Sex But Not Altitude,Modulates Phenotypic Covariations Between Growth and Physiological Traits in Adult Asiatic Toads
Ping LI ,Song TAN , ,Zhongyi YAO ,,Gaohui LIU,Jinzhong FU and Jingfeng CHEN*
1 CAS Key Laboratory of Mountain Ecological Restoration and Bioresource Utilization,Ecological Restoration and Biodiversity Conservation Key Laboratory of Sichuan Province,Chengdu Institute of Biology,Chinese Academy of Sciences,Chengdu 610041,Sichuan,China
2 University of Chinese Academy of Sciences,Beijing 100049,China
3 Key Laboratory of Bio-Resource and Eco-Environment of Ministry of Education,College of Life Sciences,Sichuan University,Chengdu 610065,Sichuan,China
4 Chinese Research Academy of Environmental Sciences,Beijing 100012,China
5 Department of Integrative Biology,University of Guelph,Guelph N1G 2W1,Canada
Abstract The pace-of-life syndrome (POLS) hypothesis predicts that most variation in life history,physiology,and behavior among individuals,populations,and species falls along a continuum from slow to fast pace of life.While there is evidence for climatic gradientmediated POLS patterns among species,this approach has rarely been explicitly used to study POLS patterns among-and within-populations.In addition,the roles of sex in POLS evolution among-or within-populations are largely unknown.In this study,we investigated the effects of altitudinal gradient and sex on the covariations between growth rate and several physiological traits closely associated with POLS (blood glucose,baseline-and stress-induced glucocorticoids (GCs),hemolysis and hemagglutination) in the Asiatic toad Bufo gargarizans.Contrary to our expectation,altitudinal gradient had no influence on the covariations between growth rate and physiological traits,neither at the among-nor withinpopulation level,indicating that these trait integrations have similar fitness payoffs across hierarchical levels.In contrast,we found evidence for sex-specific POLS composition:there was a negative covariance structure between growth rate and baseline GCs-but only in females,and a positive covariance structure between growth rate and baseline GCs-but only in females,and a positive covariance structure between growth rate and hemagglutination-but only in males.This observation indicates that these trait associations differ dramatically in advancing fitness for each sex,and supports the idea that sex-specific POLS composition could evolve in species in which the reproductive roles largely differ between the sexes.
Keywords Bufo gargarizans,constraint,glucocorticoids,immunity,metabolism,phenotypic integration,physiological pace-of-life syndrome
1.Introduction
Life history theory posits that under limiting resources,the trade-off between allocation to current versus future reproduction or survival can generate a correlative pattern between life history traits,resulting in a slow-fast life history continuum (Stearns,1989).Recently,researchers further proposed that life-history characteristics and suites of physiological (metabolic,immunological and hormonal) and behavioral traits have coevolved in response to environmental conditions forming a pace-of-life syndrome (POLS) (Dammhahnet al.,2018;Montiglioet al.,2018;Careauet al.,2008;West-Eberhard,2003;Klingenberg,2014;Ricklefs and Wikelski,2002;Speakman,2005;Tomaseket al.,2019).This functional integration among multiple phenotypic traits is primarily caused by correlational selection,and such processes have important consequences for the evolutionary and ecological study of populations (Tielemanet al.,2005;Réaleet al.,2010).
Due to global climate change,there is increasing interest in the intraspecific local adaptation of populations along geographical gradients (Tielemanet al.,2006;Pettersen,2020).Populations generally show different life-history and physiological traits across latitude/altitude ranges that result from distinct selective regimes (Conoveret al.,2009).Therefore,consistent differences in population trait means are anticipated,forming in a POLS across latitude/altitudinal gradients.Nevertheless,while there is evidence for latitude/altitude-mediated POLS patterns among species (Tielemanet al.,2006;Wiersmaet al.,2007;Londonoet al.,2015),this approach has rarely been explicitly used to study POLS patterns among-or within-populations of the same species.The few studies looking at differences in within-population syndrome structure across different environmental gradients revealed large similarities of covariation patterns,however these studies were limited to behaviors (Segevet al.,2017;Alcalayet al.,2015;Bengston and Dornhaus,2015;Pruittet al.,2008;Debecker and Stoks,2019;Branset al.,2018).Considering the distinct roles of physiology and behavior in life history evolution (Polverinoet al.,2018),whether these covariation patterns across geographic gradients also hold for physiology remains a significant gap in our knowledge.
Sex-specific optima for reproductive investment and life history scheduling could result in sex differences in mean trait expression (Wedellet al.,2006).Similarly,as a result of their different reproductive roles and the environment,each sex may come to differ in the strength of correlation among traits,or different traits may covary in males and females.According to the source of sex-specific selection on pace of life and trait covariances,four conceptual frameworks have been proposed:uniform POLS structure,sex-specific POLS with different strength trait correlation,distinct POLS structure in each sex,no POLS (Hamalainenet al.,2018).For instance,uniform POLS would be expected when sexual conflict is either completely unresolved-such that high genetic correlations between the sexes prevents the evolution of dimorphism,or when sexual conflict is absent and therefore the life history strategies of the sexes converge.However,classic evidence for these outcomes is presently scarce.
In this study,we examined how metabolic,immunological,or hormonal traits covary with one life history trait -growth -as well as the role of altitudes and sexes in the among-and within populational covariations in the Asiatic toad (Bufo gargarizans) (Ricklefs and Wikelski,2002).The distribution range of this species spans an extremely large altitudinal gradient from zero to 4300 m above sea level (Fei,2009).Their life history traits also display significant altitudinal differences (Liao and Lu,2012;Liaoet al.,2014;Guoet al.,2016),making this species suitable for studies related to climatic gradientmediated or sex-specific POLS.We measured rate of growth,baseline blood glucose concentrations (G0),two immunological indices (hemagglutination,hemolysis),and glucocorticoids (GCs,baseline/stress-induced corticosterone) in a common garden setting.As a major blood-borne source of metabolizable energy circulating in animal blood,G0 may correlate with energy-demanding processes such as growth and reproduction (Fournieret al.,1992;Tomaseket al.,2019).For innate immunity characteristics,we measured hemolysis and agglutination since they are more dependent on genetic background than adaptive immunity (Tielemanet al.,2005).We measured GC concentration because of its functional relationship with immunity and energy mobilization (Landyset al.,2006).Many studies measuring GC levels have found variable associations with reproductive traits in fish,amphibians and reptiles (Crespiet al.,2013).
In accordance with the POLS hypothesis and the recent POLS-related theoretical framework,we expected a faster physiological pace-of-life inB.gargarizansthat show higher G0 and lower GCs and innate immunological indices,and are more likely to persist at lower altitudes or in males (Réaleet al.,2010;Hamalainenet al.,2018).We postulated that covariations should be formed between growth and physiological traits,which are expected to be more pronounced at lower altitudes because warmer and more productive environments can bring up these associations (Segevet al.,2017;van Noordwijk and de Jong,1986).We also hypothesized thatB.gargarizansdemonstrates sex-specific POLS.However,we were not able to predict which sex would display the stronger covariance structures due to lack of aprioriknowledge on how these physiological traits confer to breeding an advantage in this species.
2.Materials and methods
2.1.Sampling and acclimation procedureA total of 123 adult males were collected from seven sites in western China in summer 2018 and spring 2019.These sites were divided into two gradient groups according to their relative altitude (group L<~1000m,group H>1000m,Figure S1 and Table 1).Sites 1-4 form the first transect of the Min River drainage,and sites 5-7 form the second transect along the Dadu River drainage.After sampling,all individuals were transferred to the animal room in Chengdu Institute of Biology,Chinese Academy of Sciences (CIBCAS).A constant temperature (20±1)℃ and light/dark cycle (12h:12h) was maintained in the animal room.All toads were housed individually in a plastic container (35.5cm×25cm×15cm,L×W×H) that included a piece of wet sponge (5cm×7cm) to preserve humidity and a U shape tile (15cm×14cm×7cm) for shelter.The toads were mainly fed mealworms (Tenebrio molitor) dusted with calcium powder (Exo Terra).To ensure the toads could intake fresh mealwormsad libitum,the food plate was cleared and replenished every other day.Live crickets (Gryllus rubens) were also supplied once a week.We measured the body mass and snout-vent length (SVL) once every month.
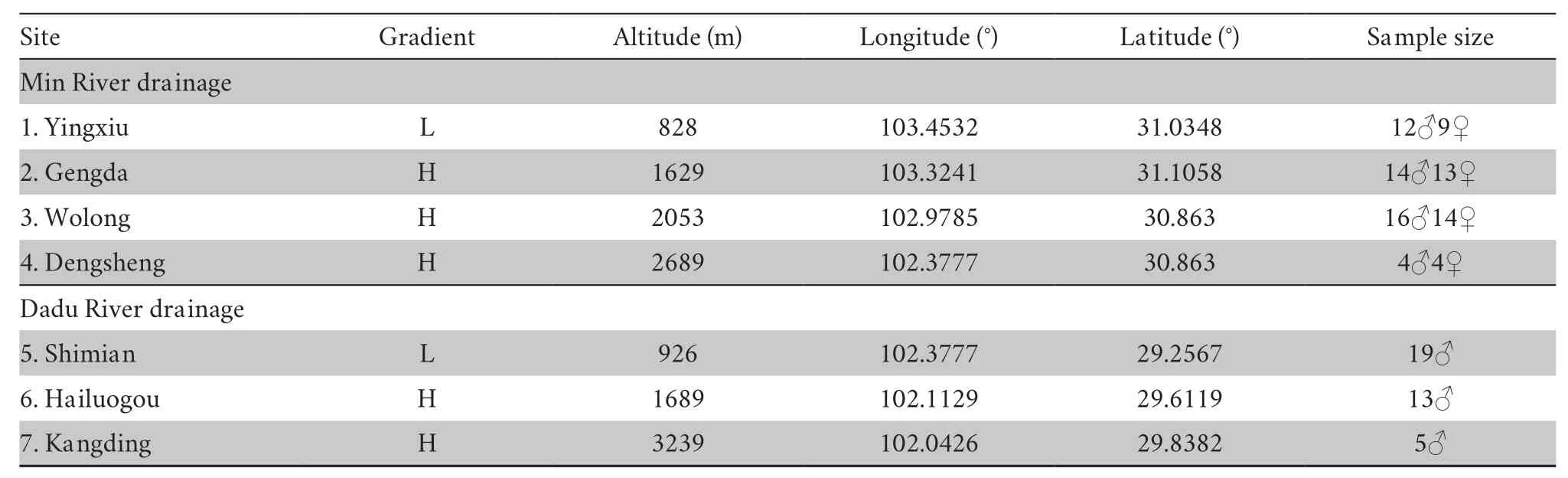
Table 1 Sample site and sample size information.
After captive acclimation of more than 4 months,each toad received intraperitoneal injections of a 50 μL mixture of the keyhole limpet hemocyanin (KLH,2 mg/25 mL,Enzo#ALX-202-064-M025) and immunological adjuvants (Sigma#F5881) (day 0).Two weeks later,all individuals were injected with 25 μL of a KLH solution again to further boost the immune system (we were not able to acquire those data in the Asiatic toads due to the low specification of the designed antibody,anti-toad IgY Ab).At day 56,we collected saliva samples for a corticosterone assay.At day 63,we sacrificed all the toads with 0.2% ms-222 solution and collected plasma by opening the abdominal cavity and drawing blood from the opening of the right ventricular cavity with a heparin-rinsed syringe.The blood sample was centrifuged at 3000 rpm at 4℃ for 5 min after chilling on ice for <1 h.The upper plasma was carefully separated and frozen for further analyses.All experimental procedures followed the guidelines of the Animal Care and Use Committee of CIBCAS.
2.2.Baseline and stress-induced corticosterone levelsTo determine the baseline corticosterone content,we collected saliva samples.A small,dry cotton ball of known weight was inserted in the toad’s mouth for 20 s;all samples were collected within 3 minutes in order to minimize the increase in corticosterone due to treatment stress (Davis and Maney,2018).To determine stress-induced corticosterone concentrations,target toads were placed in cloth bags for 60 min,and the second saliva sample was taken thereafter.We examined corticosterone content in saliva followed the method proposed by (Janinet al.,2012).We weighed the saliva-soaked cotton balls and stored them individually at -80℃.To exact corticosterone from the saliva samples,we added 1 mL methanol to each sample tube for 5 h,transferred the cotton ball and methanol to a centrifuge tube with a filter membrane and centrifuged at 8000 rpm at 4℃ for 5 min.The collected solutions were concentrated with a vacuum centrifuge for 4 h at room temperature.The dry pellets were dissolved in 60 μL pure water for radioimmunoassay.The extracted corticosterone samples were measured with Iodine [125I]-Rabbit-Cortisol Radioimmunoassay Kit (Beijing North Institute of Biological Technology,China).Fetal bovine serum was used as the intraplate control.The intra-assay coefficient of variation (CV) was<10% and inter-assay CV was<15%.The lower and upper effective limits of the corticosterone assay kit were between10 ng/mL and 500 ng/mL,respectively.
2.3.Baseline blood glucose contentThe glucose concentration in plasma was determined by a glucose oxidase assay kit (Applygen,E1010).Firstly,we diluted the plasma samples three folds with PBS buffer,then added 10 μL of the standard glucose solution (2000,1000,500,250,125,62.5,31.25,15.625 μmol/L) or plasma sample,and 190 μL of the working solution to the 96-well plate.After incubation at 37℃ for 20 min,we measured the absorbance value at 550 nm with an Enzyme standard instrument (Thermo Scientific Varioskan Flash) and calculated the glucose content based on the standard curve.
2.4.Indices of constitutive immunityComplement and natural antibody levels were measured using an erythrocyte hemolysis-hemagglutination assay (Matsonet al.,2005;Sparkman and Palacios,2009).Serial two-fold dilutions of 50 μL plasma were made with phosphate-buffered saline (PBS) in 96-well (8 rows×12 columns) round (U) bottom assay plates.Each well received 50 μL of a rabbit red blood (1%) cellsuspension.Plates were incubated for 60 min at 20℃ and then scored.Titers were estimated as the negative log2of the highest dilution factor of plasma that showed hemagglutination or lysis.Half scores were given for titers that appeared intermediate.All the samples were assayed in duplicate with positive and negative controls in each plated.
2.5.Statistical analysesAll statistical analyses were performed with IBM SPSS Statistics 21.0 software (International Business Machines Corporation) and R (Version 4.0.3).We calculated the growth rate as the body mass gain per day (g/d) during the experiment period.Four subjects died during the experiments and were hence excluded from the analysis.To achieve normality,blood glucose and corticosterone were transformed with Jonson transformation.Erythrocyte hemolysis and erythrocyte agglutination were computed with Blom rankbased normalization.
Since our experimental design was not balanced,we separated the data into two datasets and analyzed them with general linear models.Dataset I included all data on males (data from males in transect I and II),which was used to examine the effects of transect and altitude on growth,physiological variables and their covariations.Dataset II included the data of both sexes from transect I,and was used to examine the roles of sex and altitude in those phenotypic traits.
We used a univariate mixed model (UMM) to analyze the effects of altitude/transect or altitude/sex on growth and physiological variables using the“MCMCglmm”R package (Hadfield,2010).For each UMM,altitude,transect (or sex),transect (or sex) and their two-way interaction (altitude×transect or altitude×sex) were included as fixed effects,SVL or body mass as covariates to control for the effects of age (initial body mass but SVL was used for growth rate,since SVL and initial mass were correlated and only initial body mass was covaried with growth rate).Sampling site was used as a random effect.
To investigate the covariation between growth rate and physiological variables at the among-and withinpopulation level,we used a bivariate mixed model using the“MCMCglmm”R package (Hadfield,2010).We first examined the variance and covariance matrix with covariates in the models.We then examined the changes in the variance and covariance matrix caused by the inclusion or exclusion of fixed factors (altitude,transect/sex) in the bivariate model.The response and explanatory variables were scaled before analysis (mean centered on 0 and SD reduced to 1) to facilitate the interpretation of the results.Sampling site was included as a random effect on all traits.We used the following priors for the residual (V=diag(2),nu=0.002) and random effect matrices (V=diag(2) × 0.002,nu=1.002,alpha.mu=rep(0.2),alpha.V=diag(25^2,2,2)).To compute the posterior distribution,the model was run over 420 000 iterations,with a burn-in of 20 000 and a thinning interval of 100,to obtain an effective sample size between 20 001 and 419 901,with an autocorrelation level between retained iterations lower than 0.05.
3.Results
Initial body mass was positively associated with growth rate,and SVL was negatively associated with hemagglutination titer and stress-induced GCs levels (P<0.05,Table S1-S2).Moreover,there were no significant differences among altitudinal groups in growth rate and physiological variables in either dataset (P>0.05,Table S1-S2).Similarly,transect did not affect growth rate or physiological indices (P>0.05,Table S1).However,growth rate and physiological variables (excluding stress-induced GCs) varied between the sexes,with females having higher growth rate,hemolysis and hemagglutination in females,and males having with higher baseline GCs,higher blood glucose content (P<0.05,Figure 1,Table S2).
At the between-population,we did not find any significant covariance between growth rate and physiological variables (Table S3-S4).At the within-population level,growth rate significantly covaried with baseline GCs in both datasets (mean [95% credible interval]:-0.334[-0.610,-0.100] for dataset I;-0.568[-0.846,-0.323] for dataset II) (Table S3-S4).Moreover,growth rate significantly covaried with hemagglutination in both datasets (0.256[0.038,0.533] for dataset I;0.472[0.230,0.764] for dataset II) (Table S3-S4).These covariances were not modulated by transect or altitudinal group (Table S3-S4).Nevertheless,these covariances were significantly reduced when sex was included in the explained variables of MCMCglmm modeling in dataset II (-0.247[-0.426,-0.081] for the combined growth rate and baseline GCs,0.260[0.083,0.473] for the combined growth rate and hemagglutination,Table 2).Further analyses showed that the covariance between growth rate and baseline GCs was significant in females (male:-0.6[-1.562,0.921],female:-0.225[-0.477,-0.014],Figure 2),and the covariance between growth rate and hemagglutination was significant in males (male:0.288[0.0629,0.565],female:0.149[-0.047,0.390],Figure 2).
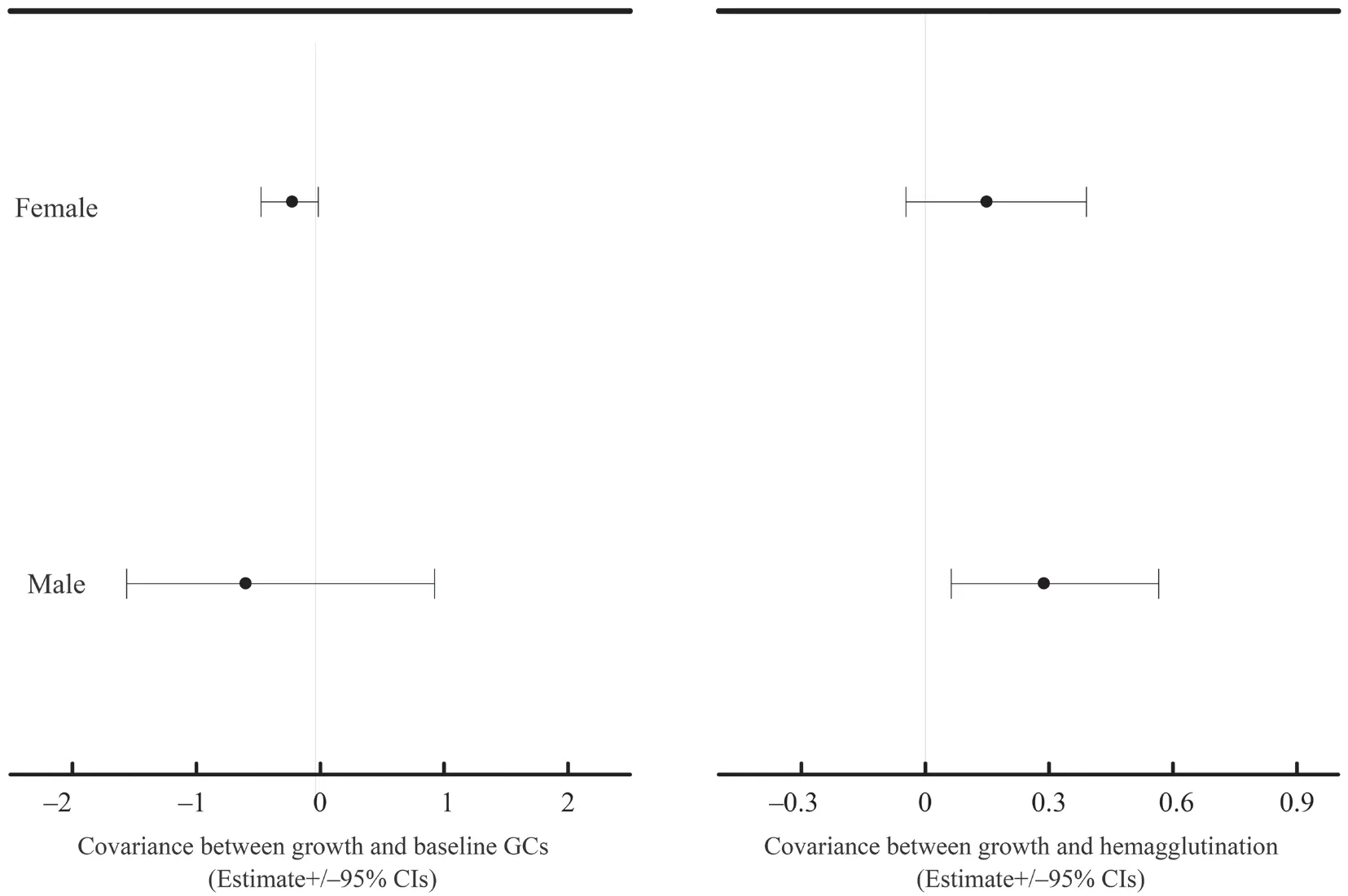
Figure 2 The estimate and 95% confidence interval for the covariances between growth rate and baseline GCs and hemagglutination for males and females of Dataset II.
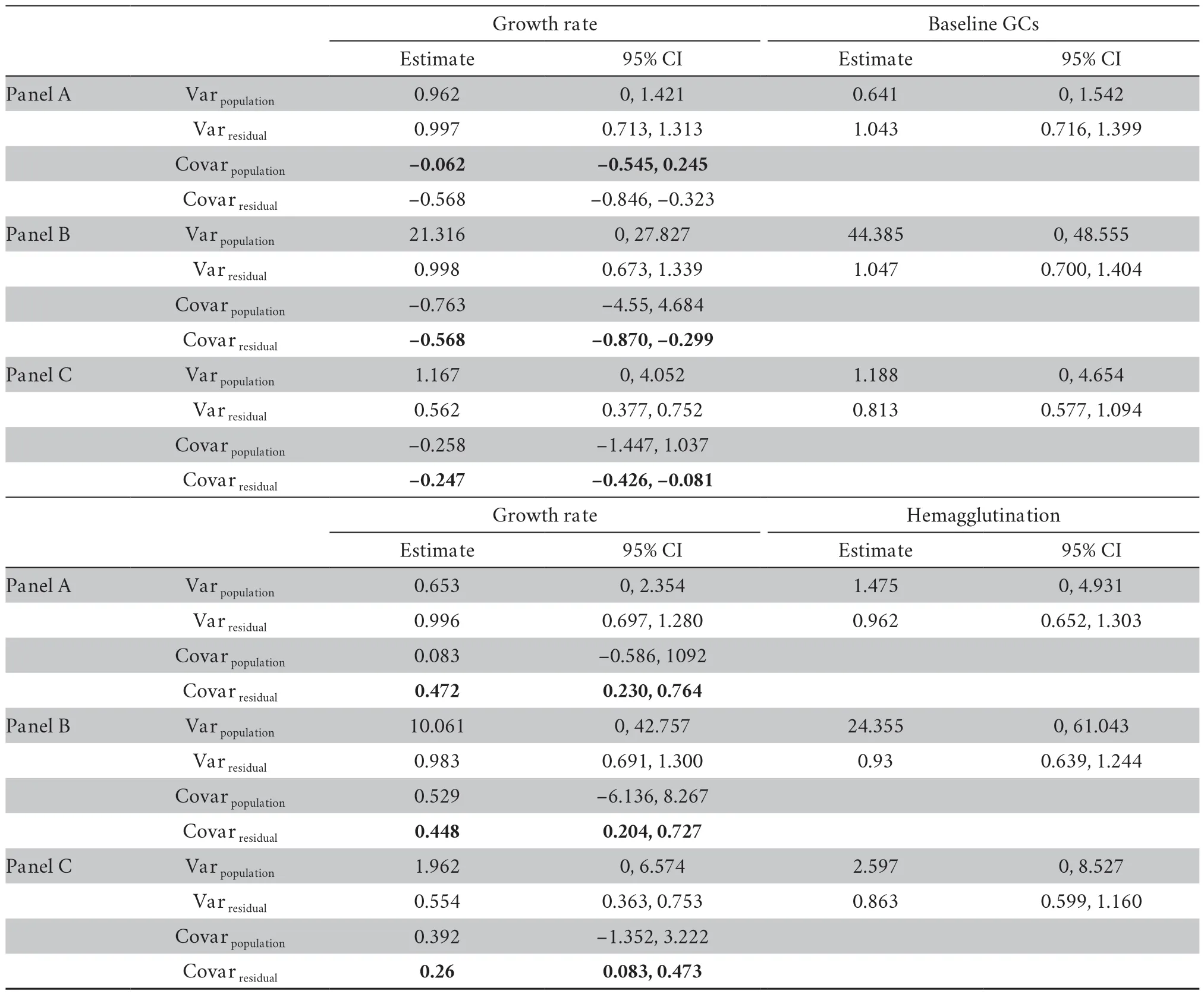
Table 2 Estimates of fixed effects and variance components for growth rate and physiological traits in of dataset II,obtained from bivariate mixed models.Panel A reports the estimates of fixed effects and variance components only with covariates included in the explanatory variables,and panel B reports the variance components after including altitude into the explanatory variables,and panel C reports the variance components after including transect into the explanatory variables.Variation in response and explanatory variables were scaled before analysis to facilitate the interpretation of the estimates.Given the structure of the data,the residual variance and covariance are interpreted as the phenotypic (co)variance within populations.The table gives the mean posterior distribution and its 95% credible interval (CI).
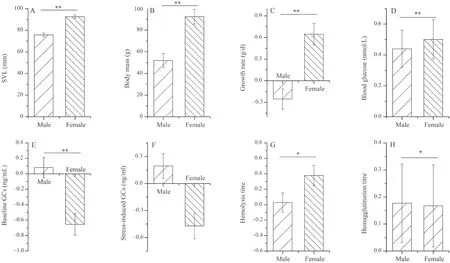
Figure 1 The effects of sex on snout-vent length (SVL) (A),body mass (B),growth rate (C),blood glucose (D),baseline corticosterone (E),stress-induced corticosterone (F),hemolysis (G),hemagglutination (H),of dataset II in Bufo gargarizans.The results are based on univariate mixed models (UMM) with altitude,sex,and their interaction as fixed effects,body mass (for growth rate) or SVL (for the other dependent variables) as covariates,and sampling site as a random effect.Data were presented as estimated mean values ± standard error.Stars refer to significant differences between sexes (*P<0.05,**P<0.01).
4.Discussion
4.1.Effects of altitude and sex on growth and physiological traitsOur results demonstrate that the life history traits and physiological traits closely related with pace-of-life inB.gargarizanswere similar along the altitudinal gradient.These similarities could be largely associated with the mixed effects of genetic adaptation and phenotypic plasticity (Conover and Schultz,1995).At high altitudes/latitudes,compensatory responses in life-history and physiological traits are expected to evolve,if the reductions in these phenotypic traits at low temperature involves a reduction in fitness,also known as counter-gradient variation (Conover and Schultz,1995;Conoveret al.,2009).For instance,although tadpoles derived from cold environments tend to intrinsically grow faster than those derived from warm environments,tadpoles experiencing cold conditions at early-life stages grow slower than those experiencing warm conditions (Seebacher and Grigaltchik,2014).Nevertheless,an earlier study revealed that the bufonids exhibit virtually no relation between size at metamorphosis and adult size (Werner,1986).This decoupling suggests that maternal effects masking the genetic adaptation in the growth ofB.gargarizanscould emerge after metamorphosis.
It is noted that in addition to growth rate,the measured metabolic,immunological,hormonal indices ofB.gargarizansdiffered significantly between the sexes,which could be associated with their sex-specific processes of ecology and evolution.The high rate of growth in females was traditionally considered to be driven by sexual and fecundity selections,and substantially contributes to sexual size dimorphism (Chouet al.,2016).The relatively low immunocompetence in males is likely to be associated with the immunosuppressive roles of testosterone content,which synchronously promote the development of sexual signals (Folstad and Karter,1992).In addition,the relatively high baseline GCs and blood glucose content in males may indicate that the males’ maintenance energy expenditure (e.g.respiration,immuno-competency,blood circulation,digestion) is higher than females’ (Tomaseket al.,2019).
4.2.Covariation in growth rate and physiological traits among and within populationsThe results of our study found strong phenotypic correlations between growth rate,and baseline GCs and hemagglutination,supporting POLS at the within-population level.However,these correlations were not found at the among-population level,indicating that the coupling between life-history and physiological traits may vary across hierarchical levels (Réaleet al.,2010).Since high baseline GCs level are suggested to indicate poor health condition,the negative correlation between growth rate andbaseline GCs could suggest an allocation trade-off between growth and survival in the Asiatic toad (Lankfordet al.,2001;Kimet al.,2011).Moreover,the positive correlation between growth rate and hemagglutination level in the Asiatic toad is congruent with the prediction of the POLS hypothesis that innate immunity is favored by fast pace-of-life (Lochmiller and Deerenberg,2000;Sparkman and Palacios,2009).
Although growth rate was integrated with baseline GCs and hemagglutination,their covariances did not change among or within-altitudinal gradient.This result does not support the prediction of POLS that trait integration is more likely to occur in warm environments,and these trait combinations have similar fitness payoffs (Reznicket al.,2000;van Noordwijk and de Jong,1986).This result is similar to that reported in a recent study ofIschnura elegansdamselfly larvae,which showed that latitude did not alter the covariation between life-history and anti-oxidative physiological traits (Debecker and Stoks,2019).On the other hand,a study on the water fleaDaphnia maganfound that the covariation structures between life history and stress physiological traits changed along an urbanization gradient (Branset al.,2018).Therefore,it seems that the impacts of anthropologic disturbance on trait integration already outweigh those imposed by climatic gradients.Nevertheless,more investigations in different species and manipulation approaches are needed to confirm this scenario.
The evolution of sexual dimorphism in POLS has been highlighted because of the obvious differences between the sexes in life history optima and consequently in the optimal expression of life history,behavioral and physiological traits involved in POLS (Immonenet al.,2018).InB.gargarizans,we detected a negative covariation between growth rate and baseline GCs in females and a positive covariation between growth rate and hemagglutination in males at the withinpopulation level;both of these results indicate a sex-specific POLS composition (i.e.,the specific traits that form a POLS differ between the sexes).Considering the obvious sex-biased reproductive lifespan inB.gargarizans,these results support the predictions that sexual dimorphism in POLS is most likely to occur in species whose reproductive roles differ between the sexes (Immonenet al.,2018;Hamalainenet al.,2018).The enhanced covariation between growth and baseline GCs in female toads may help them to invest more resources in future reproduction,while the greater covariation between growth and hemagglutination in males suggest that they are likely to be more sensitive to parasite infections than females,which is potentially associated with sex-specific hormonal immunosuppression (Despratet al.,2015).As current phenotypic trait covariances may not accurately reflect the genetic covariances,further investigations need to address the underlying genetic basis of these sex-specific POLS composition.
Overall,this study showed that adultB.gargarizansmainly displayed consistent differences in growth rate,metabolic,immunological and hormonal traits between sexes,but no altitude.Moreover,sex-but not altitude-modified the phenotypic integration between growth rate and hormonal and immunological traits at the within-population level.Our study demonstrates that the prevalence of sex-specific POLS and highlights its role in understanding the evolution,maintenance and variability of POLS.
AcknowledgementsThe study was conceived and designed by J.F.CHEN and P.LI.Experiments were performed by P.LI,S.TAN,Z.Y.YAO,J.F.CHEN;results were analyzed and interpreted by P.LI and J.F.CHEN.The paper was written by P.LI,S.TAN,Z.Y.YAO,J.F.CHEN and J.Z.FU.This work was financially supported by grants for the Biodiversity Survey and Assessment Project of the Ministry of Ecology and Environment of China (2019HJ2096001006) to J.F.CHEN,the National Natural Science Foundation of China (31370431) to,the National Natural Science Foundation of China (31729003) to J.F.CHEN,and the Sichuan Provincial Science and Technology Department (2018JY0617) to J.F.CHEN.
 Asian Herpetological Research2022年1期
Asian Herpetological Research2022年1期
- Asian Herpetological Research的其它文章
- Appendix 1
- An Annotated List of Lizards (Sauria:Squamata) Recorded from the People’s Republic of China
- High-elevation Adaptation of Motion Visual Display Modifications in the Toad-Headed Agamid Lizards (Phrynocephalus)
- Circadian Rhythm and Intersexual Differences in Auditory Frequency Sensitivity in Emei Music Frogs
- Offspring Sex Is Not Determined by Gestation Temperature in a Viviparous Lizard (Eremias multiocellata) from the Desert Steppe of Inner Mongolia
- Comparative Osteology of Two Far Eastern Species of Ratsnakes (Serpentes:Colubridae),Elaphe dione (Pallas,1773) and E.schrenckii (Strauch,1873),for the Purpose of Palaeontological Studies
