Mechanism of titanium–nitride chemical mechanical polishing?
Dao-Huan Feng(馮道歡), Ruo-Bing Wang(王若冰), Ao-Xue Xu(徐傲雪), Fan Xu(徐帆),Wei-Lei Wang(汪為磊), Wei-Li Liu(劉衛(wèi)麗), and Zhi-Tang Song(宋志棠)
1State Key Laboratory of Functional Materials for Informatics,Laboratory of Nanotechnology,Shanghai Institute of Micro-system and Information Technology,Chinese Academy of Sciences,Shanghai 200050,China
2University of the Chinese Academy of Sciences,Beijing 100049,China
Keywords: TiN,chemical mechanical polishing,mechanism
1. Introduction
With the significant increase of device integration,the requirements for interconnected metal materials become more stringent. The traditional aluminum interconnected metal materials can no longer meet the requirements of ultra large-scale integrated(ULSI).Because of the lower resistivity,better electromigration ability,lower energy consumption and higher reliability,copper is widely used as interconnected metal material for ULSI.[1–3]However, copper is easy to diffuse in integrated circuits, especially in the surface of silicon device such as source, drain, and gate.[4]Once copper diffuses into the device, it will produce toxic effect and cause device failure. Therefore,it is the best choice to fill the contact hole with tungsten.There are also some problems to be solved in the filling process of tungsten in the contact hole. On the one hand,the adhesion between tungsten and silica is relatively low. On the other hand,holes are often found on the surface of tungsten plugs in the process of the production and application. Therefore, as a common barrier and adhesion layer material, TiN plays an indispensable role in preparing the contact hole tungsten plug.The presence of tungsten is also crucial in preparing the phase change memory. A typical phase change memory cell is shown in Fig.1(a). It can be seen that during the preparation of tungsten plug,holes will be derived from the interior of the plug, which will lead to instability and even failure of phase change materials. Therefore, as shown in Fig.1(b), a TiN layer must be deposited to fill the plug hole before the phase change material is deposited. And then, as shown in Fig.1(c), the excess tungsten and TiN need removing to ensure the stability of the device.[5]This global planarization is achieved by chemical mechanical polishing process. The reaction mechanism of tungsten chemical mechanical polishing has been described in many papers.[6–9]However,the mechanism of TiN chemical mechanical polishing has not been studied in depth. Hou et al.[10]provided a method of implemeting chemical mechanical polishing of TiN film with acid CMP composition. Sun et al.[11]explored the effects of pH and oxidant concentration on the CMP process of tin film under alkaline conditions. Han et al.[12]analyzed the tribology,thermal and kinetic and established a particle indentation model to describe the polishing process of TiN film with different concentrations of hydrogen peroxide. Chathapuram et al.[13]compared the polishing processes of Ti and tin with H2O2as oxidant, and considered that the difference in chemical reaction product was mainly due to the difference in material removal rate. The mechanism of chemical mechanical polishing of titanium nitride needs further studying. Therefore,in this paper the results of chemical mechanical polishing of TiN materials with two different oxidants are compared with each other to explore the mechanism of TiN polishing process. And a slurry with high polishing rate,material selectivity and better surface quality for TiN film is designed.

Fig.1. Technology of phase change memory cell,showing(a)typical phase change memory cell structure,(b)filling of TiN layer between W layer and PCM layer,and(c)removal of excess TiN.
2. Experiment
2.1. CMP process
A 200-nm-thick TiN film was deposited on an Si wafer by Oxford FlexAL ALD instrument. The deposition process was carried out with TiCl4and N2as the source materials for Ti and N at 120?C.The 8-inch(1 inch=2.54 cm)TiN obtained after ALD deposition was then cut into 2 cm×2 cm wafers for chemical mechanical polishing. 10-wt% silica sol particles were selected as polishing abrasive. The polishing process was carried out by a CP-4 tester,and the specific process parameters are described as follows. The polishing pad was IC1010,the flow rate was 100 ml/min,the pressure was 3 psi(1 psi=0.155 cm?2),the PH was 5–6,the polishing disc rotation speed was 100 rpm,the polishing head rotation speed was 100 rpm,and the polishing time was 2 min. The polishing rate was obtained from the formula: polishing rate=?T/t,where?T is the difference in thickness of TiN film between before and after polishing. The thickness of TiN film is measured by field emission scanning electron microscope (JSM-7800F,JEOL). The NaClO and H2O2used in the polishing process are of analytical grade. In order to make sure that the PH of the slurry is in a range of 5–6, nitric acid was used to adjust the slurry PH.In addition,the chemical mechanical polishing of the thermally oxidized silicon dioxide wafer was performed through the same polishing process to determine the selectivity of the polishing slurry.
2.2. Static dissolution experiment
In order to explore the ability of different polishing slurry components to dissolve TiN film and analyze the mechanism of the polishing process,a static dissolution experiment on TiN film is required. The method of measuring the dissolution rate of TiN film is consistent with the method of measuring the material removal rate.First of all,the 2 cm×2 cm TiN film should be immersed in HF acid solution for 10 min to remove the natural oxide formed on the surface. Then the dipped TiN film should be washed with deionized water and dried out. Finally,TiN films were respectively put into the solutions with different concentrations of oxidant for static dissolution for 10 min,then they were washed and blow dried. The static dissolution rate of TiN film was calculated from the difference in thickness between before and after dissolution. The ICP test then was performed on the dissolved solution to determine the dissolution of each element. The ICP test was carried out under a Nebulizer Pressure of 200 kPa, the plasma gas flow of 15 L/min,and the power of 1.25 kW.
2.3. Potential polarization curve analysis
In order to analyze the chemical reaction in the process of chemical mechanical polishing,a dynamic potential polarization experiment is required. The polarization experiment was carried out by an Autolab electrochemical workstation. In the experiment a three-electrode corrosion system was used.The counter electrode was Pt, the reference electrode was Ag/AgCl, and the working electrode was 3 cm×3 cm of TiN fixed with a copper sheet. The contact area between the working electrode and the solution is 3.14 cm2. The scan rate was 0.1 mV/s,and the potential range was from ?0.4 V to 0.4 V.
2.4. XPS experiment
In order to explore the changes in elemental composition during chemical mechanical polishing,an XPS experiment using an x-ray photoelectron spectrometer system (Axis Ultra,Kratos, UK) is required. Before performing the XPS experiment, TiN film was immersed in the HF solution for 10 min to remove the oxide layer that might exist on the surface. And then TiN film was immersed in the oxidant solutions with different concentrations for the same time, followed by being dried. During the XPS test, a monochromated Al target with a full-spectrum pass energy of 160 eV and an elemental pass energy of 40 eV was used.The standard peak of C 1s was used to calibrate the XPS test results.
3. Results
3.1. Material removal rate and surface roughness of TiN
When it comes to chemical mechanical polishing of integrated circuits, the requirements for the slurry are to obtain a good surface quality after polishing and a relatively high selectivity between the target material and the substrate material in the polishing process.[14]Figure 2(a)shows the relationship between the material removal rate of TiN film and the concentration of oxidant. In the case of using no oxidant,the material removal rate of TiN film is 3 nm/min,which is quite low. This is because the hardness of TiN is relatively high, while the hardness of silica sol is relatively low. Therefore,when there is only pure mechanical action with using no oxidant, the removal effect of silica on TiN is quite limited. When adding H2O2as an oxidant,the material removal rate would increase significantly. It increases linearly from 3 nm/min at 0 wt%to 23 nm/min at 0.9 wt%. When the H2O2concentration is higher than 1 wt%, the material removal rate of TiN film is almost unchanged as the H2O2concentration increases. Similarly,when a small amount of NaClO is added as an oxidant to take part in the chemical mechanical polishing process,the material removal rate of TiN will increase significantly. It increases linearly from 38 nm/min at 0.3 wt% to 76 nm/min at 1 wt% and keeps stable. As the concentration of the oxidant increases,there appears a peak in the material removal rate of TiN film, indicating that there is a certain factor that restricts the rate increasing at this time. And this factor is unrelated to the type of oxidant. The difference in the type of oxidant only affects the appearance of this peak. In the CMP process of integrated circuits,obtaining a high selection ratio between the target material and the substrate material is often the key to ensuring the implement of self-stop process. It is obvious that the substrate materials of TiN are silicon oxide and tungsten.Therefore, it is necessary to further explore the effect of two different oxidants on the material removal rate of silica and tungsten. As shown in Figs. 2(b) and 2(c), with the increase of oxidant concentration, the material removal rate of silicon oxide and tungsten are very low and almost unchanged. And then,the selectivity of TiN to the substrate material can be easily obtained. As shown in Fig.2(d),when using NaClO as the oxidant,the selectivity of TiN to SiO2can reach 128:1 and the selectivity of TiN to W can reach 84:1.

Fig.2. Effects of oxidizers on TiN and SiO2 film CMP performances: (a)MRR of TiN film,(b)MRR of SiO2 film,(c)MRR of W film,and(d)MRR selectivity of TiN film to SiO2 and TiN to W film with different NaClO concentrations.
In the chemical mechanical polishing process of integrated circuits,the quality of the surface post-CMP is another important factor to evaluate the quality of the polishing process. Therefore, the surface morphology of the polished TiN film is measured by atomic force microscopy(AFM)as shown in Fig.3. Figure 3(a) shows the AFM image of the original TiN film, and it can be seen that there are obvious particles on the surface of the original TiN film. This is the material structure caused by the atomic layer deposition process. Due to the presence of surface particles,the roughness of the original TiN sheet will be relatively high, reaching 3.66 nm. The surface morphology of TiN after polishing with 1-wt%hydrogen peroxide is shown in Fig.3(b).The surface morphology of TiN after polishing with 1-wt%sodium hypochlorite is shown in Fig.3(c). It can be seen from the figure that the surface quality of the polished TiN is improved compared with that of the original TiN film. And the polished TiN surface will not produce scratches and corrosion pits. In addition, compared with the original TiN film,the number of surface particles after polishing with 1-wt%H2O2will be reduced. However,after polishing with 1-wt%NaClO,there are almost no particles on the TiN surface.

Fig.3. The AFM surface morphology of TiN film: (a)no polished,(b)polished with 1-wt%H2O2,(c)polished with 1-wt%NaClO.
3.2. Static dissolution rate of TiN film
In order to explore the reason why the removal rate of TiN film is changed, static dissolution experiments and ICP tests are performed.The relationship between the static etching rate of TiN film and the concentration of the oxidant is shown in Fig.4. It can be seen from the figure that no matter whether H2O2or NaClO is used as an oxidant for static dissolution of TiN, the dissolution rate of TiN is relatively low. Table 1 shows the ICP test results of the solution after the static dissolution experiment. After static dissolution, the Ti and N elements in the solution are relatively low. These results indicate that the solution containing H2O2or NaClO has a low dissolution effect on TiN film. In the chemical mechanical polishing process,the product of the reaction between oxidants and TiN film is not easily dissolved. In addition, the surface of TiN after dipping is analyzed by SEM image. Figure 5(a) shows the morphology of the TiN surface after dipping in a 1-wt%hydrogen peroxide solution. It can be seen that its surface is relatively dense. This may be because a dense oxide layer is formed on the TiN surface.Figure 5(b)shows the morphology of the TiN surface after dipping in a 1-wt%sodium hypochlorite solution. It is obvious that the surface is not quite dense.which means that the oxide layer formed by it is thinner than the oxide layer formed by H2O2.

Fig.4. Effect of oxidant on the static dissolution rate of TiN film.

Fig.5. SEM micrograph of TiN surface after being dipped in the solution(a)with 1-wt%H2O2 and(b)with 1-wt%NaClO.

Table 1. The ICP results of elements concentration post-etching with different oxidants.
3.3. Measurements of potentiodynamic polarization curve
There are two kinds of chemical reactions that may occur in the material surface during chemical mechanical polishing.[15]One is oxidation reaction. When the material comes to contact the polishing slurry, an oxide layer will quickly form on the surface of the material,and then the abrasive contacts the oxide layer to remove this oxide layer. The other is the corrosion reaction process.When the material contacts the polishing slurry, an oxide layer is not formed on the surface of the material. Some chemical components in the polishing slurry can only partially corrode the material. The polishing process is carried out by direct contact between the abrasive and the surface of the material.In order to explore the chemical reaction of TiN film in the process of chemical mechanical polishing,a dynamic potential polarization curve test is carried out on the TiN film.The results of the test are shown in Fig.6. The values of corrosion current (Icorr) and corrosion voltage(Ecorr)can be calculated by the Tafel method[16]from the cycle curve of Fig.6(a),and the results are shown in Fig.6(b). It can be seen from the figure that the addition of a small amount of H2O2can cause the Ecorrof the TiN to increase rapidly and the Icorrto increase slightly.And as the concentration of H2O2increases,the Ecorrand Icorrboth decrease a little. Similarly,compared with the condition without oxidant,after adding NaClO,the corrosion voltage of the TiN surface increases significantly and the corrosion current increases a little. However, with the increase of NaClO concentration, the corrosion current of TiN is almost unchanged, and the corrosion voltage increases a little as shown in Fig.6(c). The corrosion voltage refers to the potential difference between the solution and the material surface, which is determined by the oxide content in the solution and the thickness of the oxide layer on the material surface. It can be seen from the results of material removal rate and ICP that the oxide content in the solution is relatively low and almost unchanged. Therefore,the rapid increase in corrosion voltage can be regarded as a massive accumulation of TiN surface oxide and the formation of TiN surface oxide layer. The Ecorrof TiN dipped in the solution with H2O2is higher than that of TiN dipped in the solution with NaClO,indicating that H2O2can form a thicker oxide layer on the surface of TiN.The results of the low corrosion currents under the conditions of the two oxidants indicate that the oxidation reaction process rather than the corrosion reaction process occurs on the TiN surface with the addition of oxidant.

Fig.6. Tests of potentiodynamic behavior about TiN film under different oxidants: (a) Potentiodynamic polarization curves in solutions with 0.3-wt%H2O2,0.5-wt%H2O2,and 0.3-wt%NaClO,0.5-wt%NaClO,1-wt%NaClO,respectively;(b)corrosion potential(Ecorr)behaviors of H2O2 and NaClO with different concentrations;(c)corrosion current density(Icorr)behavior of H2O2 and NaClO with different concentrations.
3.4. XPS measurements of TiN film
In order to explore and analyze the specific reaction mechanism during the chemical mechanical polishing,an XPS experiment is required. Before performing the XPS experiment, TiN needs immersing in the HF solution for 10 min to remove the naturally formed oxide layer on the surface. And then the TiN film should be immersed in the solution containing 1-wt% H2O2or 1-wt% NaClO for 10 min and perform XPS measurement. The XPS results are analyzed by referring to some detailed peak position information about TiN and its bond energy analysis results.[17–19]Figure 7(a)shows the XPS spectra of N 1s. The peak at 396.09 eV is N 1s of TiN and the peak at 398.584 eV is N 1s of NOx. No matter whether the oxidant is existent, there appears NOxon the surface of TiN,which means that NOxis easily formed. Figure 7(b) shows the XPS spectra of Ti 2p. The peaks at 461.2 eV, 455.8 eV,456.58 eV, 463.079 eV, and 457.731 eV are for Ti 2p1/2of TiN,Ti 2p3/2of TiN,Ti(NxO)of TiN,Ti 2p1/2of TiOx,and Ti 2p3/2of TiOx,respectively. In addition,XPS peak separation software is used to calculate the peak content value of various elements during peak separation. The results of the atomic content of N 1s are shown in Table 2. It can be seen that the atomic content of NOxin H2O2slurry is the highest,followed by the atomic content of NOxin NaClO,and the atomic content of NOxin the original film is the lowest. The calculation result of the atomic content of Ti 2p is also shown in Table 2.There is a very strong Ti(NxO)peak of Ti in the original film.The XPS results of Ti,obtained after being immersed in hydrogen peroxide and sodium hypochlorite solution,do not have Ti(NxO)peaks,but only TiOxpeaks. And the content of TiOxin H2O2is higher than that in NaClO,which means that a layer of Ti(NxO)is easily formed on the surface of TiN without oxidant. This layer is quite stable and will not be converted into TiOx. When the oxidant comes to contact TiN,the surface of TiN undergoes an oxidation reaction to form TiOx. And the thickness of the TiOxlayer under H2O2is higher than that of the TiOxlayer formed under NaClO.

Table 2. The variation of element peak content under different conditions.
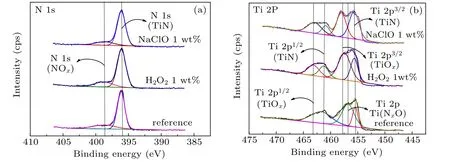
Fig.7. Dependency of XPS spectra of GSTC film surface on different oxidants in acidic slurry: (a)N 1s,and(b)Ti 2p.
3.5. Zeta potential of abrasive particle and TiN film
During the mechanical removal stage, the change of the Zeta potential on the solid surface and the Zeta potential on the abrasive surface play an important role.[20,21]The reason is as follows. On the one hand, the Zeta potential of the silica sol particles represents the stability of the silica sol. On the other hand,the relative value of the solid sample potential and the potential of the silica sol will affect the contact between them in the polishing process. Savaji et al.[22]studied the adsorption kinetics of particles and solid surfaces in detail. They pointed out in their article that the decisive effect on adsorption kinetics is the blocking function. The blocking function refers to the resistance of particles moving to a solid surface. It depends on the size of the solid surface potential, the particle radius, and the particle potential. Figure 8(a) shows the relationship between the Zeta potential of the silica surface and the oxidant concentration. Figure 8(b)shows the change of Zeta potential with the PH value for the original TiN film and oxide TiN film potential. For the solution with H2O2, the surface potential of silica sol rapidly decrease from ?20.58 mV at 0 wt%to ?34.98 mV at 0.5 wt%,and then slightly decreases from ?34.98 mV at 0.5 wt % to?39.67 mV at 2 wt%. For the solution with NaClO,the surface potential of silica sol rapidly increases from ?20.58 mV at 0 wt%to ?7.26 mV at 0.3 wt%,and then slightly increases from ?7.26 mV at 0.5 wt% to ?2.03 mV at 2 wt%. When dipped in the solution with the PH is in the range of 5 to 6,the Zeta potential of the original TiN film is in the range of?18.99 mV to ?39.72 mV and the Zeta potential of the oxidant TiN film is in the range of ?40.64 mV to ?69.02 mV,which means that the formation of an oxide layer in TiN reduces the potential of TiN film. According to the description of the blocking function,after adding H2O2the silica potential and TiN film potential decrease, thereby increasing the resistance of the contact between the silica particles and TiN film.On the contrary,after adding NaClO,silica potential increases and TiN film potential decreases slightly. Therefore, during the CMP of TiN film,the contact resistance when using H2O2as an oxidizing agent is greater than that when using NaClO as an oxidizing agent. This is the reason why the material removal rate turns higher during the CMP of TiN film with the NaClO used as an oxidant.
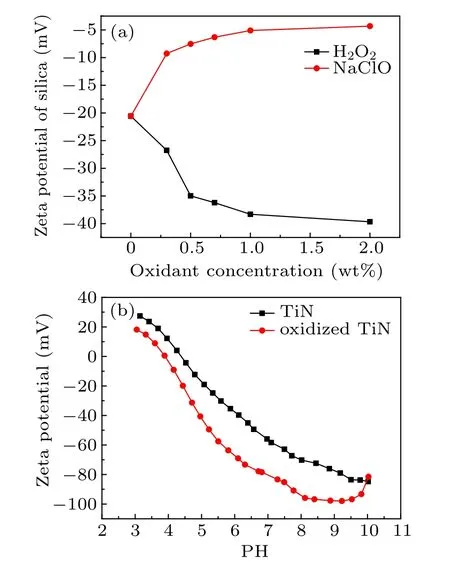
Fig.8. Zeta potential measurements of(a)silica in different oxidant concentration slurry,(b)difference between TiN film and oxidized TiN film.
4. Discussion
The above experimental results prove that when NaClO is used as the oxidizing agent for chemical mechanical polishing of TiN film, a higher material removal rate, higher selection ratio, and better surface quality can be obtained than when the H2O2is used as the oxidizing agent. It can be seen from the analysis of the potentiodynamic polarization curve and the XPS test that no matter whether H2O2or NaClO is added as oxidant,the polishing mechanism of TiN film is a cyclic reaction polishing mechanism,which means that the CMP process of the TiN film is as follows. First of all, an oxide layer is formed on the surface of TiN film as TiN film contacts the slurry. And then silica contacts the TiN film to remove this oxide layer. Finally a new TiN surface is exposed to the slurry and a new oxide layer is formed. Both the static corrosion rate and the results of ICP indicate that no matter whether H2O2or NaClO is used as an oxidant,the oxide layer formed on the surface of TiN film is stable and not easily dissolved.Based on the above analysis,when TiN film is exposed to the air,there could be a reaction on the surface of TiN film as follows:

When TiN is exposed to a slurry containing H2O2, the first reaction is
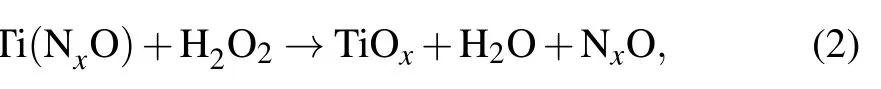
then the reaction is

Similarly,when TiN is exposed to a slurry containing Na-ClO,the first reaction is

then the reaction is
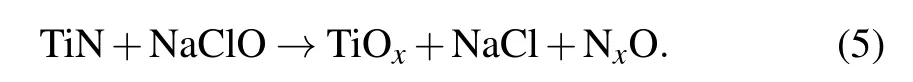
After adding the oxidant, there exists no Ti(NxO) on the surface of TIN film as indicated by Eqs.(2)and(4)that easily happen. It can follow from Figs.2 and 7 that H2O2can form a thicker oxide layer on the surface of TiN but obtain a lower material removal rate than NaCIO. This is because both the formation of oxide layer and the mechanical removal of oxide layer can affect the polishing rate in the process of mechanical cyclic reaction. Therefore, chemical–mechanical synergy is required to achieve the best results during chemical–mechanical polishing. During the mechanical action,the contact between the abrasive particles and the surface of the material to be polished plays a vital role. According to the description of the blocking function, the surface potential of silica particles and TiN film will change with the addition of oxidant,which determines the difficulty of contact between silica particles and TiN film. According to the results in Fig.8,the thicker the TiN surface oxide layer, the lower the surface potential is. Comparing with NaClO,after adding H2O2,the TiN surface oxide layer can be thickened and the surface potential of silica particles can be reduced,which greatly increases the contact resistance between the silica sol particles and the TiN surface. Therefore,a higher material removal rate can be acquired with adding the of NaClO as oxidant in the CMP process of TiN.In addition,the coordination beytween mechanical action and chemical action is also the reason why NaClO as an oxidizing agent can achieve better surface quality for TiN polishing.
5. Conclusions
Since TiN plays an important role in the barrier layer,adhesion layer and interconnection structure of the integrated circuit manufacturing process, we have made an in-depth study of the chemical–mechanical polishing of TiN.Based on experimental results and theoretical analysis,the conclusions can be acquired as follows.
(i)In the natural state,TiN is easily oxidized to form transition states Ti(NxO)and NxO.The Ti(NxO)is very stable in the absence of oxidant and will not be converted into TiOx.
(ii)Comparing with H2O2,the CMP of TiN with the addition of NaClO as oxidant can acquire a high material removal rate,material selectivity,and good surface quality.
(iii) The results of static corrosion rate and ICP demonstrate that TiN film and its oxides are insoluble in H2O2and NaClO solution.
(iv)The potentiodynamic polarization curve and XPS results indicate that the chemical–mechanical polishing process of TiN film with H2O2and NaClO as oxidant is a cyclic reaction polishing mechanism. In the polishing process, an oxide layer with high hardness and compactness can be formed on the TiN surface. All of these results indicate that the mechanical action between abrasive and TiN surface plays an important role in the TiN CMP process.According to the description of the blocking function,the addition of H2O2will greatly increase the contact resistance between the silica particles and the TiN surface. In the CMP process of TiN, the addition of NaClO can obtain a better chemical–mechanical synergy,which is the reason why the CMP of TiN with the addition of NaClO as oxidant can acquire a higher material removal rate,material selection ratio,and better surface quality.
Acknowledgement
We thank Shanghai Xinanna Electronic Technology Co.,Ltd for the help of providing the experimental platform and test platform for this research work.
- Chinese Physics B的其它文章
- Statistical potentials for 3D structure evaluation:From proteins to RNAs?
- Identification of denatured and normal biological tissues based on compressed sensing and refined composite multi-scale fuzzy entropy during high intensity focused ultrasound treatment?
- Folding nucleus and unfolding dynamics of protein 2GB1?
- Quantitative coherence analysis of dual phase grating x-ray interferometry with source grating?
- An electromagnetic view of relay time in propagation of neural signals?
- Negative photoconductivity in low-dimensional materials?

