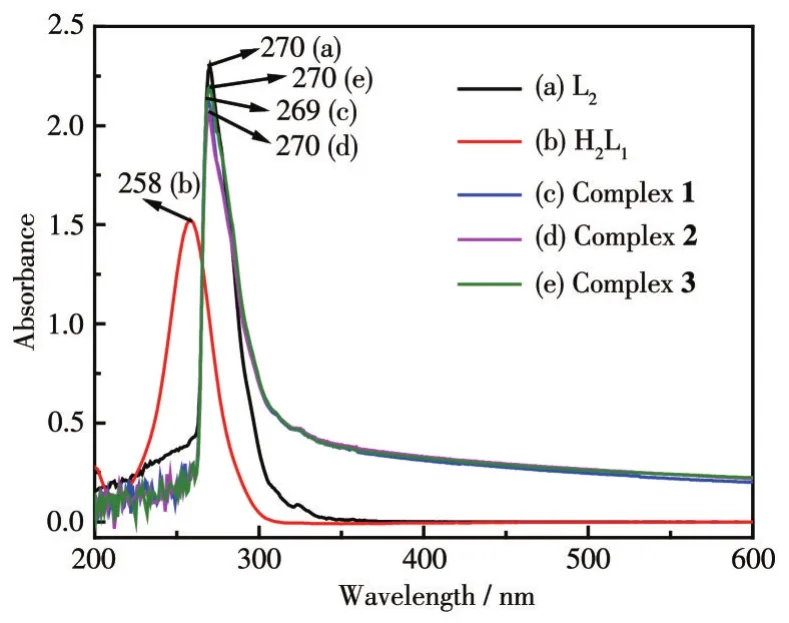
Fig.5 UV-Vis spectra of complexes 1-3 and the ligands
2.4 Fluorescence spectrum analysis of complexes 1-3
The structures of ligands H2L1,L2,and complexes 1-3 all contain nitrogenous heterocycles and the conjugatedπbonds of the heterocycles easily emit fluorescence.As shown in Fig.6,the maximum emission wavelengths of ligands H2L1,L2,and complexes 1-3 were 294,362,412,550,and 413 nm(λex=287,289,300 320,and 340 nm,respectively).The characteristic emission peaks of Dy(Ⅲ)ions disappeared for complex 1,indicating that there is no effective energy transfer from the ligand to Dy(Ⅲ)ions.After the ligand participated in the coordination reaction,the degree of internal conjugation of the complex molecule was enhanced,and the fluorescence intensity increased.The emission peaks appearing at 494,550,and 590 nm are attributed to the transitions of5D4→7F6,5D4→7F5,and5D4→7F4of Tb(Ⅲ)ions in complex 2[20],and the luminescence of complex 2 is dominated by the emission of5D4→7F5at 550 nm with the highest intensity.The emission peaks at 548,597,and 621 nm are attributed to the transitions of5D0→7F0,5D0→7F1,and5D0→7F2of Eu(Ⅲ) ions in complex 3,and the luminescence of complex 3 is dominated by the emission of5D0→7F2at 621 nm with the highest intensity.
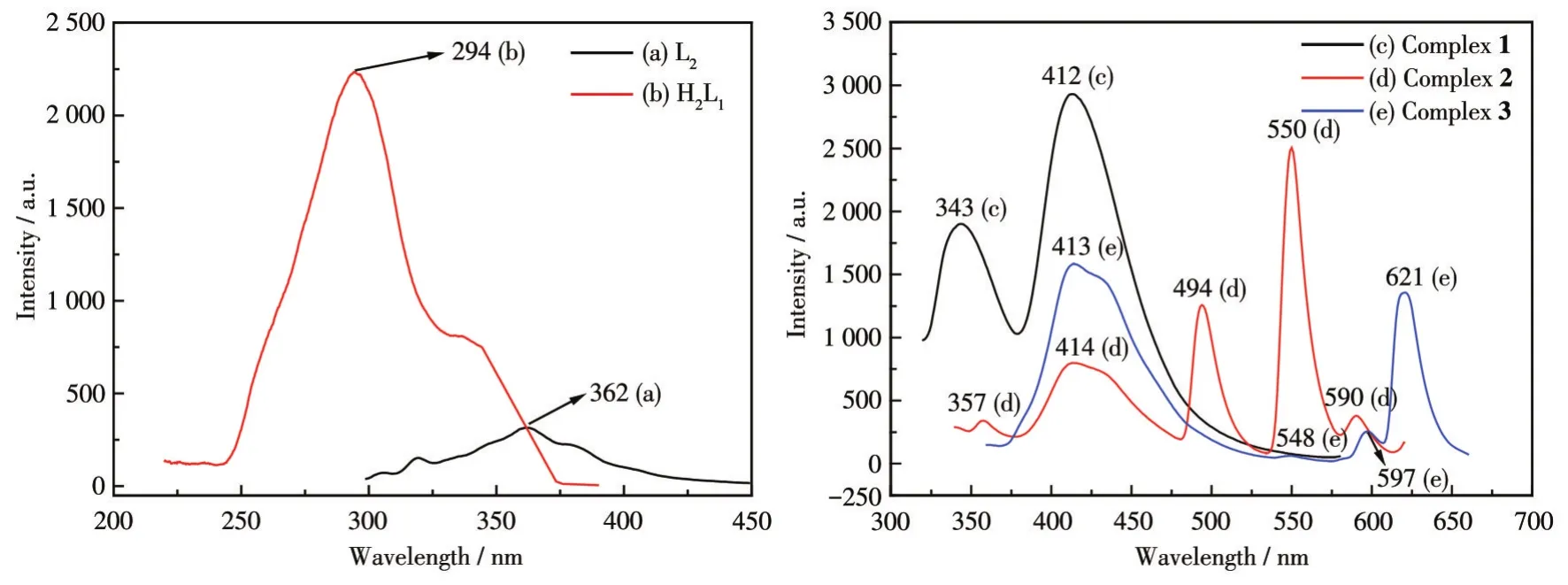
Fig.6 Fluorescence emission spectra of the ligands(left)and complexes 1-3(right)
2.5 TGA of complexes 1-3
To characterize the thermal stability of these complexes,TGA of the complexes was performed under a nitrogen atmosphere(Fig.7).Complexes 2-3 began to decompose when initially heated untila plateau appeared around 97℃,which should be caused by the decomposition of crystal water molecules and dissociative water molecules in the complex molecules.The weight loss before 100℃can be attributed to the hygroscopicity of complex 1.The decomposition occurred in a range of 260-475℃should be the decomposition of the carboxyl group in the molecule and then the weight loss curve began to decline rapidly,which was attributed to the decomposition of the ligand heterocyclic skeleton.The TG curves of complexes 1-3 at 631,574,and 604℃tended to be stable respectively.The total weight loss rate of complex 1 was 72.45%and the residual weight was 27.55%(Calcd.29.47%).The residue can be classified as Dy2O3.The residual weight of complex 2 was 26.84%(Calcd.27.14%)and the residue can be assigned to Tb2O3.The residual weight of complex 3 was 26.40%(Calcd.26.49%)and the residue can be assigned to Eu2O3.
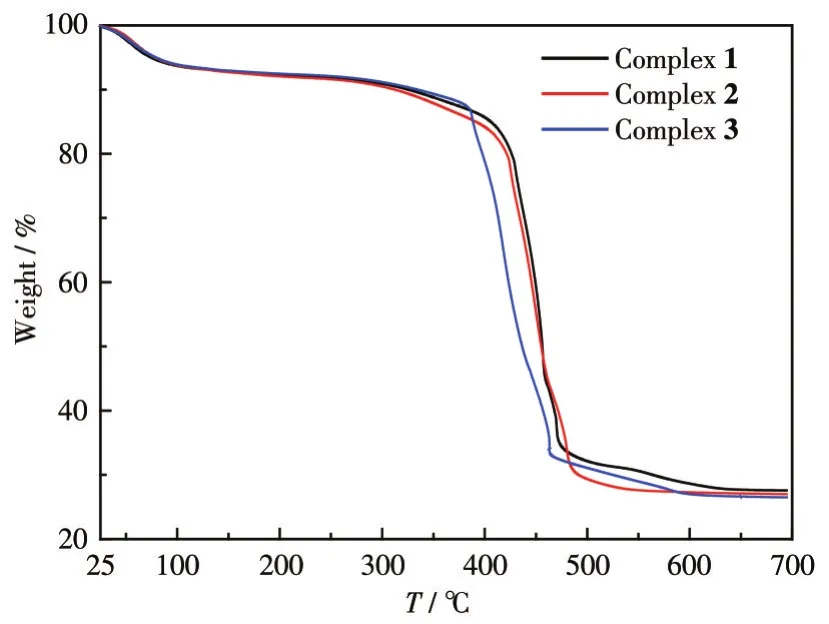
Fig.7 TG curves of complexes 1-3
3 Conclusions
In this work,three rare-earth-based pyridine acid complexes were successfully synthesized by the hydrothermal method.The structures and properties of the complexes were characterized by various analysis and testing methods and the result confirmed that the synthesized substances are all target products.Complexes 1-3 have the 1D chain structure formed by the rareearth ions and the ligands connecting infinitely in a carboxyl bridging manner.
- 無機化學(xué)學(xué)報的其它文章
- Facile Synthesis of Si@LiAlO2 Nanocomposites as Anode for Lithium-Ion Battery
- Solvent-Controlled Morphology of Ni-BTC and Ni-BDC Metal-Organic Frameworks for Supercapacitors
- 基于多金屬氧酸鹽和多壁碳納米管的雙酚A電化學(xué)傳感器的構(gòu)建與性質(zhì)
- A Fast-Responsive Mitochondria-Targeting Fluorescent Probe Detecting Hypochlorite in Living Cells and Zebrafish
- A Complex of Silver(Ⅰ)with N-O Chelating Agent and Phosphine Ligand:Design,Structures,and Biological Activity
- Synthesis,Structure,Magnetic,and Fluorescent Sensing Properties of Cobalt(Ⅱ)Coordination Polymer Based on 1-(3,5-Dicarboxybenzyl)-1H-pyrazole-3,5-dicarboxylic Acid

