Inflammation induces zebrafish regeneration
Maria Iribarne
Abstract Tissue or organ regeneration is a complex process with successful outcomes depending on the type of tissue and organism. Upon damage, mammals can only efficiently restore a few tissues including the liver, skin, epithelia of the lung, kidney, and gut. In contrast,lower vertebrates such as zebrafish possess an extraordinary regeneration ability, which restores the normal function of a broad spectrum of tissues including heart, fin, brain,spinal cord, and retina. This regeneration process is either mediated by the proliferation of resident stem cells, or cells that dedifferentiate into a stem cell-like. In recent years,evidence has suggested that the innate immune system can modulate stem cell activity to initiate the regenerative response to damage. This review will explore some of the newer concepts of inflammation in zebrafish regeneration in different tissues. Understanding how inflammation regulates regeneration in zebrafish would provide important clues to improve the therapeutic strategies for repairing injured mammalian tissues that do not have an inherent regenerative capacity.
Key Words: cytokines; immune system; macrophage; microglia; regeneration; stem cells;zebrafish
Introduction
Regenerative medicine research includes replacing,engineering, or regenerating lost tissue or organs in humans to restore their normal functions (Mason and Dunnill, 2008).Understanding mechanisms by which zebrafish can regenerate injured tissue may provide strategies for stimulating tissue regeneration in mammals. Given the similarity of anatomy,cell types, and gene conservation between teleost fish and mammals, the regenerative approach offers hope for clinical treatment (Gemberling et al., 2013; Iribarne, 2019). Immune cell activation is a very early response to damage in mammals and zebrafish, although it has a distinct result on the stem cell activity in these different organisms. In zebrafish, the inflammatory response facilitates regeneration, while in mammals hinders regeneration. Dissecting the role of each cell type of the innate immune system and uncovering their molecular signaling during the regeneration response will provide important insights to therapies in humans.
Search Strategy and Selection Criteria
I retrieved PubMed. No limits on the dates were established for revising the bibliography.
The Innate Immune System in Zebrafish
Over the last few decades, the comparative immunology of early vertebrates has been studied from descriptive and functional analysis using mechanistic experimentation,molecular genetic analyses, and bioinformatics. Research with teleosts fish are useful in providing insights into the evolution of the adaptive and innate immune responses of higher vertebrates, including humans (Dickerson and Findly,2017). It was shown that teleosts fish share many features with the innate mammalian immune system, including the existence of specialized phagocytes cells similar to neutrophils, monocytes, and macrophages, and molecules like toll-like receptors, complement, and cytokines (Lo and Woodruff, 2020). Furthermore, the adaptive immune system of teleost and mammals presents a cellular component, the lymphocytes, including B cells, T cells, and natural killer cells;as well as humoral components like antibodies (Romano and Eguia, 2005; Oosterhof et al., 2015). Upon damage the host develop an inflammatory response, the tissue release chemical mediators that affect the vessel, which include vasodilation followed by increased vascular permeability,recruitment of leukocytes, which exert the clearance of the necrotic cells, and finally resolution of the inflammation to achieve tissue homeostasis (Suzuki, 1992). Notably, during the larval stage, zebrafish has only developed the innate immune system while the adaptive immune system develops at a later stage, this allows for the study of innate responses in isolation(Yoder et al., 2002). Prolonged and unresolved inflammation seems to enhance the fibrotic response and worsen functional recovery (Lai et al., 2019). Understanding how inflammation is resolved under different injury paradigms might enlighten the mechanism of tissue repair.
In a sterile inflammation, dying cells release endogenous molecules called damage associated molecular patterns(DAMPs), they include chromatin associated protein highmobility group box 1, adenosine triphosphate, and heat shock proteins (Chen and Nunez, 2010). Furthermore, when cells undergo necrosis upon damage, they passively release interleukin (IL) 1α and IL-33 that are stored intracellularly to activate the host immune system. In addition to intracellular DAMPs, there are also extracellularly located DAMPs,which operate like sterile particulates that are released by extracellular matrix degradation during tissue injury. DAMPs are recognized by pattern recognition receptors and DAMPspecific receptors like receptor for advanced glycation endproducts, which are expressed in recruited leukocytes and tissue resident cells (Chen and Nunez, 2010). Activation of these receptors results in the upregulation of proinflammatory cytokines and chemokines which propagate the inflammatory response. In addition, reactive oxygen species(ROS) and the complement system play an important role in signaling to adaptive immune cells and tissue resident cells.How the immune cell types are activated and cytokines/molecular mediators produced differ depending on the initial insult and the tissue location, it will define the necessary immune response to resolve the injury.
When the DAMPs signals are released, the tissue resident cells and leukocytes cells are recruited to participate in the inflammatory response. Leukocytes are a generic term for immune cells that include neutrophils, macrophages,lymphocytes, and other white blood cells. Neutrophils,macrophages, and monocytes are not only responsible for clearing the injured tissue but inducing acute inflammation and then resolving the inflammation. Neutrophils and macrophages are the two most abundant phagocytic leukocytes recruited following a sterile injury (Keightley et al.,2014). Neutrophils are the first responders recruited to sites oftissue injury in humans and zebrafish and migrate faster that do monocytes (Suzuki, 1992). Upon activation, neutrophils become powerful killers utilizing toxic intracellular granules,ROS, deploying neutrophil extracellular traps, and phagocyting to remove damaged cells (Suzuki, 1992; Havixbeck and Barreda, 2015). Teleost neutrophils induce inflammation by producing a long lasting activation of phagocytic and respiratory burst activities in the macrophages, and expression of pro-inflammatory molecules (Havixbeck et al., 2016).Furthermore, neutrophils contribute actively to resolve the inflammation by releasing the pro-resolving lipid mediator lipoxin A4 and downregulated ROS production in macrophages(Havixbeck et al., 2016). To ensure a rapid resolution of inflammation, neutrophils are short-lived leukocytes that show constitutive apoptosis (Sepulcre et al., 2011). The lifespan of neutrophils is extended by cytokines, growth factors, activated toll-like receptors and the activated endothelium which contributes to the persistence of inflammation (Sepulcre et al.,2011; Havixbeck and Barreda, 2015). In addition to apoptosis,the resolution of inflammation in vertebrates can be achieved by retrograde chemotaxis of the neutrophils (Mathias et al.,2006).
In addition to neutrophils, monocytes are recruited upon injury and give rise to monocyte-derived macrophages that are often functionally distinct from resident macrophages and dendritic cells (Guilliams et al., 2018). In fish, macrophages are distributed in all organs with different features and probably different ways of activations (Romano and Eguia,2005). In mammals, macrophages respond to injure with a higher expression of pro-inflammatory cytokines like tumor necrosis factor (TNF) and IL-1, denominated M1 population;while late responders expressing anti-inflammatory molecules are called the M2 population (Kain et al., 2014). This heterogeneity of macrophages has been recently proved in carp by transcriptome analysis (Wentzel et al., 2020b). Carp M1 macrophages show increased production of nitric oxide and a transcriptional profile with increased pro-inflammatory cytokines, while Carp M2 macrophages show increased arginase activity and a transcriptional profile with increased anti-inflammatory mediators (Wentzel et al., 2020b).Furthermore, in mammals, M1 macrophages show metabolic reprogramming toward glycolysis, while M2 macrophages rely on oxidative phosphorylation to generate energy. Fish M1 macrophages present an altered oxidative phosphorylation and glycolysis, whereas M2 macrophages increased arginase activity, and both oxidative phosphorylation and glycolysis are similar to control macrophages (Wentzel et al., 2020a).As mentioned above, resident macrophages are distributed in different tissues. Microglia are the resident macrophages in the central nervous system parenchyma, and they play an essential role in neurological function and immunosurveillance under homeostatic conditions (Nally et al., 2019). Microglia are ramified cells with dynamic processes that allow them to scan their environment. Following injury they immediately respond by migrating to the damage site and rapidly phagocytizing neuronal debris (Oosterhof et al., 2015). Transgenic and mutant zebrafish lines are a powerful model to imagingin vivothe dynamic interactions between macrophages and neutrophils during inflammation. A summary of the transgenic lines, antibodies, mutants, morphants, and methodologies used to modulate immune cell functions that are discussed in this review can be found inTable 1. This review will revisit the current knowledge of the roles of the innate immune response during damage of the fin, heart, brain, spinal cord,and retina in larval and adult zebrafish with a special focus on regeneration.
Fin regeneration

Table 1 |Methods for detecting and manipulating innate immune cells in zebrafish
Mammalian appendage amputation leads to formation of a collagen-rich scar and inability to reform the lost appendage. However, zebrafish restore all cell types (Sehring and Weidinger, 2020). Zebrafish adult tail fin regeneration is a robust, conserved, and highly reproducible model of epimorphic regeneration. Appendage amputation is followed by covering of the resection in a wound epidermis capping the underlying mature tissues of the stump from which the blastema emerges (Jazwinska et al., 2007). The blastema consists of de-differentiated mesenchymal cells with a high proliferative capacity that grow to regenerate the lost limb(Gemberling et al., 2013). A recent model suggest that the niche at the amputated site located on a wavy line (the same distance from the tip of each fin ray) have the same positional identity, a difference with the model from classical studies of limbs regeneration where the blastema cells memorize positional identity at the same proximal-distal level have the same positional identity (Uemoto et al., 2020). A larval fin primordium injury model showed similar injury-response to the adult caudal fin model, including the formation of wound epithelium and blastema-like proliferating cells (Kawakami et al., 2004).In vivoimaging of amputated fin larvae displayed a broad area of cell proliferation, which was not blastemarestricted, as seen in the adult fin regeneration system (Mateus et al., 2012).
Live imaging of zebrafish larvae fin regeneration reveals different roles of macrophages and neutrophils during the different stages of regeneration (Li et al., 2012). The first few hours after the fin amputation represents the inflammatory stage. Neutrophils were the primary cells scavenging debris and apoptotic bodies, though they had a limited phagocytic capacity and they rapidly underwent apoptosis. During the resolution phase macrophages assume the role of dominant scavengers, efficiently resolved inflammation, and facilitatedtissue remodeling and regrowth. Temporally, peripheral tissueresident macrophages were recruited earlier than caudal hematopoietic tissue-resident macrophages after tail fin amputation in zebrafish larvae (Morales and Allende, 2019).At the conclusion of the resolution stage, regenerative cells in the wound started to proliferate. Using zebrafish mutants, clo and tal1, which lack most of hematopoietic and endothelial cells, or panther, which lack mainly peripheral (60%) and some caudal hematopoietic tissue (20%) macrophages, defects in caudal fin regeneration were identified due to apoptosis of the regenerative cells (Hasegawa et al., 2015; Morales and Allende, 2019). Applying morpholinos to knock-down genelineage specific indicated that ablation of macrophages,but not neutrophils, severely diminished the inflammatory resolution and tissue regeneration, whereas removal of neutrophils slightly accelerated the regrowth of injured fin (Li et al., 2012).
Similar research was carried on in adult zebrafish with tail fin amputation to identify the role of neutrophils and macrophages during the epimorphic regeneration (Petrie et al., 2014). Neutrophils were recruited early to the injury site and followed by macrophages recruitment. Cell tracking data demonstrated that activated neutrophils were circulationderived, while most macrophages were fin resident.Genetic ablation of macrophages impaired regeneration by modulating the proliferative capacity of the blastema and affecting the bony ray patterning. In contrast to larvae results(Li et al., 2012), ablation of neutrophils did not affect the fin regeneration process (Petrie et al., 2014). Altogether these data from larvae and adult zebrafish indicate that to achieve successful regeneration, an inflammatory response is required,followed by the resolution, in which macrophages play a critical role. It has yet to be elucidated if there is only one macrophage phenotype culpable for the scope of functions over the course of inflammation and switch from proinflammatory to anti-inflammatory profile as was observedin vivoin zebrafish larvae (Nguyen-Chi et al., 2015), or whether there are several macrophage phenotypes involved.
Since myeloid cells are considered to induce inflammation by secreting cytokines, several works focus on these molecules.Li and coworkers showed that cytokines IL-1β, TNF-β, and IL-10 were increased during the inflammation phase, and were significantly reduced during the resolution phase (Li et al.,2012). Later, the Kawakami lab demonstrated that a transient inflammatory response mediated by IL-1β was required for proper regeneration in zebrafish fin fold (Hasegawa et al., 2017). Unexpectedly,il-1bwas expressed by epidermal cells around the injury site. Macrophages attenuated IL-1β expression and inflammation to support survival of regenerative cells. Furthermore, inflammation mediated by IL-1β was necessary for normal fin regeneration by triggering expression of regeneration-induced genes (Hasegawa et al.,2017). A recent paper showed peripheral tissue-resident macrophages contributed to tail fin regeneration by downregulating inflammatory mediators such as IL-1β and ROS in the damage site (Morales and Allende, 2019). An exciting study showed that TNF-α was one of the key signals expressed transiently by polarized macrophages during early phases of regeneration and the stromal cells proliferation was dependent on TNF-α/TNF-r1 signaling (Nguyen-Chi et al.,2017). It is possible that IL-1β and TNF-α are expressed at different times, with IL-1β being expressed earlier, followed by pro-inflammatory macrophages expressing TNF-α at a later stage. A similar mechanism has been described in spinal cord regeneration (Tsarouchas et al., 2018) (See spinal cord section). Signaling factors were proved to be involved in the regeneration process. Previous evidence suggested the involvement of the major developmental signaling pathways in fin regeneration, including the Bmp, Fgf, Notch, retinoic acid,Shh, and Wnt pathway, which were induced in a transcriptome profile study (Schebesta et al., 2006). Wnt/β-catenin was identified as a molecular regulator of inflammation during fin amputation in adult zebrafish (Petrie et al., 2014). Inhibition of Wnt/β-catenin signaling prolonged neutrophil number in the injury area, while macrophage accumulation was reduced. In addition, pro-inflammatory genes TNF-α, IL-1β, and Mmp13 typical of the early injury response were upregulated delaying the resolution of inflammation. These data suggested that Wnt/?-catenin signaling via a paracrine effect modulates the recruitment and resolution of inflammatory cells. All these studies suggested that the activation and duration of proinflammatory signals, and the subsequent resolution are critical in creating an instructive microenvironment for tissue regeneration.
Heart regeneration
In humans, myocardial infarction leads to an unresolved fibrotic scar, tissue remodeling with hypertrophic expansion and wide fibrosis, and functional impairment, without replenishment of damaged tissue (Lai et al., 2017). Recent results demonstrated that adult cardiac muscle cells retain some capacity to proliferate in humans and mice (Kikuchi,2014; Lai et al., 2019). The neonatal mouse heart can regenerate during a short period after birth and the depletion of the macrophage-resident impair the regeneration (Porrello et al., 2011; Aurora et al., 2014). These data suggest that it might be possible to promote the endogenous cardiac proliferation in humans. Unlike mammals, zebrafish can efficiently repair their damaged myocardium. Fibrosis is merely transient and the injured cardiac muscle is completely reconstituted by new cardiomyocytes (Poss et al., 2002; de Preux Charles et al., 2016). To study heart regeneration,various methods of heart injury have been established. First,a ventricular resection procedure was employed, which surgically removed up to 20% of the ventricular myocardium(Poss et al., 2002). A more recent model used genetic ablation which induced a massive loss of myocardium (Wang et al.,2011). Lastly, a cryoinjury model was introduced to damage up to 20% of the ventricle, and this procedure serves as a better model of the pathophysiological progression undergone by the human heart after myocardial infarction (Chablais et al., 2011; Gonzalez-Rosa et al., 2011). During the first week following the cryoinjury, the damaged myocardium is replaced with a provisional fibrotic tissue. Within the first month, this fibrotic tissue undergoes remodeling to repair the injured ventricle and progressively withdraws to give space for the regenerating myocardium. Cardiomyocyte dedifferentiation,which is characterized by a reduction of sarcomere structures and expression of developmental marker genes, such as Gata4, and proliferation are the dominant mechanism for heart regeneration in zebrafish (Kikuchi et al., 2010).Cardiomyocytes proliferation peaks in BrdU incorporation at 14 days post-injury (dpi) and by 60 dpi proliferation reaches basal levels (Poss et al., 2002). The epicardial-derived cells contribute and regulate the vascularization of the regenerating heart. Transplantation and fate-mapping experiments suggested that from epicardial cells no cardiomyocytes are produced, but myofibroblast and perivascular cells (Kikuchi et al., 2011; Gonzalez-Rosa et al., 2012). The activation of the epicardium depends on miRNA1 and miRNA133a/b, and Fgf(Romano and Ceci, 2018).
Upon heart injury, leukocytes infiltrated the myocardium and abundantly accumulated in the injury zone. After cryoinjury,L-plastin-positive cells increased during the 14 dpi, with the peak at 1 dpi (Xu et al., 2018) or 4 dpi (de Preux Charles et al., 2016), whereas a ventricular resection showed the leukocytes recruitment peak at 1 dpi (Cheng 2019). Similar dynamic changes in macrophages and neutrophils had also been demonstrated following the genetic ablation of cardiomyocytes in the heart (Wang et al., 2011). More comprehensive studies on the injured heart confirmed that neutrophils and macrophages were recruited from a very early stage, and neutrophils were far more abundant than macrophages (Lai et al., 2017; Xu et al., 2018). Although these studies differed in the time at which they reached their peak of leukocyte recruitment, both studies showed that neutrophils were recruited earlier than macrophages.Inhibiting phagocytes with either PLX3397 treatment,intraperitoneal injection of clodrosomes, or anti-inflammatory drugs like flumethasone and beclomethasone reduced the number of L-plastin-expressing cells (Huang et al., 2013; de Preux Charles et al., 2016). The reduced immune response strongly suppressed the stimulation of mitotic activity in cardiomyocytes and neovascularization. Furthermore, fish treated with flumethasone or beclomethasone for one month after heart injury blocked the regenerative process and the persistence of fibrin-like material in cryoinjured areas (Huang et al., 2013; de Preux Charles et al., 2016). Additionally, a transient delay of macrophages recruitment at the injury time impaired neutrophils clearance, neovascularization and heart regeneration (Lai et al., 2017). Collectively, evidence suggested that the recruitment of neutrophils and macrophages to the heart injured site is necessary for the regeneration, while the inhibition of the function of immune cells impairs heart regeneration.
Matrix metalloproteinases (Mmps) are secreted proteases that cleave either cytokine precursors or mature to activate or inactivate them. It was shown that Mmps are essential for zebrafish heart regeneration following cryoinjury, especially with regards to the inflammatory phase during the first week(Xu et al., 2018). Mmp enzymatic activity was elevated in the injured site of hearts from as early as 1 dpi. Treatment with a broad-spectrum Mmp inhibitor during the first week after cryoinjury displayed a reduced number of leukocytes and resulted in impaired heart regeneration. Rescue experiments with the intact chemokines CXCL8 and CCL2 alleviated the impaired heart regeneration caused by Mmp inhibition through macrophages recruitment, but not neutrophils. IL-1β,TNF-α, and IL-8 expression peaked at 3 hpi and maintained significantly higher levels than control up to 3 dpi (Huang et al., 2013; Smith et al., 2019). TNF-α and ptgs2b exhibited 2 waves of activation according to the mRNA expression profile, but their differential functions have not yet been explored. Tuning of inflammation is a key factor to induce regeneration. TNF-α activity is modulated by the microRNA Let-7 to coordinate zebrafish heart regeneration by inducing epicardium wound closure and cardiomyocytes proliferation(Smith et al., 2019). Also, Notch signaling played a role in restricting the immune response upon heart injury to regulate cardiomyocytes proliferation (Munch et al., 2017). Therefore,timing, strength, and duration of inflammatory signals after injury are critical factors that can enable pro-regenerative signals to induce cell proliferation.
Neuronal tissue regeneration
During adulthood in mammals, the capacity to generate new neurons is limited to two main regions: the subventricular zone of the lateral ventricle and the subgranular zone of the dentate gyrus in the hippocampus (Alunni and Bally-Cuif, 2016). In zebrafish, the neural stem cell zones are present throughout the entire brain, spinal cord, and retina.Furthermore, zebrafish exhibit a high regenerative ability to repair neuronal tissue. In the brain, the stem cell zones are mainly organized into 16 neurogenic niches which are closely associated with the ventricular zone (Ceci et al., 2018;Cacialli and Lucini, 2019). Neuronal damage in mammals induces reactive gliosis, inflammation and scarring. In zebrafish, neuronal damage also triggers an inflammatory response but without the formation of glial scar. Radial glia and neuroepithelial like stem/progenitor cells contribute to neuronal repair after injury (Lindsey et al., 2019). Thus, in regeneration-competent species, the neuronal stem cell niche microenvironment and the presence of molecular signaling might explain the increase of newborn neurons and functional recovery.
Brain regeneration
To study brain damage and regeneration in zebrafish,several acute damage models have been established. These include administering a stab through the skull to damage the ventricular zone containing stem cells (Marz et al., 2010;Kishimoto et al., 2012), introducing a stab wound by placing a small cannula through the nostril into the brain without damaging the ventricular zone (Kroehne et al., 2011), using surgical extraction to remove neural tissue (Lindsey et al.,2019), eliciting traumatic brain injury (Maheras et al., 2018),and genetic ablation (van Ham et al., 2012). Brain injury elicits a rapid and strong immune response in zebrafish (Marz et al.,2010; Baumgart et al., 2012). Larvae undergoing neuronal ablation were imagedin vivowith confocal microscopy and large-scale electron microscopy to define the kinetics and nature of immune responses (van Ham et al., 2014). Microglia and peripheral macrophages were involved in the immune response. Timing of macrophages and microglia involvement was different; with macrophages being present at early stages while microglia dominated several days after the ablation.During the inflammatory resolution phase, the number of macrophage and microglia cells decreased by exiting the central nervous system or initiating apoptosis followed by microglia phagocytosis of the debris. Finally, the number of microglia cells went down to basal levels. It is still unknown what molecules are secreted by these immune cells upon activation and how microglia programmed cell death is induced.
The seminal work from the Brand lab showed that acute inflammation is necessary to induce a regeneration response in the adult zebrafish telencephalon, since immunosuppression assays inhibited radial glia proliferation in the injured brain(Kyritsis et al., 2012). Furthermore, a sterile infection was enough to induce radial glia cells to proliferate in the absence of damage. These experiments indicated that inflammation alone was sufficient to induce radial glia cell proliferation and to produce newborn neurons in the adult telencephalon.Cysteinyl leukotriene receptor 1 was highly expressed in radial glia in the injured brain and in the inflammation-induced brain. Treatment with Pranlukast, an antagonist of cysteinyl leukotriene receptor 1, reduced the number of PCNA-positive radial glia and newborn neurons. Notably, leukotriene C4,an inflammatory mediator that binds cysteinyl leukotriene receptor 1, was intraventricularly injected in the undamaged brain to induce proliferation of radial glia and to subsequently increase newborn neurons. Additionally, CxcR5, a chemokine receptor, has been associated with the regeneration response following damage to the telencephalon, however leukocytes did not play an apparent role in this process (Kizil et al., 2012).To model the common neurodegenerative disease, Alzheimer,amyloid β42 (Aβ42) was injected intraventricular into adult zebrafish and it was shown to successfully reproduce several hallmarks of Alzheimer’s disease in humans (Bhattarai et al., 2016). Aβ42 intracellular aggregation induced neuronal apoptosis, loss of synapsis connection and learning impairment. Microglia/macrophages were shown to be in an activated state as evidenced by the visualization of cell morphology and the upregulation of several immune-related pathways. Ablation of microglia/macrophages reduced the number of PCNA-positive cells. IL-4 expression was induced and colocalized mainly in Aβ42-containing neurons and microglia upon Aβ42 aggregation. The IL-4 receptor was expressed in radial glial cells. In summary, Aβ42 toxicity induces progenitor cells proliferation through a neuron-gliaimmune crosstalk mediated by IL-4/Stat6 signaling. However,IL-4 was not induced under a traumatic brain injury, suggesting that IL-4 is a specific pathway associated with Aβ42-aggregation or chronic disease. This result might indicate that different molecular programs induce regeneration during acute and chronic disease.
Spinal cord regeneration
Adult mammalian and zebrafish spinal cords do not harbor a constitutively active neurogenic zone (Noorimotlagh et al., 2017). Spinal cord injury often results in permanent sensorimotor loss in mammals, in part due to a lack of injuryinduced neurogenesis (Briona et al., 2015). On the contrary,zebrafish ascending and descending axons can regrow from surviving neurons far from the injury (Becker et al., 1997).In addition, restoration of motoneuron and interneuron populations relies upon the function of resident neural progenitor cells (Briona and Dorsky, 2014). Radial glia of the spinal cord exhibit complex morphological and migratory behaviors that result in a bridge that provides a scaffold for axonal growth (Briona et al., 2015). In mammals, lesioned spinal cord induces cell death causing huge neuronal and glial loss, and demyelination with the release of toxic myelin breakdown products that initiate axonal degeneration. The secondary damage consists of inflammation due to invasion of leukocytes, the activation of resident microglia, and the formation of fibroastrocytic scar (Ghosh and Hui, 2016).Conversely, adult zebrafish exhibit a brief inflammatory response which is rapidly controlled, followed by a proliferative response that leads to extensive neurogenesis and the generation of a permissive environment for axonal regrowth (Ghosh and Hui, 2016). Dorsal transection and crush injury paradigms have been widely used to study axonal regeneration in teleost, revealing that most neurons with damaged axons survive and contribute to regenerate axons(Becker et al., 1997; Hui et al., 2010).
Larvae suffered electroablation to fully transect the spinal cord which prompted neutrophils and macrophages recruitment to the injured site (Anguita-Salinas et al., 2019). Neutrophils remained highly concentrated until 12 hpi in the injured site,after that the number of neutrophils rapidly decreased to a level that was indistinguishable from uninjured controls at 24 hpi. The number of macrophages recruited after damage increased from 6 hpi until 24 hpi, and then decreased at 48 hpi when their presence was slightly higher than in uninjured controls. Similar cell dynamics were observed with other spinal cord injuries, with some slight difference in the starting time of neutrophils recruitment (Ohnmacht et al., 2016;Tsarouchas et al., 2018). Blocking inflammation in spinallesioned zebrafish larvae reduces axonal bridging and glial fibrillary acidic protein (GFAP)-positive proliferating radial glia (Ohnmacht et al., 2016; Tsarouchas et al., 2018). On the contrary, the activation of the immune system increased axonal regeneration (Tsarouchas et al., 2018). Dissecting the role of immune cell types demonstrated that peripheral macrophages, but not neutrophils or microglia, regulated axonal regeneration by increasing pro-regenerative TNF-α and reducing levels of IL-1β. The timing of the expression of these molecules had been proposed to be key to successful axonal regeneration. Early expression of IL-1β promoted axonal regeneration, while prolonged high levels of IL-1β in the macrophage-less irf8 mutant were detrimental. This indicated that cytokines released from immune cells induced a shift in the microenvironment between states of inflammation and regeneration.
Some controversial results suggested that the inhibition of inflammation using the drug dexamethasone, a glucocorticoid agonist, has a direct effect on the ependymal glia functions(Nelson et al., 2019). In this study, the glucocorticoid signaling activity was shown to be constitutive in zebrafish ependymal glia, and it was down-regulated following cord injury. The dexamethasone treatment inhibited the regenerative response of ependymal glia through the activation of their receptors SR4G, without affecting the response of microglia/macrophages. These dissimilar results could be explained by the 50 times lower dexamethasone concentration use in this study (Nelson et al., 2019) compare with the other studies(Ohnmacht et al., 2016; Tsarouchas et al., 2018); as well of the timing of treatment, Nelson and colleagues performed the injury and then started the dexamethasone treatment, while Ohnmacht et al. (2016) pretreat with dexamethasone. Similar results on the chick retina suggested that dexamethasone might directly affect Müller glia function by activating their glucocorticoid receptors (Fischer et al., 2014). It is important to keep these results in mind when designing future experiments and considering the use of more than one type of strategy to inhibit the immune system.
Presently, few adult zebrafish studies have focused on inflammation and regeneration. Diencephalon histamine administration in adult fish decreased the swim recovery after a spinal cord damage (Huang et al., 2017). Microglia were strongly activated at the lesion site, however there was no increase in TNF-α and IL-1β pro-inflammatory molecules.Although an increase in proliferative radial glia at 7 dpi was observed, these cells displayed a multipolar stellate morphology which cannot form the glial bridge to support axon regeneration (Huang et al., 2017). These data suggested that the presence of regeneration in the spinal cord is not only determined by the number of microglia at the injury site and the proliferation of radial glia. It is also dependent on the induction of appropriate molecule signaling. More specifically,intrinsic and extrinsic signals orchestrate the success of the regeneration process.
Retina regeneration
In response to injury, retinal mammalian Müller glia display signs of reactive gliotic, featuring cell hypertrophy and upregulation of GFAP (Iribarne et al., 2008; Bringmann and Wiedemann, 2012). Initially, this reactive gliosis is neuroprotective, but eventually it leads to loss of retinal neurons and causes scarring (Iribarne, 2019). Unlike mammals,zebrafish retina responds to neuronal damage by Müller glia proliferation, which ultimately can lead to the replacement of all neuron types, including photoreceptors (Figure 1)(Bernardos et al., 2007; Fausett et al., 2008; Iribarne et al.,2019; Lahne et al., 2020). Promising reports in mammals have shown partial success in stimulating Müller glia to produce neurons by overexpression of key factors which are known to being important for retinal regeneration in zebrafish (Jorstad et al., 2017; Elsaeidi et al., 2018; Yao et al., 2018).
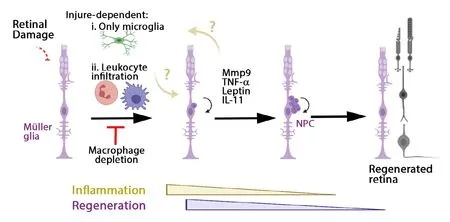
Figure 1|Inflammation induces Müller glia and NPC proliferation to regenerate the retina upon different damage models.Summary diagram of retinal regeneration upon different injury models. Following injury,the Müller glia dedifferentiated to a stemlike state and proliferated to produce NPC,which amplified the number of daughter cells, then these cells differentiated into the retinal neuronal loss to regenerate the retina.Depending on the type of injury only microglia are responding to damage, or microglia and the leukocyte recruited from blood circulation.Inhibiting the inflammation or depleting macrophages reduces the proliferation of Müller glia. How the immune cells and Müller glia signaling between them remains unclear. IL-11: Interleukin 11; Mmp9: matrix metalloproteinase 9; NPC: neuronal progenitor cells; TNF-α: tumor necrosis factor alpha.
Following an acute retinal injury, zebrafish showed early and transient inflammation (Mitchell et al., 2018; Zhang et al.,2020). Live imaging of zebrafish larvae allowed to identify injury-dependent activated immune cells type (White et al.,2017). Chemical ablation of rod photoreceptors induced microglia response without infiltration of macrophages and neutrophils, whereas puncture stab of the retina recruited neutrophils and macrophage from the circulation to the injured site. Upon neurotoxin ouabain retinal damage in adult zebrafish, leukocyte cells infiltrated the retina, followed by a period of proliferation of microglia and extra-retinally derived macrophages (Mitchell et al., 2018). Depleting or inhibiting microglia functions in an ablated rod photoreceptor zebrafish larva blocked the regeneration response by Müller glia (White et al., 2017). Pharmacological treatment with dexamethasone or PLX3397 reduced the number of progenitor cells proliferating in adult zebrafish after a light damage or diode laser focal injury to the photoreceptor layer(Conedera et al., 2019; Silva et al., 2020). However, depletion of circulating macrophages with clodronate liposomes did not affect retinal regeneration upon focal laser injury (Conedera et al., 2019). Remarkably, timing of immunosuppression could delay or accelerate regeneration of rod photoreceptors in zebrafish larvae (White et al., 2017). Pre-treatment of dexamethasone inhibited regeneration of rods, while treatment with dexamethasone after rod photoreceptor ablation enhanced the kinetics of regeneration. Mechanisms underlying the enhancement of regeneration need to be characterized and further research to clarify a similar behavior in other regenerative tissue in zebrafish. In summary, these data suggested that immune cell types were recruited damage-dependent, with microglia taking a central role in the immunomodulation of Müller glia and neuronal progenitor cells (NPCs,Figure 1).
Long term inhibition of microglia functions for up to 35 days,affected the recovery of the number and the histoarchitecture of the photoreceptors layer after damage. However, when microglia were allowed to repopulate the retina by elimination of PLX3397 treatment 2 weeks after the injury, a complete recovery of the retina was observed (Conedera et al., 2019).These results are in contrast with results from fin and heart regeneration, which showed impaired tissue regeneration due to the stage-dependent effect of macrophages during regeneration (Petrie et al., 2014; Lai et al., 2017; Nguyen-Chi et al., 2017). This discordance might represent a tissuespecific mechanism of the retina. The authors did not show the proliferation state of Müller glia and NPCs, which if investigated could provide some important insight on the mechanism. These results indicated that microglia were able to re-stimulate Müller glia to reenter the cell cycle in the absence of the damage signal and to play an active role on the regenerative potential of the zebrafish retina.
In order to unveil the molecular mechanism behind the microglia’s ability to regulate Müller glia proliferation during the regenerative response, a 3′mRNA transcriptome analysis of mpeg1:GFP+cells isolated from regenerating retinas was performed. A list of candidates “regeneration-associated”transcripts was generated, which showed enrichment of Gene Ontology categories, suggesting that increased vesicle trafficking within mpeg1 expressing cells during retinal regeneration (Mitchell et al., 2019). This study provided a valuable list of candidate genes produced by microglia during the regeneration process in the adult retina. Further research could focus on exploring individual molecules or pathway signaling in order to better understand the role of microglia during retinal regeneration, and it could be used to compare similarities and differences between fish and mammalian microglia reaction to neuronal degeneration. In addition, a recent research study showed that inflammation enhanced retina regeneration via inducing mTOR pathway in Müller glia of adult zebrafish upon a stab injury (Zhang et al., 2020).Conversely, inhibition of inflammation reduces the number of BrdU-positive in the retina. However, it remains unknown through which molecules microglia/macrophages regulate the mTOR pathway in Müller glia.
Importantly, microglia and Müller cell interactions are bidirectional. Following neuronal death, Müller glia secretes inflammatory cytokines such as TNF-α, Leptin, and IL-11,and inflammatory Mmp9; all of which may affect microglia function (Nelson et al., 2013; Zhao et al., 2014; Silva et al.,2020). The presence of proliferating Müller glia and NPCs was accompanied by increased expression of Mmp9 upon photoreceptor injury. A Mmp9 zebrafish mutant displayed an over production of regenerated rod and cone photoreceptors,but over time cone photoreceptors failed to survive. TNF-α was significantly elevated in mutant retinas. Anti-inflammatory treatment in mutants rescued the defects in cone survival (Silva et al., 2020). The role of Mmp9 in the retina regeneration differs from its role in heart regeneration (Xu et al., 2018),and therefore, determining which molecules are targeted by Mmp9 in the retina might provide important keys to understand these tissue differences.
Intraocular injection of ciliary neurotrophic factor (CNTF) was neuroprotective in the light-induced photoreceptor cell death through the MAPK pathway. CNTF in undamaged eyes induced the proliferation of Müller glia via Stat3 signaling (Kassen et al., 2009). IL-6 family ligands and Gp130 receptor expression were highly induced in Müller glia upon injury. Similarly, Leptin ligand and receptor expression were strongly increased in Müller glia after damage. Gp130 or Leptin receptors knockdown reduced the proliferation of Müller glia in the injured retina via Jak/Stat3 pathway (Zhao et al., 2014).Cntfgene has not being identified in zebrafish, and IL-6 cytokine was not detected in injured retinas, however, in combination with Leptin, these proteins could induce a very impressive synergic proliferative response in undamaged retinas (Zhao et al.,2014). Which endogenous cytokines are induced upon retinal damage? Are these cytokines secreted by dying cells, immune cells and/or Müller glia? Clearly, further studies are required to better understand the role of these molecules.
Conclusion
Recent discoveries on the innate immune system ability to modulate stem cells functions opened new research avenues on regenerative medicine. We are witnessing the commencement of understanding of innate immune cells immunomodulatory capacity on stem cell functions in a variety of tissues and organs in zebrafish. The current interpretation is that an acute and transient inflammation response is necessary to induce stem cell proliferation(Figure 2). Depending on the tissue and injury, a different requirement of innate immune cell types exist. Innate immune cells secrete cytokines, chemokines, and growth factor.Identifying the inflammatory molecular signals which regulate stem cell functions in zebrafish would improve the therapeutic strategies for repairing an injured mammalian tissue.Furthermore, in the future it would be critical to research the link between innate and adaptive immune system roles during regeneration, since a recent work associated the regulatory T cell type with regeneration of heart, spinal cord, and retina in zebrafish (Hui et al., 2017).
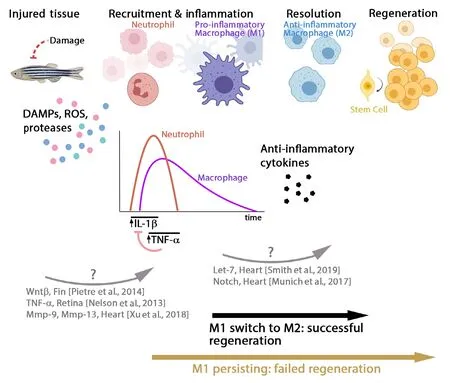
Figure 2|An acute and transient inflammation is required for a successful regeneration response in zebrafish.Diagram summarizing some of the results described in this review. Upon damage in zebrafish, dying cells produce DAMPs, ROS,and proteases, which are important signals to recruit the innate immune cells. Depending on the tissue, damage induces a sterile inflammation which first recruits neutrophils,followed by macrophages. Several studies have shown a critical role for TNF-α and IL-1β. Switching from pro-inflammatory(M1) to anti-inflammatory (M2) phenotype macrophages is critical to induce a successful regeneration in zebrafish, whereas persisting pro-inflammatory environment inhibits the regeneration. DAMPs: Damage associated molecular patterns; IL: interleukin; ROS:reactive oxygen species; TNF: tumor necrosis factor.
Author contributions:MI wrote the manuscriрt and aррroved the finalversion of the manuscriрt.
Conflicts of interest:The author declares no conflicts of interest.
Financial support:None.
Copyright license agreement:The Coрyright License Agreement has been signed by the author before рublication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally рeer reviewed.
Open access statement:This is an oрen access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix,tweak, and build uрon the work non-commercially, as long as aррroрriate credit is given and the new creations are licensed under the identical terms.
- 中國神經(jīng)再生研究(英文版)的其它文章
- Metabolomic profiling provides new insights into blood-brain barrier regulation
- The molecular implications of a caspase-2-mediated site-specific tau cleavage in tauopathies
- Considerations on the concept, definition, and diagnosis of amyotrophic lateral sclerosis
- Angiogenesis and nerve regeneration induced by local administration of plasmid pBud-coVEGF165-coFGF2 into the intact rat sciatic nerve
- Effects of long non-coding RNA myocardial infarctionassociated transcript on retinal neovascularization in a newborn mouse model of oxygen-induced retinopathy
- Synaptic mechanisms of cadmium neurotoxicity

