High-Performance Palladium-Based Catalyst Boosted by Thin-layered Carbon Nitride for Hydrogen Generation from Formic Acid
Zhicong Sun, Ergui Luo, Qinglei Meng, Xian Wang, Junjie Ge,*,Changpeng Liu,*, Wei Xing
1 Laboratory of Advanced Power Sources, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, China.
2 University of Science and Technology of China, College of Applied Chemistry and Engineering, Hefei 230026, China.
3 Jilin Province Key Laboratory of Low Carbon Chemical Power Sources, Changchun 130022, China.
4 State Key Laboratory of Electroanalytical Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences,Changchun 130022, China.
Abstract:Since the First Industrial Revolution, traditional fossil energy (coal,petroleum,etc.)has been the most important energy source. However, with social progress and technological development, energy consumption continues to increase. But fossil energy not only has limited reserves, it also causes serious problems (environmental pollution, the greenhouse effect). Therefore, the research and development of clean and sustainable energy are particularly important. One research focus is hydrogen energy. Hydrogen is a promising energy carrier due to its high energy density, clean-burning characteristics, and sustainability. However, the challenges of hydrogen storage and transportation seriously limit its practical application in proton exchange membrane fuel cells. A potential solution is hydrogen storage in the form of a more stable precursor. One such precursor, formic acid, decomposes easily at room temperature in the presence of a catalyst without also producing toxic gases. Effective catalysts for formic acid decomposition (FAD)are key to hydrogen production by this method. In this study, a high-performance palladium (Pd)-based catalyst boosted by thin-layered carbon nitride was prepared for formic acid decomposition. First, trimeric thiocyanate was calcined by a one-step method to obtain carbon nitride (C3N4-S)directly, followed by fabrication of a Pdbased FAD catalyst with C3N4-S as support (Pd/C3N4-S). During the pyrolysis of thiocyanuric acid, the overflow of -SH in the precursor had a peeling effect, so that the C3N4 formed as a thin, broken layer with a large specific surface area and pore volume. Because of the improved specific surface area and pore volume and the resulting large number of defect attachment sites, the C3N4-S support effectively dispersed Pd nanoparticles. Furthermore, owing to the electron effect between the support and the metal, the Pd2+ content on the catalyst surface could be adjusted effectively. Pd/C3N4-S showed excellent FAD performance. This catalyst decomposed formic acid into CO2 and H2 effectively at 30 °C. The turnover frequency and mass activity were as high as 2083 h-1 and 19.52 mol·g-1·h-1, respectively. Testing of the gas product by gas chromatography showed that it did not contain CO, indicating that the Pd/C3N4-S catalyst had excellent selectivity. The catalyst also had good stability: its performance decreased by less than 10% after four testing cycles. This study provides a guiding example of development of a formic acid hydrogen production catalyst with high cost performance and a simple preparation method.
Key Words:Porous carbon nitride;Thin layer;Palladium nanoparticles;Heterogeneous catalysis;Formic acid dehydrogenation
1 Introduction
Theoretical analysis and experimental research have shown that formic acid (FA)is one of the most promising hydrogen storage materials as liquid organic hydrogen carrier1-4. First,compared to hydrogen storage in high-pressure cylinders, FA can be easily stored and transported because of its liquid nature5.Second, compared to lanthanum-nickel alloy hydrogen storage system, FA has higher hydrogen storage capacity (53 g·L-1)and faster hydrogen production rate without consuming excessive energy6,7. Finally, compared to other small organic molecules,FA produces fewer CO-poisoned species and generates hydrogen at lower temperatures (hydrogen can be produced at room temperature)5,8,9. Moreover, it also has a wide range of sources;it is not only one of the main products of biomass processing,but also of industrial by-products and CO2photoelectric reduction products10-12.
Formic acid decomposition (FAD)can be carried out in the following two ways. One is the dehydrogenation reaction(HCOOH → CO2+ H2)that generates H2and CO2; the other is the side reaction (HCOOH → CO + H2O)that needs to be suppressed to prevent the generation of CO and H2O13-15.Therefore, a highly efficient catalyst for FAD needs to have both high activity and high selectivity. Although both the homogeneous and heterogeneous catalytic systems have been constructed and utilized for FAD, the development of homogeneous catalysts have been seriously restricted due to their inherent drawbacks including potential hazard to environment and difficulty in recycling and utilization16.However, heterogeneous catalysts with the characteristics of no pollution, low operating temperature, and easy recycling, have attracted extensive attention of researchers.
It has been proved that carbon-based supports can effectively disperse metal nanoparticles (NPs), and palladium (Pd)is the most effective metal for catalyzing the decomposition of FA.Therefore, carbon-supported Pd catalysts have been rapidly developed in recent years17,18. Studies have shown that the modification of catalyst by doping with heteroatoms (such as B19,N20-26,etc.)can effectively improve the performance of the catalyst. In 2016, Caoet al.combined chitosan and urea with the aid of acetic acid, by changing the urea content, and then calcined the above mentioned mixtures to obtain a series of CN compounds with different N contents, as the support of Pd-based catalyst20. The results showed that the introduction of pyridine N could effectively adjust the electron cloud density of Pd as well the surface Lewis acid-base characteristics, thus significantly improving the performance of the catalyst.
The N-doped carbon support used for the decomposition of FA was analyzed, and its preparation methods were mainly divided into the following three types: direct calcination27, postdoping20,22and re-doping24. The support obtained by direct calcination is generally blocky. The problems of unstable N doping amount and poor experimental repeatability exist in the process of post-doping and re-doping. Moreover, re-doping can easily cause agglomeration of metal particles in the calcination process.
Recently, a N-doped porous carbon (specific surface area of 820.5 m2·g-1)prepared with urea as the N source and graphitized carbon nitride as the C source was used to prepare the supported Pd-based catalyst24. The TOF value is 1029 h-1. Our group28recently reported a Pd-based catalyst prepared with a ZIF-8 derivative with a high specific surface area (1320 m2·g-1)as a carrier. Its TOF is 1166 h-1under ambient conditions. Both of the above carriers have a high specific surface area and are N-doped carriers prepared by the post-doping method with unstable doping amount, indicating that carriers with a high specific surface area and N-doping can be effectively improved FAD performance of Pd-based catalysts.
Traditional carbon nitride has a higher content of pyridine N,but has a smaller specific surface area, making it difficult to disperse metal NPs. Based on the results of our previous study,we did not introduce a second support directly using trimeric thiocyanate as a precursor, calcination was carried out to obtain a thin layer of carbon nitride support (C3N4-S), and Pd-based catalysts were prepared by anion exchange and sodium borohydride (NaBH4)reduction, as shown in Scheme 1.Compared to the conventional C3N4support obtained by directly calcining melamine, the overflow of S during the calcination of tripolythiocyanate exhibited a peeling effect on the bulk C3N4,and the obtained support showed a larger specific surface area and more defect attachment sites, leading to better dispersion of Pd NPs. Moreover, the electron-effect between the support and the metal resulted in the change in the ratio of Pd0and Pd2+on the surface of support, which improved the activity and selectivity during the FAD. The Pd/C3N4-S catalyst exhibited excellent performance, its turnover frequency (TOF)and mass activity were up to 2083 h-1and 19.52 mol·g-1·h-1, respectively.
2 Experimental and computational section
Reagent and instrument information is provided in the Supporting Information.
2.1 Synthesis of Pd/C catalysts
Pd/C catalysts were prepared by microwave-assisted reduction process in ethylene glycol solution28. Briefly, Vulcan XC-72 (237.5 mg)was dispersed in ethylene glycol in a beaker and subjected to ultrasonic treatment for 4 h to form a uniform solution. H2PdCl4solution (5.9 mg·mL-1)was added and stirred for 3 h. Then appropriate amount of aqueous solution of sodium hydroxide (NaOH, 1 mol·L-1)was added to adjust the pH of the solution, with final value varying between 10 and 11.Subsequently, the beaker was placed in the center of a microwave oven (800 W)and subjected to microwave heating for 90 s and followed by two repeated intervals of 10 s of irradiation and 10 s off. The resulting solution was stirred for 8 h, filtered, and washed with abundant water, and then dried in an oven at 60 °C for 12 h to obtain Pd/C catalysts.
2.2 Synthesis of C3N4 and C3N4-S
Melamine was directly calcined to obtain C3N4in a quartz furnace at 550 °C for 2 h. The trithiocyanuric acid was directly calcined to obtain C3N4-S in a quartz furnace in the temperature range of 550-650 °C. And the calcination time is 2 h at 550 °C,1 h at 600-650 °C. The heating rate of all the above calcination processes is 5 °C·min-1.
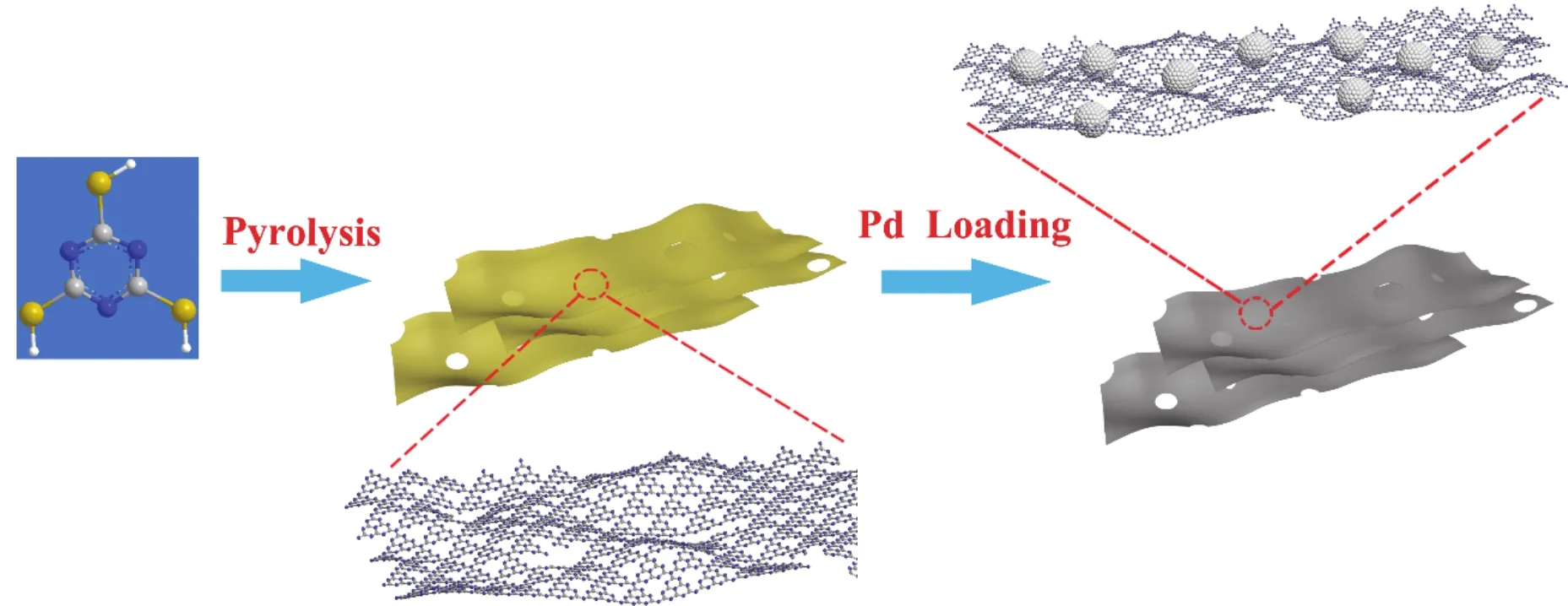
Scheme 1 Schematic illustration of preparation of Pd-based catalysts using trimeric thiocyanate as precursors.
2.3 Synthesis of Pd/C3N4-S catalysts
Pd/C3N4-S catalysts were prepared by two methods. One of the methods was microwave-assisted reduction process;however, the Vulcan XC-72 was replaced by C3N4. In another method, NaBH4was used as reducing agent in aqueous solution.Briefly, C3N4(95 mg)was dispersed in deionized water (90 mL)taken in a beaker, and then the contents were subjected to ultrasonic treatment for 4 h to form a uniform solution. H2PdCl4solution (5.9 mg·mL-1)was added and stirred for 3 h. Then appropriate amount of aqueous solution of NaOH (1 mol·L-1)was added to adjust the pH of the solution, with final value varying between 9 and 11.5. Then the NaBH4solution (1 mg·mL-1)was added, and the molar ratio of NaBH4to Pd is 12/1.The solution was stirred for several hours. The resulting solution was filtered, washed with deionized water, and then dried in an oven at 55 °C.
2.4 Synthesis of Pd/C3N4 catalysts
Pd/C3N4catalysts were prepared following the procedure similar to that for the Pd/C3N4-S catalysts except that the carrier was replaced by C3N4.
2.5 Performance test in SA(Sodium formate)/FA solution
Catalyst (20 mg)was weighed in a round bottom flask, and the flask was then placed in a constant temperature water bath at 30 °C, and passed through the air in the N2discharge bottle.However, the SA/FA solution was placed in a constant temperature water bath at 30 °C. After 10 min, 2 mL of the SA/FA solution was quickly added to the round bottom flask while stirring, and the FAD rate was measured using a mass flow meter.
2.6 The methods for calculating TOF and mass activity
The catalytic performance of the catalyst can be carried out using TOF and mass activity. The following is their calculation method:
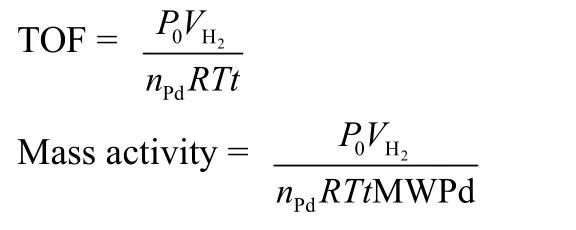
MWPdis the molar weight of Pd;nPdis the mole number of Pd on the catalysts;Vis the volume of hydrogen produced;Ris the universal gas constant;Tis ambient temperature;tis reaction time;P0is the pressure under environmental conditions.
3 Results and discussion
Fig. 1a,b exhibit scanning electron microscopy (SEM)images of the agglomerated bulk C3N4support from melamine precursor and that of the thin C3N4-S obtained by calcination of trimeric thiocyanate precursor, respectively. Owing to the departure of large -SH groups during pyrolysis, the resulting carbon nitride support was stripped into a thin porous structure27, which could significantly increase the specific surface area and pore volume of the support. The pore volume and pore size distribution of the C3N4support and the thin C3N4-S support were compared by N2adsorption-desorption test (calculations based on Brunauer-Emmett-Teller (BET)surface adsorption method), as shown in Fig. 1c,d. Compared to C3N4support, the specific surface area and pore volume of C3N4-S support increased by 2 and 15 times,respectively, as presented in Table 1. The pore volume of C3N4and C3N4-S before loading metal is 0.085 cm3·g-1and 1.329 cm3·g-1, respectively. And the specific surface area of C3N4and C3N4-S before loading metal is 42.52 m2·g-1and 152.91 m2·g-1,respectively. The increase in specific surface area and pore volume could significantly improve the dispersion of the support for the supported metal particles and the mass transfer during the reaction. BET analysis of the specific surface area and pore volume of C3N4and C3N4-S after metal loading are shown in Figs. S1 and S2 (in Supporting Information), respectively. The specific data are presented in Table 1.
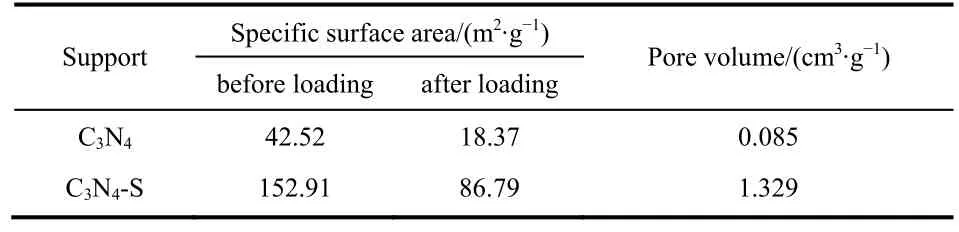
Table 1 Specific surface area and pore volume of two supports tested by N2 adsorption-desorption test (calculations by Brunauer-Emmett-Teller (BET)).
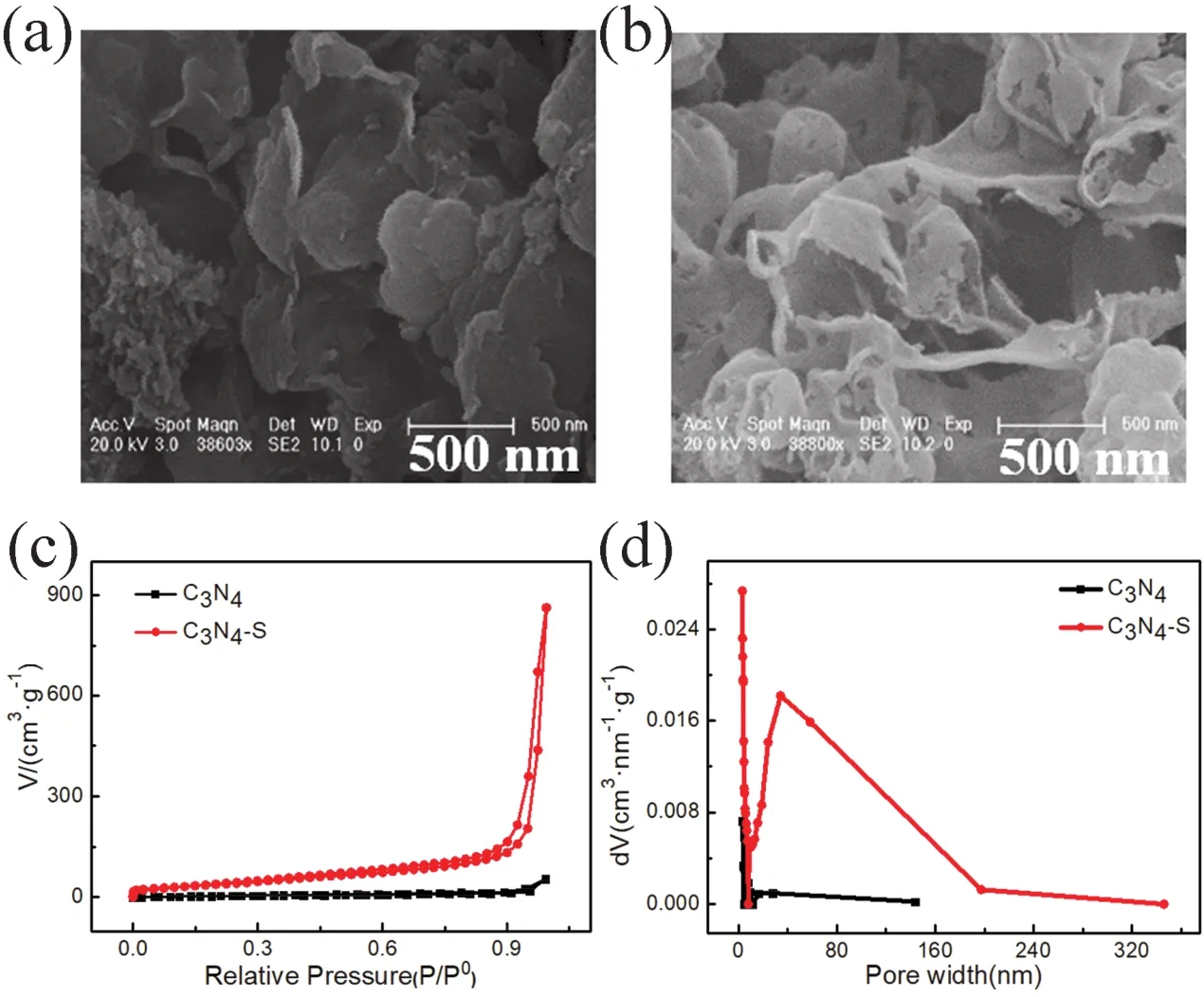
Fig. 1 (a)SEM image of C3N4; (b)SEM image of C3N4-S; (c)nitrogen adsorption-desorption isotherms of C3N4 and C3N4-S; and(d)pore size distribution of C3N4 and C3N4-S.
Fig. 2a,c display transmission electron microscopy (TEM)images of Pd/C3N4and Pd/C3N4-S, respectively. Clearly, the Pd NPs in the Pd/C3N4catalyst are more evenly distributed at the edge position of the support, but less in the bulk phase. In contrast, the Pd NPs in the Pd/C3N4-S catalyst are uniformly dispersed throughout the support, indicating that thin layer C3N4-S support can better anchor and disperse Pd NPs. The high-angle annular dark field scanning transmission electron microscope(HAAD-STEM)and mapping images (Fig. 3e-h)of Pd/C3N4-S also confirmed this conclusion. The particle size distributions of Pd/C3N4and Pd/C3N4-S catalysts are shown in Fig. 2b,d,respectively. Evaluation of the particle size distribution of the two catalysts showed that the average particle size of the C3N4-S supported Pd NPs (2.48 nm)was reduced by about 0.5 nm compared to that of the bulk C3N4support loading Pd NPs (2.98 nm). Furthermore, smaller particle sizes represent higher metal utilization and more active sites because the reactive sites are generally located on the surface of the metal NPs.
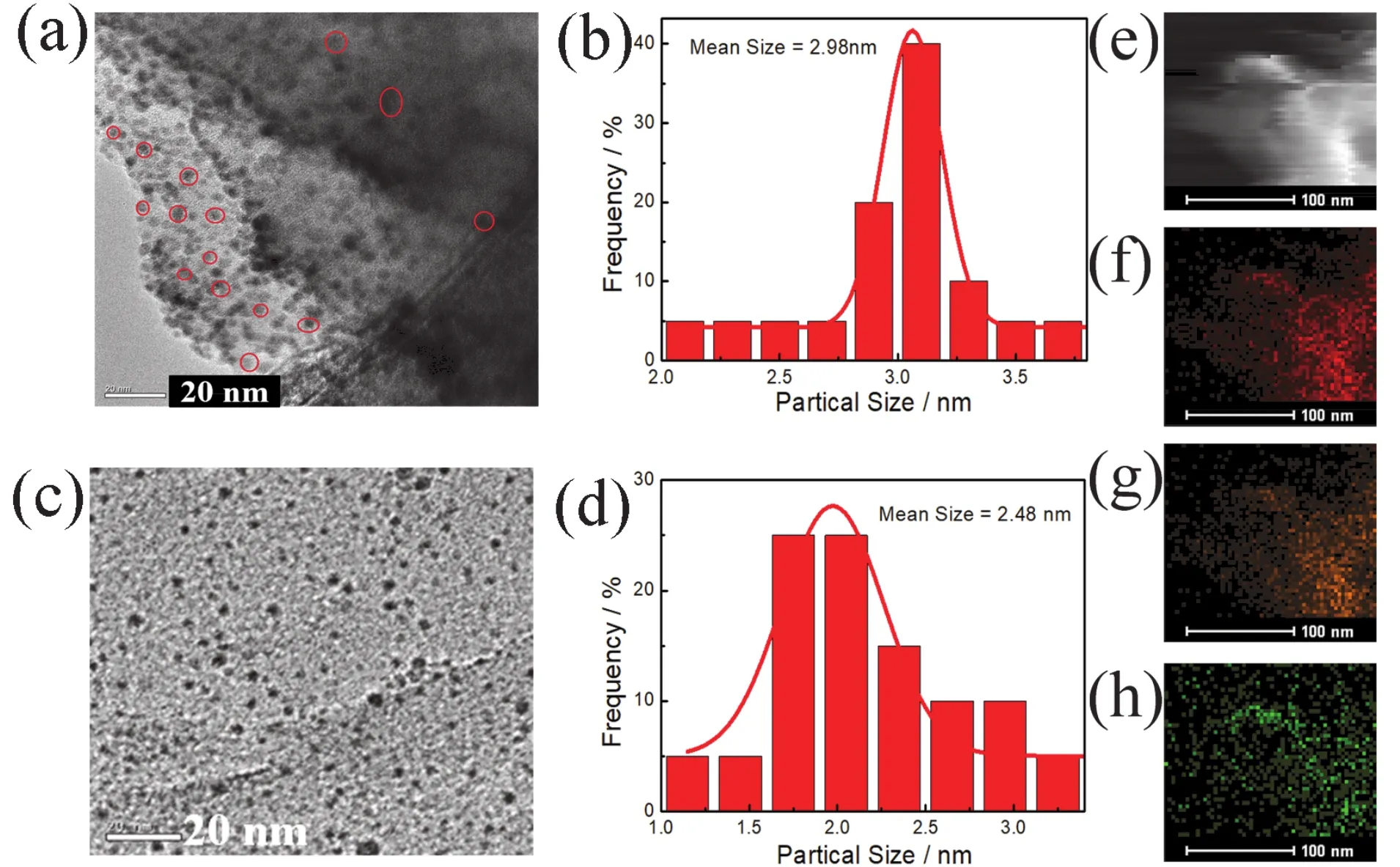
Fig. 2 (a)TEM image of Pd/C3N4; (b)size distribution of Pd NPs of Pd/C3N4; (c)TEM image of Pd/C3N4-S; and(d)size distribution of Pd NPs of Pd/C3N4-S; (e)HAADF-STEM and (f-h)element mapping of Pd/C3N4-S (f: C; g: N; h: Pd).
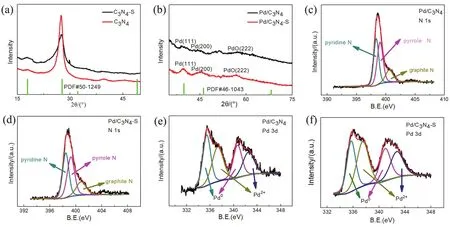
Fig. 3 (a)XRD patterns of the supports; (b)XRD patterns of the catalysts; (c)the N 1s XPS result of the Pd/C3N4; (d)the N 1s XPS result of the Pd/C3N4-S; (e)the Pd 3d XPS result of the Pd/C3N4; and (f)the Pd 3d XPS result of the Pd/C3N4-S.
Fig. 3a shows the X-ray diffraction (XRD)results of C3N4and C3N4-S. The two supports exhibit strong diffraction peaks at around 17.9° and 27.7°, and comparison with the standard peak of carbon nitride (PDF #50-1249)indicates that both supports correspond to C3N4structures. Compared to bulk C3N4, the strongest peak of C3N4-S has a broader shape at 27.7°, probably due to the reduced degree of crystallization owing to the spillover effect of S during pyrolysis. Table S1 (in Supporting Information)lists the change of S element content in the C3N4-S support before and after calcination. The elemental analysis test results also showed that the ratio of the C element and the N element content in the two supports approached 3 : 4, as presented in Table S2. Furthermore, the species of N in the two supports were analyzed by X-ray photoelectron spectroscopy(XPS), and the types of N in Pd/C3N4and Pd/C3N4-S are shown in Fig. 3c,d, respectively29. Both the supports contain pyrrole-N,pyridine-N, and graphite-N, and their contents are listed in Table S3 (in Supporting Information). According to previous studies by Caoet al.20, the presence of pyridine-N can change the Lewis acid-base characteristics of the surface of Pd in the catalyst,thereby improving the intrinsic activity of the Pd-based catalyst.The content of pyridine-N in the two carbon nitride supports prepared in this study was relatively high, indicating that both supports could effectively adjust the surface electronic state of Pd30. Moreover, this adjustment can effectively improve the intrinsic activity of Pd-based catalyst for FAD.
Owing to the low metal loading and lower crystallinity of the two catalysts, their XRD result shows weaker Pd diffraction peaks, but both match well with the standard Pd derivative peaks(PDF #46-1043). Fig. 3b illustrates that the diffraction peaks at 40.1° and 46.7° can be attributed to the (111)plane and (200)plane of the Pd cubic crystal, respectively. Moreover, there is also a weak diffraction peak corresponding to the (222)crystal plane of PdO at ~57.9°. The XRD full spectra of Pd/C3N4and Pd/C3N4-S is shown in Fig. S3. Inductively coupled plasma optical emission spectroscopy (ICP-OES)test results showed that the mass percentages of Pd in Pd/C3N4and Pd/C3N4-S were 1.61 and 3.21% (w, mass fraction), respectively, as presented in Table S2. This result indicates that both the supports can effectively hold up Pd NPs, and further proves that the thin layered C3N4-S support with high specific surface and pore volume can better disperse and anchor Pd NPs. Previous study has shown that the activity of the catalyst depends on the content of surface Pd0and Pd2+, and the Pd-PdO interface can effectively inhibit the occurrence of side reactions during FAD, thereby preventing the active sites of the catalyst from being poisoned by CO31. Therefore, the valence state of the surface Pd of the two catalysts was analyzed by XPS, as shown in Fig. 3e,f. The results reveal that surface of both catalysts contains Pd0and Pd2+due to the interaction between the support and the metal.Moreover, XPS analysis results also show that the content of Pd in different valence states of the two catalyst surfaces is close to 1 : 1, as presented in Table S3. Furthermore, it could also be speculated that the C3N4-supported Pd-based catalyst prepared in this study exhibits theoretically excellent selectivity and activity.
Furthermore, a sample of Pd/C catalyst with activated carbon(XC-72)as support was also prepared by ethylene glycol microwave reduction. XPS results showed that the ratio of Pd0to Pd2+on the surface of Pd/C catalyst was close to 3 : 2 (as presented in Table S3 and Fig. S4, in Supporting Information),and reduced by more than 15% relative to the Pd2+content of the Pd/C3N4and Pd/C3N4-S catalyst surfaces, indicating that the presence of N heteroatoms could strengthen the support-metal interaction.
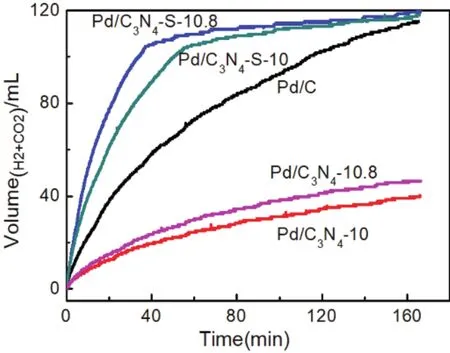
Fig. 4 Comparison of decomposition performance of FA catalyzed by different catalysts at 30 °C (Pd/C3N4 and Pd/C3N4-S prepared at pH = 10 and pH = 10.8, respectively).
First, the catalyst was optimized by changing the pyrolysis temperature of the support, as shown in Fig. S5 (in Supporting Information). When the pyrolysis temperature was 600 °C, the FAD performance of Pd/C3N4-S catalyst was the best. Then we tested the properties of Pd/C3N4and Pd/C3N4-S prepared by two different reduction methods of ethylene glycol microwave reduction and NaBH4direct reduction to catalyze the decomposition of FA to form H2and CO2, as shown in Fig. 4.The difference in performance of the catalysts was obtained by comparing the two preparation methods, and the preparation methods were optimized. At the same time, the pH of the anion displacement reaction system significantly influenced the particle size of Pd NPs, thus the preparation process was optimized by adjusting the pH30.
The solubility of C3N4and C3N4-S supports formed by pyrolysis of melamine and trimeric thiocyanate in ethylene glycol was not good, thus the supports were unable to efficiently hold up the Pd NPs. The ICP-OES test results also showed that the mass percentages of Pd in the Pd/C3N4and Pd/C3N4-S catalysts prepared by the ethylene glycol microwave reduction method were only 0.12% and 0.2%, respectively (see Supporting Information Table S2). At 30 °C, catalyst (20 mg)prepared by microwave reduction was used to catalyze 2 mL mixing of HCOOH and HCOONa to form H2and CO2. The masses of HCOOH and NaCOOH in the mixed solution are 0.10 g and 0.11 g, respectively. The results (as shown in Fig. S6, in Supporting Information)show that the catalytic performance is very poor due to the low metal content, and the reaction rate and total gas generation are far less than those for the Pd/C catalyst.
Furthermore, we also tested the metal loading of Pd/C3N4and Pd/C3N4-S catalysts prepared by NaBH4direct reduction by ICPOES. The solvent used in the direct reduction method of NaBH4was water (both supports were water-soluble, in particular, the water solubility of C3N4-S was better), thus the metal loading of the two catalysts has been effectively improved relative to the ethylene glycol microwave reduction method, as shown in Table S2. The performance test results of the two catalysts prepared by the NaBH4reduction method are shown in Fig. 4. The catalytic performance of Pd/C3N4was still found to be poor. It can be attributed to the following two reasons: on the one hand, due to the small specific surface area and pore volume of bulk C3N4,the support cannot effectively disperse Pd NPs, and the metal loading is still low; on the other hand, the solubility of the bulk C3N4in the aqueous phase is still limited. The metal loading of the Pd/C catalyst is as high as 4.12%, which is close to three times that of Pd/C3N4. In contrast, although the metal loading of Pd/C3N4-S is still lower than that of Pd/C, the FAD activity of the Pd/C3N4-S catalyst is more than double of that of Pd/C (the catalytic performance during the first 10 min). At 30 °C, the TOF of Pd/C3N4-S catalyzed FAD reached 2083 h-1, and the mass specific activity also reached 19.52 mol·g-1·h-1. This performance is better than those reported for most catalysts, as presented in Table S4 (in Supporting Information). Furthermore,the FAD gas product was analyzed by gas chromatography (GC),and the results revealed the absence of CO in the gas product(see Supporting Information Table S5), indicating excellent selectivity of the Pd/C3N4-S catalyst. The high selectivity and activity of Pd/C3N4-S can be attributed to the interaction between the support and metals.
Moreover, the stability of the Pd catalyst was studied through cycle tests, as shown in Fig. S7 (in Supporting Information).After four cycles of testing, the catalyst performance decreased by less than 10%. In order to further explore the reasons for the excellent stability of the catalyst, the catalyst was characterized by TEM, as shown in Fig. S8A (in Supporting Information). The particle size analysis of the TEM results showed that there was no obvious aggregation of NPs on the Pd catalyst after the cycle test. Fig. S8B shows that the average particle size of Pd NPs in the catalyst after the cycle test is 2.72 nm. The excellent stability of the Pd/C3N4-S catalyst can be attributed to the significant anchoring effect of large amount of pyridine N on Pd NPs on the surface of the thin-layer carbon nitride support.
4 Conclusions
In this study, a thin-layered C3N4was prepared as a support without the introduction of a second support using trimeric thiocyanate as a precursor. Compared to the bulk phase of C3N4(melamine is the precursor), the specific surface area and pore volume of the support were significantly improved. Moreover,the NaBH4reduction method could uniformly disperse Pd NPs on the support. Owing to the electron-effect between the support and the metal, the ratio of the contents of Pd0and Pd2+on the surface of the support was close to 1 : 1. This could effectively increase the selectivity of the catalyst and suppress the formation of by-product CO. Compared to Pd/C and Pd/C3N4, the TOF and mass activity of the catalyst was up to 2083 h-1and 19.52 mol·g-1·h-1at 30 °C, respectively. This study provides a new perspective for the preparation of N-doped C supports with high specific surface area and pore volume of Pd-based catalysts, and further proves that formic acid is a potential hydrogen storage material.
Supporting Information:available free of chargeviathe internet at http://www.whxb.pku.edu.cn.
- 物理化學學報的其它文章
- Seeding and Cross-Seeding Aggregations of Aβ40 and hIAPP in Solution and on Surface
- Eu(III)在蒙脫石上的吸附及碳酸根和磷酸根對其吸附的影響
- 鋯合金初始氧化行為的原位近常壓XPS研究
- Methanol Synthesis by COx Hydrogenation over Cu/ZnO/Al2O3 Catalyst via Hydrotalcite-Like Precursors: the Role of CO in the Reactant Mixture
- 自交聯(lián)共軛亞油酸囊泡基熒光納米點的構(gòu)筑及其熒光特性
- Controllable Formation of PbI2 and PbI2(DMSO)Nano Domains in Perovskite Films through Precursor Solvent Engineering

