Plasma-assisted ammonia synthesis in a packed-bed dielectric barrier discharge reactor:roles of dielectric constant and thermal conductivity of packing materials
Jin LIU(劉進(jìn)),Xinbo ZHU(竺新波),*,Xueli HU(胡學(xué)理) and Xin TU(屠昕)
1 Faculty of Maritime and Transportation,Ningbo University,Ningbo 315211,People’s Republic of China
2 Department of Electrical Engineering and Electronics,University of Liverpool,Liverpool L69 3GJ,United Kingdom
Abstract In this article,plasma-assisted NH3 synthesis directly from N2 and H2 over packing materials with different dielectric constants(BaTiO3,TiO2 and SiO2)and thermal conductivities(BeO,AlN and Al2O3)at room temperature and atmospheric pressure is reported.The higher dielectric constant and thermal conductivity of packing material are found to be the key parameters in enhancing the NH3 synthesis performance.The NH3 concentration of 1344 ppm is achieved in the presence of BaTiO3,which is 106% higher than that of SiO2,at the specific input energy(SIE)of 5.4 kJ·l-1.The presence of materials with higher dielectric constant,i.e.BaTiO3 and TiO2 in this work,would contribute to the increase of electron energy and energy injected to plasma,which is conductive to the generation of chemically active species by electron-impact reactions.Therefore,the employment of packing materials with higher dielectric constant has proved to be beneficial for NH3 synthesis.Compared to that of Al2O3,the presence of BeO and AlN yields 31.0% and 16.9% improvement in NH3 concentration,respectively,at the SIE of 5.4 kJ·l-1.The results of IR imaging show that the addition of BeO decreases the surface temperature of the packed region by 20.5% to 70.3°C and results in an extension of entropy increment compared to that of Al2O3,at the SIE of 5.4 kJ·l-1.The results indicate that the presence of materials with higher thermal conductivity is beneficial for NH3 synthesis,which has been confirmed by the lower surface temperature and higher entropy increment of the packed region.In addition,when SIE is higher than the optimal value,further increasing SIE would lead to the decrease of energy efficiency,which would be related to the exacerbation in reverse reaction of NH3 formation reactions.
Keywords:dielectric barrier discharge,ammonia synthesis,packing materials,thermal conductivity,dielectric constant
1.Introduction
Ammonia(NH3)is the world’s second largest chemical product and a vital precursor in the synthesis of many products.It is also an environmentally friendly fuel and excellent hydrogen storage carrier.Therefore,ammonia is an ideal alternative to fossil fuel[1-6].Conventional industrial-scale NH3synthesis via Haber-Bosch(HB)method consumes huge amount of energy(1%-2%of world’s primary energy supply)and requires large occupied area due to the severe working conditions,e.g.lasting high temperature(500°C-600°C)and high pressure(20-40 MPa),which limit the application HB method in medium and small scale NH3synthesis[7].In the past 100 years,researchers have devoted great efforts to develop more sustainable alternatives for HB method,including biochemical process,electro-chemical process and non-thermal plasma(NTP)assisted process[8,9].
Among the NH3synthesis methods,NTP is particularly promising due to its characteristics of compact system,high energy efficiency(EE)and flexibility to combine with renewable energy sources.A variety of NTPs have been investigated for NH3synthesis,including dielectric barrier discharge(DBD)[10-14],radio frequency discharge[15,16]and glow discharge[17]plasmas.Recently,the DBD plasma has attracted a wide spread attention.The DBD reactor can be combined with packing materials,i.e.the packed-bed DBD reactor,to increase the reaction yield and EE.The combination of plasma and packing materials often leads to a synergistic effect,i.e.the enhancement in the reaction performance generated by the interactions between the plasma and the packing materials[18-20].To enhance the plasma-assisted NH3synthesis process,researchers have investigated the effect of different packing materials in the packed-bed DBD reactor.Akayet alemployed microporous silica-supported nickel catalysts packed with BaTiO3for NH3synthesis in a DBD reactor.When the ratio of catalyst increased from 0%to 100% in the catalyst-BaTiO3pellet mixture,the nitrogen conversion rate increased from 7.3% to 11.2%[21].Mizushimaet alevaluated the effects of Al2O3membranesupported transition metal catalysts(Ru,Ni,Pt,and Fe)on the synthesis of NH3using DBD plasma.Ru showed significant catalytic effects on the NH3formation in N2-H2plasma,although it had a lack contribution of the N2dissociation[22].Wanget aldemonstrated that the synergistic effect of plasma and a Ni/Al2O3catalyst appeared to be evident from 400°C,and the loaded Ni supported more chemically active sites[23].
Although the synergistic effect of plasma-assisted NH3synthesis in a packed-bed DBD has long been investigated,there are only few studies on the influences of packing materials’ electrical properties on the discharge behavior and its contribution to NH3synthesis.In the presence of packing materials,the electrical characteristics of the DBD reactor would be affected together with the chemical processes in plasma region,e.g.the onset voltage,electron energy of plasma and uniformity of filamentary discharges,etc[7,8,24-29].The dielectric constant of packing materials played a dominant role in the plasma discharge process.Guet alreported that the amplitude of the current peak increased in presence of materials of higher dielectric constant in a coaxial DBD reactor.At the AC discharge frequency of 1 kHz and voltage amplitude of 2 kV,the maximum current amplitude increased 20 times when the relative dielectric constant of packing materials increased from 10 to 15[24].Duanet alinvestigated the effects of dielectric materials on the decomposition of CO2in a DBD reactor.CaO exhibited a superior promotional effect on the conversion of CO2compared a reactor with other packing materials,the conversion of CO2was highly improved in the CaO packed reactor,and reached the highest value of 32.8%,which was approximately twice the conversion of CO2in the SiO2packed reactor and the Al2O3packed reactor.The results indicated that the dielectric constant would affect the conversion of CO2[30]
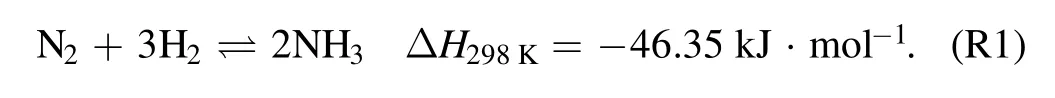
Another key factor is the thermal conductivity of the packing materials,which greatly affects the heat transfer process in the packed-bed DBD reactor.It has been reported that heat generates inside of packed region then transfers to environment and the flowing gas when the plasma is lighted in the packed-bed DBD,and heat production is proportional to the discharge power in a packed-bed DBD reactor[31].The temperature of packed region would vary depending on different thermal conductivity of the packing materials.As an exothermic reaction,low temperature is beneficial for the forward reaction for NH3synthesis(R1).Previously,Sunet alinvestigated the effects of ambient temperature and other operation parameters and reported high temperature would enhance the heat build-up effect,which is beneficial for NH3synthesis at the beginning of discharge[32].However,very few works have reported the influence of thermal conductivity of packing materials on NH3synthesis in a DBD reactor when the reaction has been in the steady state.
In this work,several packing materials(BeO,AlN,SiO2,TiO2,BaTiO3and SiO2)with different relative dielectric constant and thermal conductivity are selected to study the effects of the dielectric constant and thermal conductivity of these packing materials on the process of plasma-assisted NH3synthesis.The effects of packing materials on the fundamental characteristics of the NH3synthesis in the DBD reactor are investigated through electrical and thermal diagnostics.We also illustrate that increasing specific input energy(SIE)or packing the materials of higher dielectric constant could enhance the reduced electric field(E/N)and change the electron energy distribution function(EEDF)by calculation through solving the Boltzmann equation using BOLSIG+.A complete understanding on the effects of dielectric constant and thermal conductivity of packing materials on NH3synthesis has been proposed.
2.Experimental
2.1.Experimental setup
The DBD reactor is a coaxial cylindrical structure,as shown in figure 1.A 30 mm long stainless-steel mesh is wrapped around the quartz tube with an external diameter of 10 mm and an inner diameter of 8 mm.A stainless-steel rod with an outer diameter of 4 mm is placed in the center of the quartz tube and it acts as an inner electrode.The inner electrode is connected to the high voltage output of the power supply.An external capacitance(Cm=0.47 μF)is connected between the outer electrode and the ground.
Zero grade(purity>99.999%)N2and H2are provided from gas cylinders and regulated by mass flow controllers(Sevenstars D07-B).Before being introduced to the reactor,the two gas streams are mixed in a mixing chamber.The DBD reactor is supplied by an AC high voltage power supply(Suman CTP-2000K).The gas feed ratio is the molar ratio of N2/H2,which is fixed at 1:1.The total gas flow rate is fixed at 100 ml·min-1.The frequency of AC discharge(f),which is kept in the range of 9.2-9.8 kHz in this work.The applied voltage(Ug)and the voltage across measuring resistance are measured by high voltage probe(Tektronix TPP0502)to obtain theU-Icurve.The voltage(Uc)on the external capacitor is measured to obtain the charge generated in the discharge.All of the electrical signals are sampled by a fourchannel digital oscilloscope(Tektronix TDS2024C).Various electrical parameters of plasma could be obtained from the typicalQ-ULissajous figure as illustrated by figure 2.

Table 1.Relative dielectric constant and thermal conductivity of the packing materials.
A series of packing materials with different dielectric constants and thermal conductivities include BaTiO3,TiO2,SiO2,Al2O3,AlN and BeO.These materials are nonporous ceramics with high purity(>99%)and purchased from Beilong Electronic Co.Ltd China.The relative dielectric constant and thermal conductivity of the packing materials are measured by the manufacturer and the value is given in table 1.Before using,these materials are crushed and sieved to 40-60 meshes,followed by ultrasonic cleaning in ethanol for 1 h.Then the materials were heated in an argon flow at 350°C for 2 h,and then cooled to room temperature in argon flow.After pretreatment,the packing materials are placed into the plasma region while the length of packed region is 12 mm.Quartz wool is evenly placed on both ends of the packing materials to hold them.
The surface temperature of the reactor is measured by an IR camera(UNI-T UTi165A),while the average surface temperature of DBD reactor is calculated by UTi thermal analyzer software.The temperature of inlet and outlet gas mixtures could be measured by dual-channel digital thermometer(UNI-T UT320D).The outlet NH3concentration is analyzed by dualchannel gas chromatography(Fuli 9790Ⅱ),which is equipped with a thermal conductivity detector.The chromatography column was a packed column for ammonia separation produced by Wuhan Puli Technology.Co.Ltd China.The outlet NH3concentration is measured three times and the average value is given.The EEDF is calculated by BOLSIG+[33].
2.2.Parameter calculation
To better understand the effects of dielectric constant and thermal conductivity on NH3synthesis,a simplified model of the packed DBD reactor is established.The regions of packing materials and gas phase are equivalently considered as two parallel plate capacitors,as shown in figure 3.
The relationship of relative dielectric constant and dielectric constant is as follow:

where ε,εrand ε0are dielectric constant,relative dielectric constant and the dielectric constant of vacuum.
The plasma discharge power(Pdis)can be calculated by:

whereCmis external capacitance(0.47 μF),fis the frequency of AC discharge andAis the area of the Lissajous figure.
The equivalent gap thicknesses in the gas(dg)and solid(dp)region are determined by equations(2)and(3):

wheredgapis the gap width between internal face of quartz tube and the surface of inner electrode,lis the length of packed region andDis the outer diameter of quartz tube.
The volume of the void of the packed region can be calculated by:

whereVgasis the volume of the void in the packed region,Vpackingis the volume of the packed region.In this work,the value of measured void fraction coefficient(β)is measured at 0.43±0.01.
Cdrepresents the capacitance of the quartz tube and the capacitance of the gap isCgap.The equivalent capacitance of the quartz tube is calculated according to the topology of a coaxial capacitor,as shown in equation(5)

where ε0is the dielectric constant of vacuum(8.85×10-12F·m-1),ε is the dielectric constant of quartz tube andxis the wall thickness.
The equivalent capacitance of the gas(Cg)and solid(Cp)regions is calculated in the form of a parallel plate capacitor:

where εgis the relative dielectric constant of mixed gas(εg=1 when the gas feed ratio is 1:1);dganddpare the equivalent gaps of gas and packing materials respectively;Sis the surface area of outer electrode of packed region

The relationship betweenCcellandCgapcan be calculated as follows:
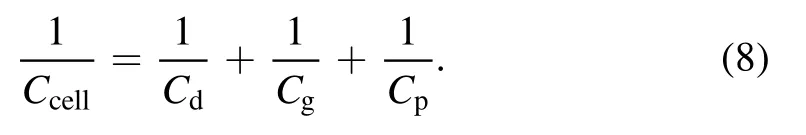
The gas voltage(Ug)can be calculated as follows:

The average strength of electric field(E)is defined as:
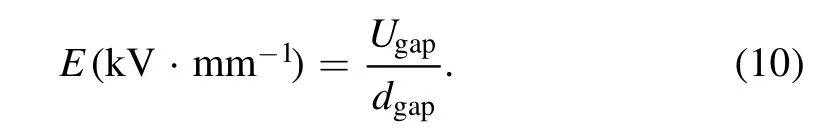
The average electric field strength of gas phase(Egas)can be calculated by:
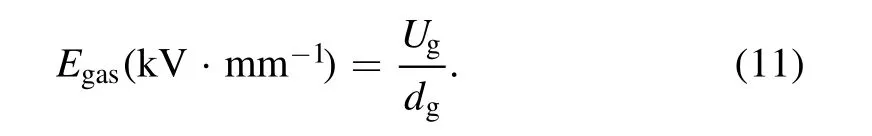
The breakdown voltage of DBD reactor could be calculated by:
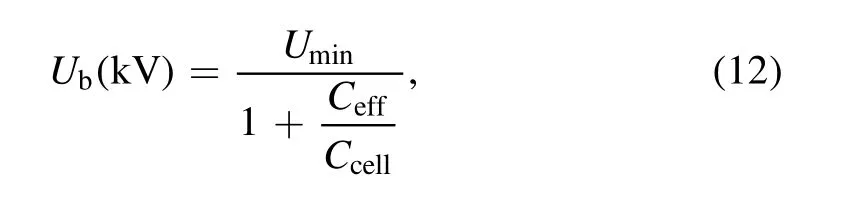
whereUmin,CcellandCeffcan be determined by Lissajous figure(as shown in figure 2).
SIE is defined as the ratio of the plasma absorbed power to the gas flow rate:

whereQis the total gas flow rate.
The EE of the plasma-assisted NH3synthesis process is defined as follow:

whereMis the molar mass of NH3,Coutdenotes the outlet NH3concentration andQafteris the outlet gas flow rate after the reaction and is measured by a soap film flow-meter.
3.Results
3.1.Effect of SIE on NH3 synthesis
Figure 4 presents the effects of SIE on NH3synthesis over different packing materials.As presented in figure 4(a),when the SIE increases from 3 to 5.4 kJ·l-1,the NH3concentration increases from 112 to 1344 ppm in the BaTiO3packed reactor,and the increment of NH3concentration is only 283 ppm when the SIE increases in the range of 5.4-10.8 kJ·l-1.Meanwhile,the increment of NH3concentration in the TiO2packed reactor is 1019 ppm when the SIE increases from 3 to 5.4 kJ·l-1,which is 351 ppm when the SIE increases from 5.4 to 10.8 kJ·l-1.As shown in figure 4(b),for the case of packing BeO,the concentration increases from 478 to 1707 ppm with the SIE increasing in the range of 3-10.8 kJ·l-1.
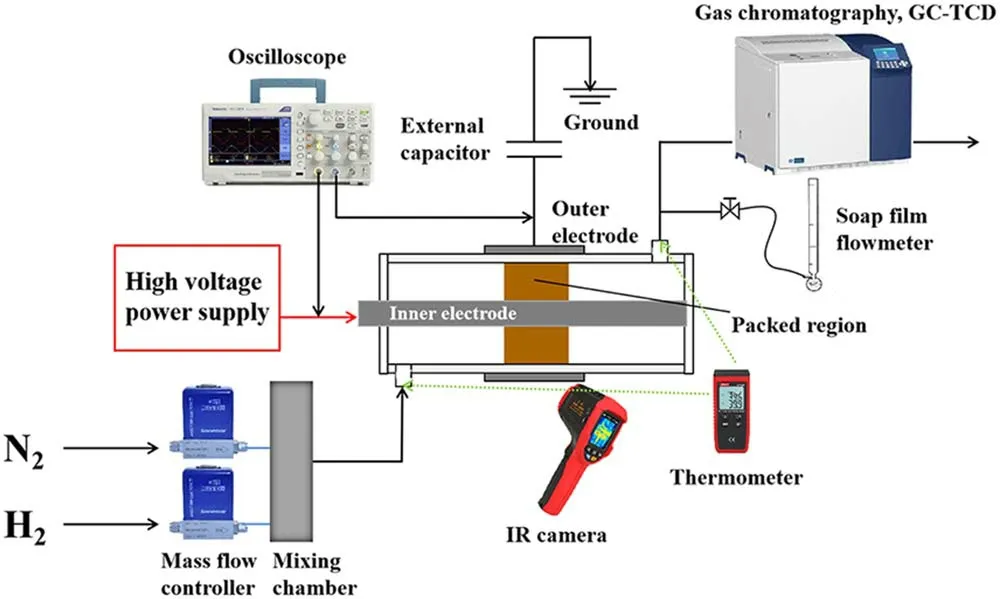
Figure 1.Experimental setup for the plasma-assisted NH3 synthesis.
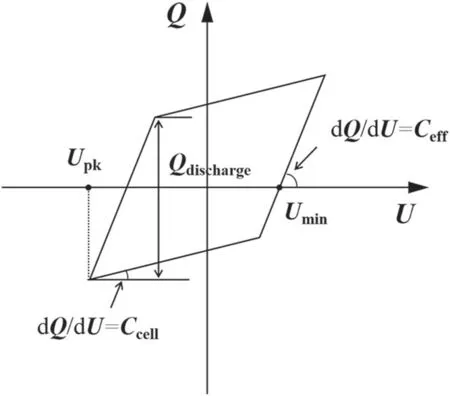
Figure 2.Schematic figure for the derivation of electrical parameters from a Q-U Lissajous.
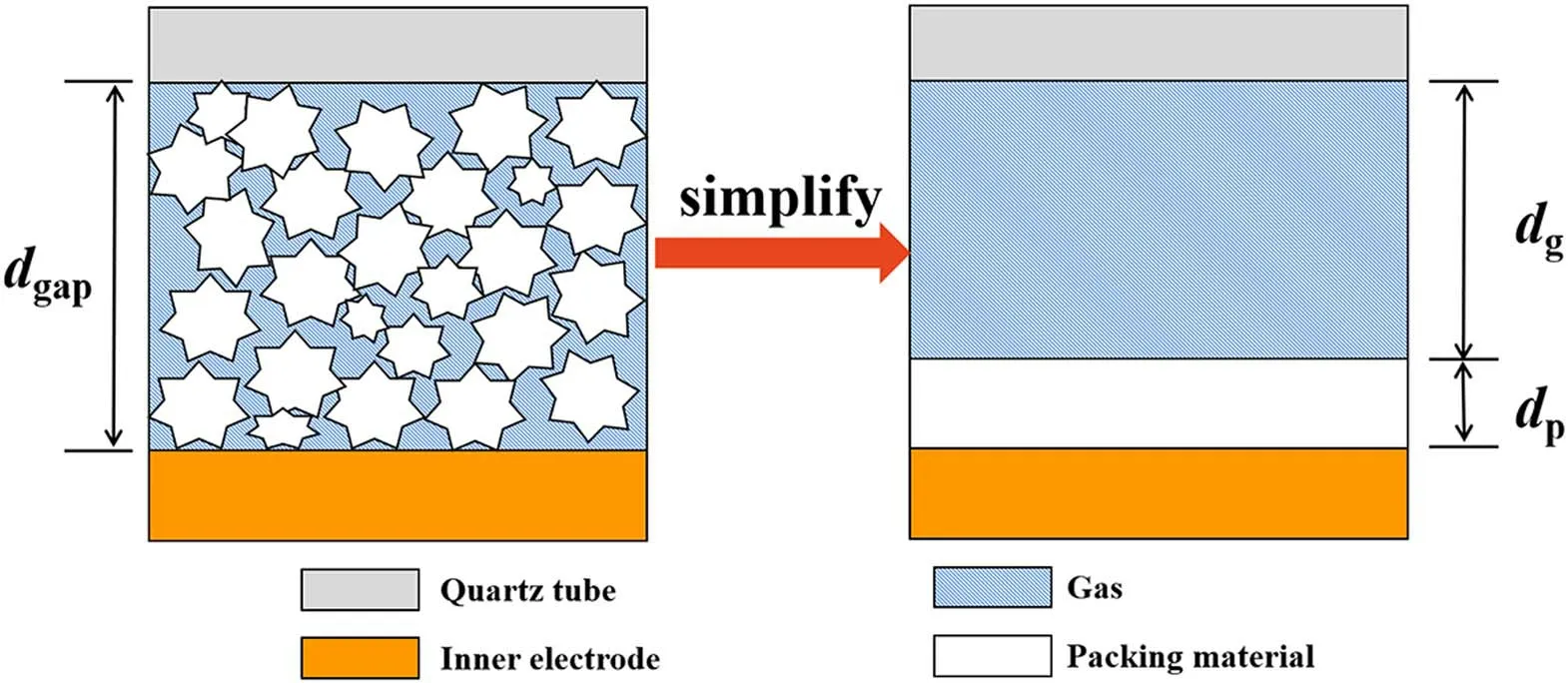
Figure 3.The simplified model of packed-bed DBD reactor.

Figure 4.Effects of SIE on NH3 concentration over different packing materials.
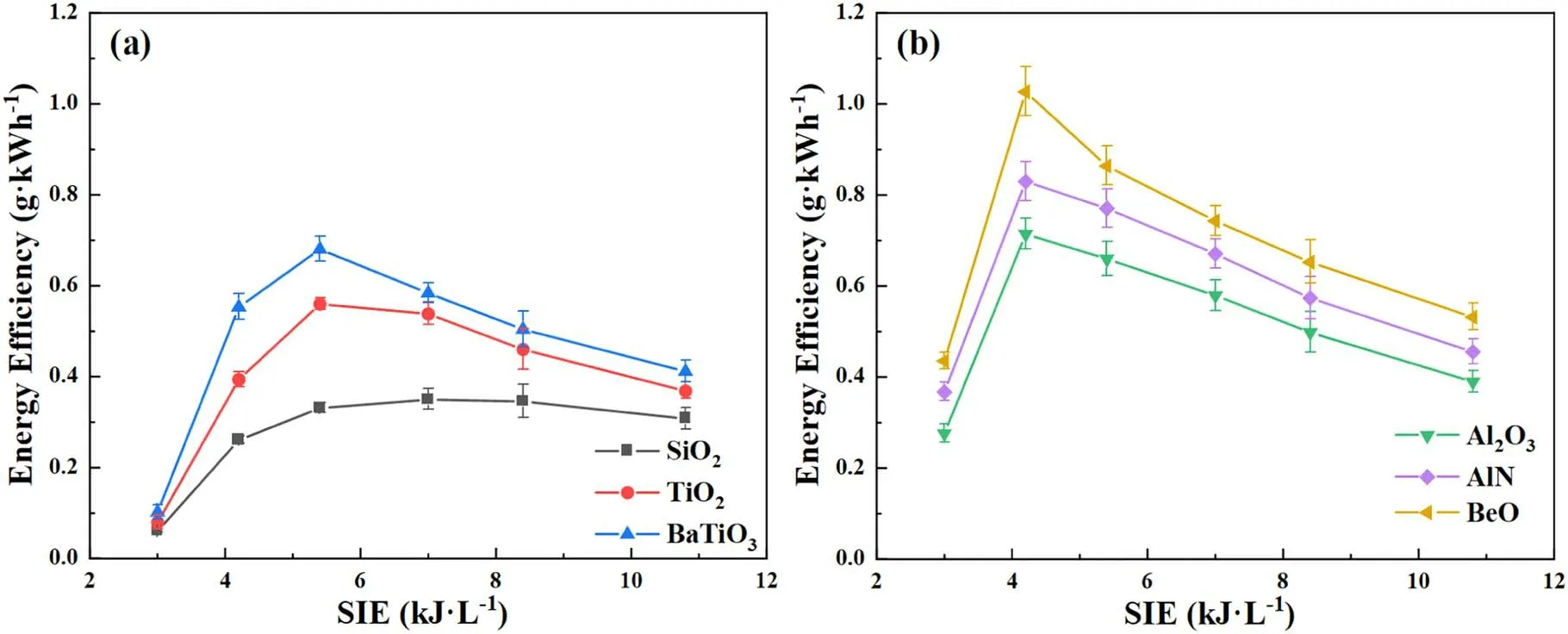
Figure 5.Effects of SIE on energy efficiency over different packing materials.
In summary,higher NH3concentration is achieved over BaTiO3and BeO peaked reactors.The NH3concentration increases rapidly,but when the SIE is higher than a certain value(the optimal value),the trend of NH3concentration changes from rapid increase to slight increase with the increasing SIE,this trend is pronounced as shown in figure 4(a).
Figure 5 shows the effect of SIE on EE in the presence of different packing materials.As shown in figure 5(a),the highest EEs of 0.68,0.56 and 0.33 g·kWh-1for BaTiO3,TiO2and SiO2packed reactors,respectively,are observed at the SIE of 5.4 kJ·l-1.Meanwhile,the EE is always higher in presence of BaTiO3,compared to that of TiO2and SiO2.As presented in figure 5(b),the EE follows the ascending order of Al2O3<AlN<BeO,indicating higher thermal conductivity of packing materials is beneficial for NH3synthesis.Therefore,it can be deduced that higher EE is achieved by employing packing materials with higher thermal conductivity or higher dielectric constant.

Figure 6.Relationships between voltage and current for different packed materials reactors(SIE=5.4 kJ·l-1).
For all of the cases,the EE increases initially,however,when the SIE is higher than the optimal value,the energy efficient would decrease.As shown in figure 5(a),the EE decreases dramatically when the SIE is in the range of 5.4 and 10.8 kJ·l-1,which decreases from 0.68 to 0.41 g·kWh-1in presence of BaTiO3and from 0.56 to 0.37 g·kWh-1in presence of TiO2.Similar phenomenon is also presented in figure 5(b).This observation may be related to electronimpact NH3decomposition and the inhibition of NH3synthesis by high temperature in the packed region.
3.2.Discharge behaviors in different materials packed reactors
Figure 6 shows the current profiles in different materials packed reactors.In packed-bed DBD reactors,the discharge would transfer from filamentary discharge to a combination of surface discharge on the surface of the packing materials and spatially limited microdischarges generated in the void space[34,35].At the SIE of 5.4 kJ·l-1,the intensity of time-averaged microdischarges current in BaTiO3packed reactor is 18.3 mA,which is about 2 times and 1.8 times higher than those of SiO2and TiO2,respectively.This phenomenon was also reported in various plasma-assisted processes[35-37].Obviously,the current intensity increases in presence of the materials with higher dielectric constant.Moreover,the breakdown voltage is increased in presence of packing materials with higher dielectric constant as determined from both Lissajous figures and current profiles.Compared to that of BaTiO3(εr=7200),the breakdown voltage decreases from 1.15 to 0.77 kV in presence of SiO2(εr=3.80).

Figure 7.Q-U Lissajous figures for different materials packed reactors(SIE=5.4 kJ·l-1).
The time-averaged microdischarge current intensity is approximately equal when Al2O3,AlN and BeO are employed as shown in figure 6(b).The current intensities in BeO and AlN packed reactors are 9.2 mA and 9.1 mA,respectively,which are close to the case of Al2O3packed reactor(8.8 mA).A slight increasing number of microdischarge is obtained in the presence of BeO and AlN,which may be related to the increasing electron density at lower temperature of discharge region.
Figure 7 shows the Lissajous figures for different materials packed reactors at the SIE of 5.4 kJ·l-1.The shape of the Lissajous figure is varied when different packing materials are employed,which suggests the changes in the discharge characteristics includingVpk,Ceff,andQdischarge(quantity of the discharged charges per half-cycle).At the SIE of 5.4 kJ·l-1,the applied voltage of the DBD reactor increases from about 13.6 kVpk-pkfor SiO2packed reactor to about 15.8 kVpk-pkfor the case of BaTiO3.TheQdischargeincreases in the presence of packing materials with higher dielectric constant,which is 0.32 μC for BaTiO3packed reactor and 0.23 μC for SiO2packed reactor.Meanwhile,higherCeffof 88.39 pF is achieved in BaTiO3packed reactor,which is 11.8% higher than that of SiO2.The large effective capacitance suggests that the addition of higher dielectric constant materials benefits to achieve the fully-bridged discharge.The higherCeffproves that the employment of BaTiO3could bridge the gap between the electrodes and enhance the charge transfer between them[38].In contrast,the Lissajous figures for BeO,AlN and Al2O3packed reactors are approximately the same,indicating the employment of these materials is of no effect in varying electrical characteristics of plasma.

Figure 8.(a)Surface temperature distribution of SiO2 packed reactor at different SIE(discharge time=1.5 min),(b)reactor surface temperature distribution at SIE of 5.4 kJ·l-1(discharge time=5 min,1-6 reactors are packed with BaTiO3,TiO2,SiO2,Al2O3,AlN and BeO,respectively).
3.3.Surface temperature distribution of the reactors
The surface temperature of plasma region in steady state is notably affected by the SIE as shown in figure 8(a).When the SIE increases from 3 to 10.8 kJ·l-1,the average reactor surface temperature of packed region(inside of the dotted box)increases from 19.1°C to 78.3°C,which increases 4.1 times when the SIE increases in the range of 3-10.8 kJ·l-1.Similar observations were also reported by Kimet aland Tirumalaet al[31,39].We find the average surface temperature of the packed region is significantly higher than other area covered by the outer electrode,indicating that the presence of packing materials contributed to generate intensified microdischarge.Figure 8(b)presents the IR imagings of the packed region of the plasma reactor in the presence of different packing materials at the SIE of 5.4 kJ·l-1.The average surface temperature of the packed region is obtained from figure 8(b)and summarized in figure 9.The reactors packed with BaTiO3,TiO2and SiO2are of similar average surface temperature of the packed region,which is 96.2 °C,91.2 °C and 90.6 °C,respectively.In contrast,average surface temperature of the reactor packed region decreases dramatically in presence of packing materials with higher thermal conductivity.Compared to the Al2O3packed reactor,the presence of BeO leads to a decrease of average surface temperature from 88.4 °C to 70.3 °C,this suggests that the heat transfer is significantly enhanced by employing packing materials with higher thermal conductivity.
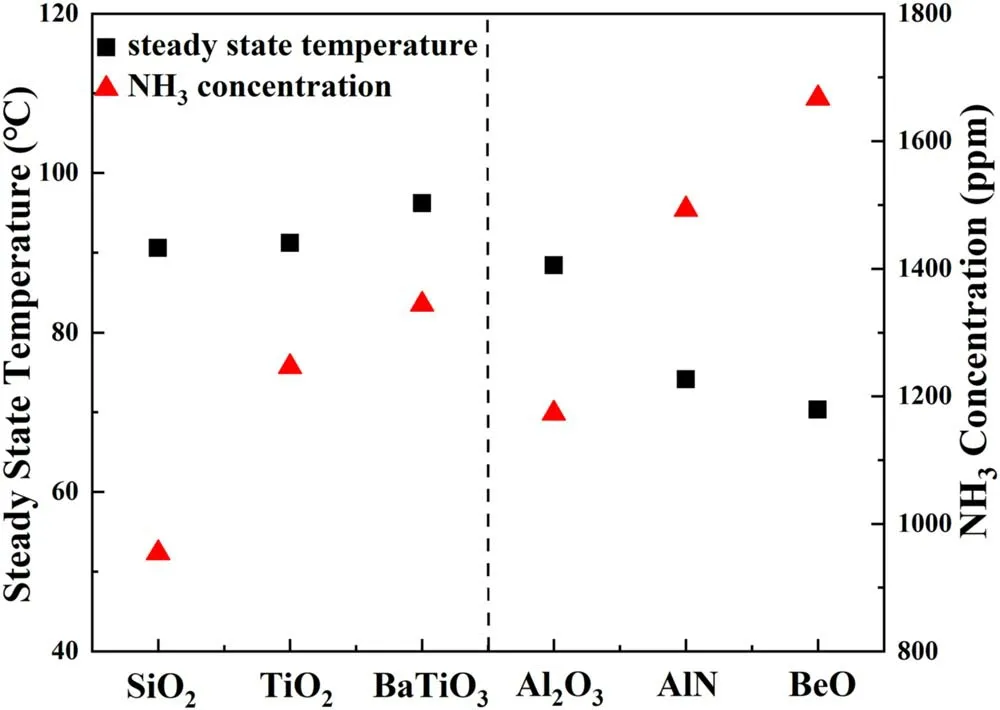
Figure 9.Relationships between NH3 concentration and average surface temperatures in steady state with different packing materials(discharge time=5 min,SIE=5.4 kJ·l-1).

Figure 10.(a)Mean energy as a function of reduced electron field strength(E/N),(b)electron energy distribution function.
4.Discussions
4.1.Correlations of packing materials with higher dielectric constant to the ammonia synthesis
The NH3concentration and EE in the presence of BaTiO3were 1344 ppm and 0.68 g·kWh-1,respectively,which were about 106% higher than those of SiO2,respectively,at the SIE of 5.4 kJ·l-1.Meanwhile,electrical characteristics of DBD reactors changed dramatically in presence of packing materials with different dielectric constants.As described in figure 7,theCeffof reactor,as well asQdischarge,was in an ascending order of SiO2<TiO2<BaTiO3,indicating that the presence of BaTiO3leads to an expansion of microdischarge in the gas phase.Thus,more energy could be injected into the plasma for chemical reactions[38,40].
According to our calculation,Egaswould be improved by the higher dielectric constant of packing materials.To further understand the relationships between electron energy and reduced electric field strength,EEDF and mean electron energy are calculated by BOLSIG+.As shown in figure 10,with increasingE/N,the mean electron energy is improved as well.Meanwhile,the EEDF is found to shift with an increase in electron density in the high-energy tail of the distribution function,indicating the increase fraction of highly energetic electrons.In our case,at the SIE of 5.4 kJ·l-1,theE/Nis 1.62 times higher in the presence of BaTiO3,compared to that of SiO2.The higherE/Ndirectly results in the increase of mean electron energy,which is about 1.41 times while the proportion of energetic electrons would also increase as confirmed in figure 10(b).Similar phenomenon was also reported by Meiet alin CO2decomposition experiment,at the discharge power of 50 W,the reduced electric filed strength generated by packing BaTiO3was 1.37 times higher than which was generated by SiO2packed and 1.53 times to the non-packed reactor.Compared to that of the SiO2and nonpacked reactors,The EE of the BaTiO3packed reactor was 1.75 times and 1.35 times higher than those of the SiO2and non-packed reactors,respectively[35].It was also reported by Chenet al[29]and Duanet al[30]that higher electric field and consequently higher mean electron energy would be obtained in presence of packing materials with higher dielectric constant in a packed-bed DBD reactor.
Since the quantity of energetic electrons and energy injected into plasma were improved in presence of materials with higher dielectric constant,electron-impact reactions would be enhanced and consequently contribute to the generation of chemically active species including excited species likeand(e.g.(R2)-(R5)).These chemically reactive species would recombine and form NHx(x=1,2)species,which were regarded as the most important intermediate products of plasma-assisted NH3synthesis process[23],and accelerate NH3formation(e.g.(R4)).

Hence,the NH3concentration,as well as EE,was observed to follow the ascending order of dielectric constant of packing materials,i.e.SiO2<TiO2<BaTiO3.However,in plasma-assisted NH3synthesis process,the positive reactions towards NH3formation would coexist with the reverse reactions of NH3decomposition.Thus,NH3decomposition is inevitable during the whole process and unfavorable for NH3formation.These reverse reactions would cause the decomposition of NHx·(x=1,2 and 3)species and directly lead to the decrease of EE,which would be exacerbated in the presence of materials with higher dielectric constant.In particular,when the SIE is higher than the optimal value,e.g.5.4 kJ·l-1for BaTiO3,TiO2and SiO2packed reactor in this work,the EE decreases dramatically in presence of BaTiO3,compared to that of SiO2.This phenomenon is aligned with many works[7,41,42].
Since the dielectric constant of Al2O3,BeO and AlN was similar,an increasing amount of microdischarge were generated when materials with higher thermal conductivity were packed at the same SIE.This phenomenon was related to the decrease of average temperature in discharge zone,which would contribute to improve the electron density,promote the formation of filamentary microdischarge and further contribute to the electron-impact reactions for NH3synthesis[37,43].
4.2.Correlations of packing materials with higher thermal conductivity to the ammonia synthesis
It has been reported the NH3concentration would increase with the increasing discharge time and eventually stabilize,then the maximum NH3concentration would be achieved[7,32].At the beginning of plasma discharge duration,heat storage inside the packed region is conducive to accelerate the synthesis reaction to reach the steady state[44].However,NH3synthesis reaction(R1)is an exothermic reaction.According to Le Chatelier’s principle,lower ambient temperature is conductive to the NH3synthesis process.To further understand the effect of thermal conductivity on NH3synthesis,our discussion is carried out under the condition that NH3synthesis reaction has been in the steady state.
Since the NH3synthesis process is in steady state,the heat generation and dissipation in the whole reactor would reach thermodynamic equilibrium.Meanwhile,both inlet and outlet gas flow rates are fixed,and the pressure inside the reactor is also a constant(atmosphere pressure).The reactor is simplified as a thermodynamic steady flow system with a stable temperature field.
According to the second low of thermodynamic,the entropy increment can be calculated as follows:
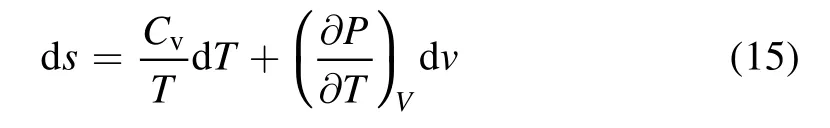
whereSis entropy of the plasma region(J mol-1·K-1),Cvdenotes specific heat capacity at constant volume of the mixed gas(J kg-1·K-1)andTis the average temperature of the packed region(°C).
The relationship between the NH3generation and the entropy increment is as follows:

whereJis the relative reaction react rate of per molar NH3generation(mol ml·min-1);Ais the relative chemical entropy increase of per molar NH3generation(J mol·K-1).
According to the simplified model,the heat transform quantity(Qtrans)could be calculated by:

where λtis the total thermal conductivity of solid phase and gas phase(W m-1·K-1),Aisois the area of the isothermal surface of packed region(m2),tis the temperature of isothermal surface(°C),σ is the distance from isothermal surface to reactor outer surface(m),τ is the heat transfer time(s).
In plasma-involved cases,the energy balance equation can be described as follows:

wherePreactionis the power consumption of the reactions andPgasis the power absorbed by the gases.
The power consumption of reactions(Preaction)can be calculated as:

whereQmis the mass flow rate of the mixed gas,αiis the mass fraction of the one gas components,hiis the specific enthalpy of one of the gas components,imeans one of the gas components(e.g.N2,H2and NH3)andgis the total species number of the gas components.
The discharge power absorbed by feed gas can be calculated by:

whereTis the temperature of gas,Cp(i)is the heat capacity at constant pressure of the gas.
In this work,the presence of BeO achieved both a higher NH3concentration of 2102 ppm and EE of 1.02 g·kWh-1,which were about 30%higher than that of Al2O3.Meanwhile,the heat transfer process would be enhanced in the presence of materials of higher thermal conductivity.At the SIE of 5.4 kJ·l-1,the heat transfer quantity(Qtrans)was increased by 32.5% in the BeO packed reactor compared to the reactor packed with Al2O3.As a result,the addition of BeO significantly decreases the average temperature to 70.3 °C.Therefore,with the enhanced heat transfer process,compared to that of Al2O3,a higher entropy increment would be achieved with BeO,according to equations(15)-(18).
NH3synthesis reaction is a process of entropy reduction.According to the second law of thermodynamics,this process is spontaneous and needs an extra entropy increase process with the aim of increasing the system entropy to benefit the forward reaction of(R1).In plasma-assisted process,the processes of gas molecules electron-impact decomposition and the heat transfer process were considered as the entropyincreasing process[45,46].Considering that the temperature difference between the inlet and outlet gases of the reactor was small(<0.1 °C)and could be neglected in this work,furthermore,the ambient temperature was almost the same,it would be thermochemically feasible that the process which heat transfers to the environment was the dominate process of entropy increase of the plasma-assisted NH3synthesis process.Therefore,compared with that of Al2O3,both higher NH3concentration and EE were achieved in presence of BeO because of both the entropy increment and lower temperature of the packed region,which contributed to NH3synthesis reaction.
5.Conclusions
Plasma-assisted NH3synthesis has been carried out in different materials(BeO,AlN,SiO2,TiO2,BaTiO3and SiO2)packed DBD reactors.The effects of the dielectric constant and thermal conductivity of packing materials on NH3synthesis have been investigated.
The presence of materials with higher dielectric constant materials could improve the electron energy and energy injected to plasma,which would be beneficial for the generation of chemically active species by electron-impact reactions and accelerate NH3formation.Therefore,both NH3concentration and EE were following the ascending trend of SiO2<TiO2<BaTiO3.The higher NH3concentration of 1344 ppm was achieved in presence of BaTiO3,followed by that of TiO2of 1106 ppm and SiO2of 654 ppm.Meanwhile,the NH3synthesis EE of 0.68 g·kWh-1was achieved with BaTiO3,which was 105%higher than that of SiO2at the SIE of 5.4 kJ·l-1.
The presence of materials with higher thermal conductivity significantly enhanced the heat transfer process and decreased the average temperature of packed region.Compared to that of Al2O3,the quantity of heat transfer was increased by 32.5%in presence of BeO.The entropy of the packed region was also increased as result.Meanwhile,the lower temperature of the packed region(70.3°C)was also obtained in the BeO packed reactor,which was 20.5%lower than that of Al2O3,at the SIE of 5.4 kJ·l-1.Both the entropy increment and the lower temperature have proved to be conductive to forward reaction of NH3synthesis.Therefore,the presence of materials of higher thermal conductivity achieved higher NH3concentration and EE,which increased with different packing materials in the order of Al2O3<AlN<BeO.
Acknowledgments
The authors are grateful for the financial support from National Natural Science Foundation of China(No.51976093)and K C Wong Magna Fund in Ningbo University.
ORCID iDs
 Plasma Science and Technology2022年2期
Plasma Science and Technology2022年2期
- Plasma Science and Technology的其它文章
- Application of local Monte Carlo method in neutronics calculation of EAST radial neutron camera
- Effect of gas pressure on ion energy at substrate side of Ag target radio-frequency and very-high-frequency magnetron sputtering discharge
- Numerical simulation of hydrogen arcjet thruster with coupled sheath model
- An electromagnetic wave attenuation superposition structure for thin-layer plasma
- A fast convergence fourth-order Vlasov model for Hall thruster ionization oscillation analyses
- Surface modification of silicone rubber by CF4 radio frequency capacitively coupled plasma for improvement of flashover
