Efficient decomposition of sulfamethoxazole in a novel neutral Fered-Fenton like/oxalate system based on effective heterogeneous-homogeneous iron cycle
Chen Wng,Yubei Liu,To Zhou,Mingjie Hung,Jun Mo,b,Xiohui Wu,b,*
a School of Environmental Science and Engineering, Huazhong University of Science and Technology, Wuhan 430074, China
b Key Laboratory of Water and Wastewater Treatment (HUST), MOHURD, Wuhan 430074, China
Keywords:
Fered-fenton like
Heterogeneous iron cycle
Iron-oxalate complexes
Surface electron transfer
Persulfate
ABSTRACT
In this study, efficient sulfamethoxazole (SMX)degradation was demonstrated in a novel neutral Fered-Fenton like/oxalate (electro-Fe2+/PDS/Ox, Fered-FL/Ox) system adopting pre-anodized Ti@TiO2 cathode.Optimization of operational parameters was conducted and the whole reaction mechanism based on the critical solid-liquid interfacial reactions was explored.An efficient neutral heterogeneous-homogenousiron cycle would exist in the Fered-FL/Ox system,depending on the formation of specific C--O--Tibonds through the inner sphere surface complex(ISSC)of Fe(C2O4)33-.It would induce ultrafast electron transfer from the cathode to the FeIII core, effectively accelerating the neutral Fenton-like reactions and complete mineralization of SMX with relative low dosage of ferrous catalyst and applied voltage.The result of this study is expected to supply a good alternative in treating complex neutral industrial wastewaters
As one of the widely used antibiotics,sulfamethoxazole(SMX)has received increasing attentions on frequent occurrence and high persistence in the aquatic environment [1].Fenton oxidation process, is a classic and one of the most efficient advanced oxidation processes (AOPs) in treating hazardous and/or hardlydegradable pharmaceuticals and personal care products(PPCPs)as reported in the recent years [2,3].Despite that most Fenton technologies can produce·OH of high oxidation potential (2.8 eV)for rapid and non-selective oxidation of the target recalcitrant organic pollutants, challenges such as acidic reaction circumstances, overload of Fe2+and low utilization of H2O2limit their applications in real wastewater treatment [4,5].
Electro-Fenton(EF)process is a good alternative Fenton process in which H2O2is generated electrolytically via two electron cathodic reduction of oxygen in acidic medium [6].In the past decades, a mass of researches have focus on how to raise the onsite cathodic generation efficiency of H2O2, while few studies thought about the simultaneous reduction of Fe3+to Fe2+that would also lead to more efficient utilization of H2O2catalysis as well as lower production of iron sludge [7].Recently, a method named Fered-Fenton has been developed by adopting cathodic Fe3+reduction instead of H2O2generation [8].Generally, Fered-Fenton is cost-effective than common EFs, since the applied voltage and current intensity for the reduction of Fe3+/Fe2+is lower than O2/H2O2on cathode [9].
Circumstance pH tends to be another factor affecting the application of Fenton technologies in treating actual neutral industrial wastewaters because either Fe2+or Fe3+cannot persist in the bulk solution.Several additives such as hydroxylamine (HA)[10,11],organic chelates[12,13]could effectively expand the work pH range of Fenton or Fenton-like systems up to neutral since they would maintain essential neutral Fe(III)/Fe(II)recycles.It has been reported that neutral Fenton-like systems based on iron-ligands have good efficiencies on the degradation of pollutants, but also caused more difficult regeneration of Fe2+because of the lower redox potential of Fe3+-ligands than free Fe3+[14].Furthermore,most related studies used ligands to prompt the in-situ chemical oxidation adopting heterogeneous solid catalysts,e.g.,iron oxides,wherein the interfacial surface binding reactions between ligands and metal ion in the solid catalyst have been intensively concerned[15].In addition, utilization of H2O2in neutral conditions is questioned since·OH will be scavenged by increased carbonate[16].Peroxydisulfate (PDS) is a suitable and favorable alternative powder oxidant since it can be activated to produce sulfate radicals(SO4·-) of higher redox potential (E0=2.5-3.1 V) and longer lifetime (3-4×10-5s) as compared to·OH [17,18].
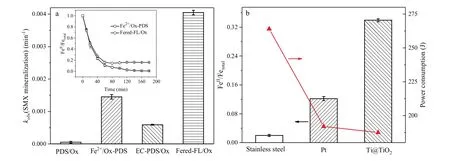
Fig.1.(a) Comparative kobs(SMX mineralization) at 3 h for PDS/Ox, Fe2+/Ox-PDS, EC-PDS/Ox and Fered-FL/Ox systems.Inset shows the related evolution of FeII/Fetotal in Fe2+/Ox-PDS and Fered-FL/Ox systems.Initial conditions: 10 mg/L SMX, pH 6.5, 0.5 mmol/L PDS, 0.1 mmol/L Fe2+, 0.4 mmol/L Ox, -0.7 V applied voltage and 25°C.(b)Evolution of FeII/Fetotal and the related power consumption at 3 h with comparative electrodes.Initial conditions: pH 6.5, 0.1 mmol/L Fe3+, 0.4 mmol/L Ox, -0.7 V applied voltage and 25°C).
Therefore, in this study, a novel neutral Fered-Fenton like/Ox(Fered-FL/Ox) system has been established for more efficient degradation of SMX by using the oxidant PDS, the ligand oxalate and the work cathode pre-anodized Ti@TiO2electrode.Optimization of operational parameters and exploration of the whole reaction mechanism based on the critical solid-liquid interfacial reactions were proceeded.
All experiments were conducted in a divided threeelectrodes cell reactor with a saturated calomel (SCE)reference electrode, a counter electrode of graphite rod(d=6 mm), and the working electrode of Ti@TiO2sheet(20 mm×20 mm×0.2 mm) which was pre-anodized with mixed solution of 0.5 mmol/L Fe3+and 2 mmol/L Ox at a weak voltage for 10 min.10 mmol/L sodium sulfate (Na2SO4) was chosen as the electrolyte.The Design-Expert 8.0.6 software(Stat-Ease Inc., USA) was used to design the experiments to conduct central composite design (CCD) and to establish response surface methodology RSM models for optimizing four main experiment parameters, i.e., initial pH, cathode voltage,dosage of Ox and Fe2+at five levels.Upon the optimized parameters and neutral circumstance, attenuated total reflection Flourier transformed infrared spectroscopy (ATR-FTIR) and X-ray photoelectron spectroscopy (XPS) were used to explore the improved heterogeneous-homogenous degradation mechanism based on the Fered-FL/Ox system adopting the Ti@TiO2electrode.
Degradation of SMX in four comparative systems, i.e., PDS/Ox,Fe2+/Ox-PDS, electro-PDS/Ox (EC-PDS/Ox) and the Fered-FL/Ox,were carried out under the conditions initial sulfamethoxazole(SMX)of 10 mg/L,pH of 6.5 and Ox of 0.4 mmol/L in solution.It was found that the time-dependent mineralization of SMX in all systems could be applied with pseudo first-order reaction kinetic(R2>0.95).
Fig.1a shows that the Fered-FL/Ox system could achieve significant synergistic mineralization of SMX as compared to the other three systems.Its kobs(SMX mineralization) was 4.1×10-3min-1which was about 81, 6.9 and 2.8 times larger than the PDS/Ox, EC-PDS/Ox and Fe2+/Ox-PDS systems,respectively.It indicated that Fe2+could effectively catalyze PDS in producing sulfate radical and oxidizing SMX, while the Fered-FL/Ox system would lead to an efficient iron cycle based on the electrochemical reduction of Fe3+-Ox in the neutral reaction circumstance.As presented in the inset of Fig.1a, the added Fe2+was rapidly consumed and used up at 60 min in Fe2+/Ox-PDS system, while a stable [FeII]/[Fe]totalratio of 0.16 could be kept in the Fered-FL/Ox system even the reaction proceeded to the reaction time of 180 min.This result indicated that the electroregeneration efficiency of FeII/Ox in the Fered-FL/Ox system could cause continuous catalytic decomposition of PDS and effective mineralization of SMX.
Fig.1b compares the electro-reduction efficiency of 0.1 mmol/L FeIII-Ox at pH of 6.5 using three common metal electrodes/cathodes,i.e.,Ti@TiO2(20 mm×20 mm×0.2 mm),Pt(square flag,20 mm×20 mm×0.2153 mm), and stainless steel (mesh,20 mm×20 mm×0.1897 mm).It can be seen that the Ti@TiO2electrode achieved a [FeII]/[Fe]totalof 0.34, whereas the Pt and stainless steel could only achieve the[FeII]/[Fe]totalof 0.12 and 0.02,respectively.Meanwhile, it was interesting to note that the corresponding power consumption of the three electrodes presented a converse behavior.Ti@TiO2cathode could reduce 0.033 mmol/L Fe(III) by consuming only 98 J.It indicated that Ti@TiO2electrode would be very effective in the electroregeneration of ferric in such iron-based Fenton like systems.It is well known that Pt is inert and commonly have a surface oxide layer due to the spontaneous passivation,while the ATR-FTIR of the commercial stainless steel in adsorbing FeIII-Ox exhibited that its surface structure would be more restrict to the solid-liquid interfacial reaction in the Fered-FL/Ox system.Therefore, the significant difference in the three metal electrode might ascribe to the potential surface-binding reactions of FeII/FeIII-Ox complexes depending on the specific surface structure which would affect the interfacial electron transfer [18].More detailed description in the related mechanism will be discussed in the below content.
Thereafter,parameters optimization for the SMX degradation in the Fered-FL/Ox system adopting the Ti@TiO2cathode was conducted with the four factors and five levels CCD as presented in Table 1.Thirty experimental runs have been designed with the SMX degradation efficiency (kobs) as the response.
After the step-wise model fitting by the software, a response surface model for the SMX degradation in the Fered-FL/Ox system could be concluded as expressed by Eq.(1).

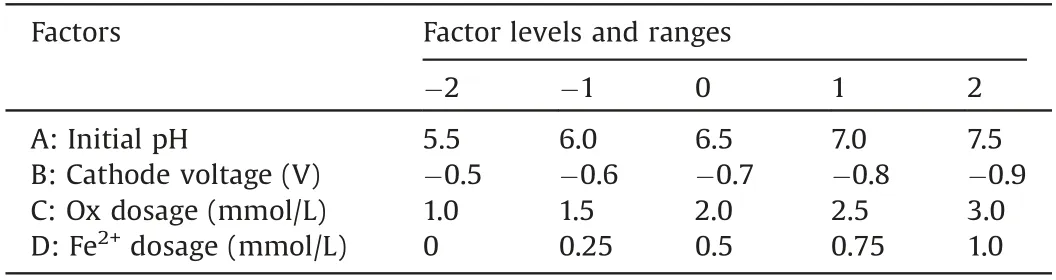
Table 1 The CCD design and response of SMX degradation(kobs)in the Fered-FL/Ox system.
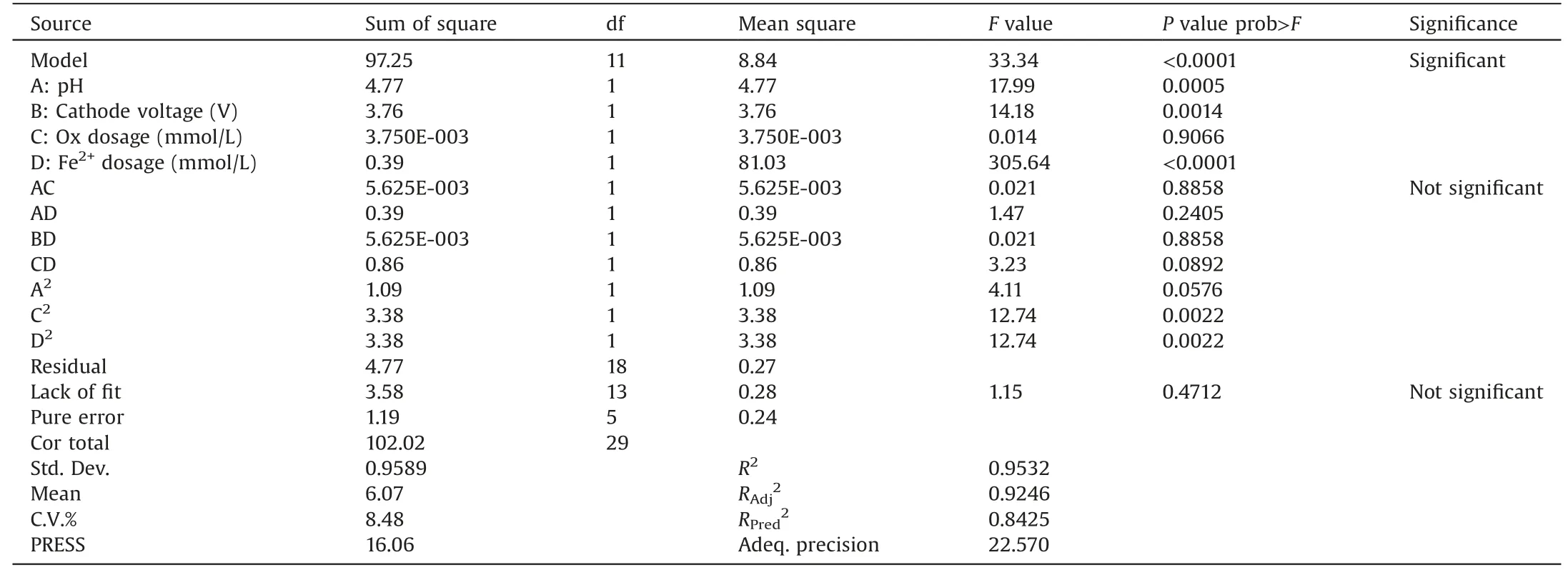
Table 2 ANOVA (analysis of variance) for the RSM model.
Table 2 variance (ANOVA) of the obtained model.It indicated that this model could be successfully applied for the SMX degradation in the Fered-FL/Ox system.The model value of“prob>F” and F value were <0.05 and 33.34, respectively.This indicated that both the model terms and the model were significant were significant, there was only a 0.01% chance that a model value this large could occur due to noise.In addition, it should be noted that the“l(fā)ack of fit P-value”was 0.4712(>0.1000),suggesting that the lack of fit was not significant and the model had a good predictability.Besides,the value of R2and“Adeq.precision”reached 0.9532 and 22.57 (>4), respectively, indicating that this model could be used to navigate the design space with an adequate signal and it had a good degree of fitting.
According to the expression (1), the results of different experimental parameters could be optimized under the remaining experimental conditions: 20 mg/L SMX, 10 mmol/L Na2SO4,0.5 mmol/L PDS.As shown in Table 3, for achieving "the best degradation efficiency",the optimal parameters were predicted as pH of 5.79,cathode voltage of-0.745 V,Ox dosage of 1.98 mmol/L,Fe2+dosage of 0.985 mmol/L, by set all related parameters "in range".An estimated value kobs(SMX) of 1.34×10-2min-1was obtained, which was close to the actual value of 1.44±0.06×10-2min-1in the Fered-FL/Ox system.Another trial for achieving the target "the most cost-effective" was also calculated by the model, where the parameters were set as “pH equal to 7”,"the cathode voltage minimize"(-0.5 V)and"others in range".The model estimated kobs(SMX) was 1.25×10-2min-1which was also very close to the related actual value of 1.23±0.06×10-2min-1.The above result indicated that the precision of the model was good enough in advising the degradation of pollutants in the Fered-FL/Ox system.In addition,optimal parameters of Ox dosage was 2.45 mmol/L, [Fe2+]dosage was 1.0 mmol/L for the case of "most cost-effective" were noted.The values were slightly higher than the case "best efficiency".It was because that the catalysis activity of FeII-Ox complexes would be lower in the neutral circumstances than the acidic circumstances [19].
As a result,the related RSM analysis between the initial pH and[Ox]affecting kobs(SMX) was further conducted.It was observed that raising pH would lead to obvious inhibition in the kobs(SMX)since Fe3+tended to precipitate when [Ox]was too low to effectively maintain the aqueous Fenton like reaction.On the contrary, overloaded free Ox would competitively adsorb on the Ti@TiO2layer,causing less electrochemical reduction of the soluble Fe(C2O4)33-complex.Therefore,more but appropriate amounts of[Fe2+]and[Ox]would be needed in achieving efficient degradation of SMX in the neutral circumstance as compared to the acidic,since more Fe2+would be benefit for the activation of PDS but need more Ox to maintain the effective iron cycle in the Fered-FL/Ox system.
To reveal the interfacial reaction mechanism in the Fered-FL/Ox system depending on the Ti@TiO2electrode, XPS and ATR-FTIR characterizations were conducted for the three adopted cathodes,which were pre-adsorbed for 10 min in the neutral solution of 0.5 mmol/L Fe3+and 2 mmol/L Ox under a weak voltage of-0.01 V.Under the neutral conditions,Fe(C2O4)33-would be the dominate FeIII-Ox complex and would be absorbed to cathode and then may interact with specific cathode surface to accelerated the process of reduction.From Fig.2, it can be seen that the obtained Fe 2p spectra of the three electrodes with pre-adsorbed FeIII-Ox were rather different.The case of the stainless steel electrode presented an extremely low intensity of Fe 2p indicating difficult adsorption of FeIII-Ox on its surface.As for the cases of Pt and Ti@TiO2,the two broad peaks (711.2 and 713.6 eV) and the small shoulder at 709.2 eV could be attributed to the adsorbed FeIII-Ox and its electrochemical reduction products FeII-Ox, respectively [19,20].This result indicated that FeIII-Ox could be effectively adsorbed on the two cathodes surface and reduced.It was interesting to note that the intensity ratio of Fe2+/Fe3+on the Ti@TiO2electrodeincreased significantly from 28.2% to 53.5% as compared to the Pt electrode.This indicated that the adsorbed FeIII-Ox species would be more easily electrochemically reduced depending on the Ti@TiO2surface, probably due to TiO2had more amounts of surface hydroxyl than Pt to combine with FeIII-Ox and then easily to start a reduction reaction at a weak voltage.As a result,the electron transportation between FeIII-Ox and the cathode could be accelerated.

Table 3 The verification for the optimized RSM model.
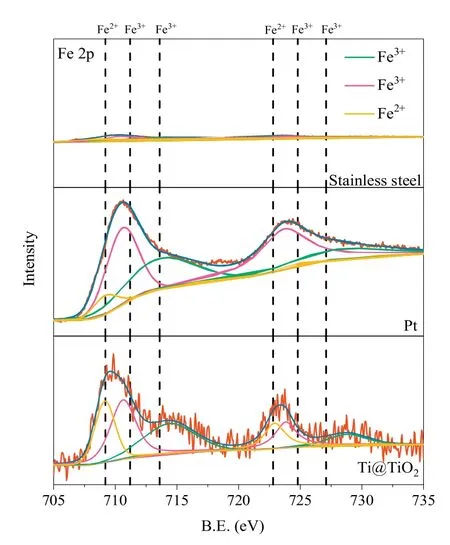
Fig.2.The XPS Fe 2p spectra of the stainless steel/Pt/Ti@TiO2 cathodes with preadsorption in the presence of 0.5 mmol/L Fe3+ and 2 mmol/L Ox with a voltage of-0.01 V for 10 min.
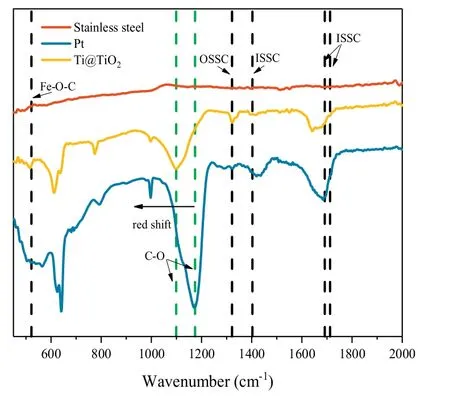
Fig.3.ATR-FTIR spectra of the stainless steel/Pt/Ti@TiO2 cathodes with preadsorption in the presence of 0.5 mmol/L Fe3+and 2 mmol/L Ox with a voltage of-0.01 V for 10 min.
The corresponding ATR-FTIR examination was further conducted to verify the critical role of cathode surface hydroxyl in the Fered-FL/Ox system.As shown in Fig.3, the FTIR patterns of the three electrodes were also different.In general, the formation of FeIII-Ox complexes could be roughly divided into two types, one was the outer sphere surface complex(OSSC)and the other was the inner sphere surface complex (ISSC) [21].In the neutral Fered-FL/Ox system,FeIII-Ox complexes would be formed as Fe(C2O4)33-in consistent with the relatively large Langmuir equilibrium constant.From Fig.3,it can be seen that few peak was found in the case of the stainless steel electrode,verifying this electrode could not adsorb FeIII-Ox effectively.While in the cases of Ti@TiO2and Pt electrodes,the peak at 1394 cm-1and the peaks at 1710,1690 cm-1were clearly observed, which corresponded to the υ (C--O)stretching vibrations involving predominantly the oxygen atom not bonded or bonded to Fe in ast ructure of FeIII-Ox complexes [22,23].Another peak at 528 cm-1represented the Fe--O--C ring in FeIII--Ox complexes [23,24].The above finding proved that either the Ti@TiO2or the Pt could adsorb FeIII--Ox onto their surfaces through the ISSC.Meanwhile, the symmetric stretching vibration of the C-O bond at 1315 cm-1represented that a small portion of Ox also coordinated to FeIIIvia OSSC [25].The peak at 1169 cm-1represented the tensile vibration of the C--O bond [26].It indicated that the bond between the FeIII--Ox and the electrode surface was formed through the excess sites on the oxalic acid bonding to the surface --OH.
It was worth noting that an obvious red-shift of the C--O peak at 1109 cm-1in the case of Ti@TiO2electrode, suggesting a potential"electron-rich"phenomenon in the bond[27].This would be attributed to the easier electron transfer through the surface C--O--Ti bond.Therefore, it could be concluded that the surface characteristics of the Ti@TiO2electrode would be more benefit for the electrochemical reduction of FeIII--Ox and lead to the more efficient SMX degradation.
Fig.4 illustrates the proposed reaction mechanism in the neutral Fered-FL/Ox system depending on the Ti@TiO2cathode.At first,aqueous neutral Fenton like oxidation of PDS occurred in the presence of FeII--Ox and PDS where SO4·-would be the dominant oxidant [28].Simultaneously,the efficient electrochemical reduction of the formed FeIII--Ox complexes would happen through a series of solid-liquid interfacial reactions.Herein, the main FeIII--Ox species Fe(C2O4)33-could be hydrated and electrostatically interacted with the Ti@TiO2cathode surface through ISSC and form specific C--O--Ti bonds, which would induce ultrafast electron transfer from the cathode to the FeIII[29,30].It would cause efficient electrochemical generation of FeII-Ox which easily detached to the bulk solution due to its low affinity with the surface hydroxyl groups [30].The appended FeIIspecies would continuously activate PDS and lead to efficient Fenton like oxidation of SMX.
Therefore,an efficient neutral heterogeneous-homogenous iron cycle would exist in the Fered-FL/Ox system adopting the Ti@TiO2cathode.It would effectively accelerate the neutral Fenton-like reactions and complete mineralization of SMX with relative low dosage of ferrous catalyst and applied voltage.The result of this study is expected to supply a good alternative in treating complex neutral industrial wastewaters.
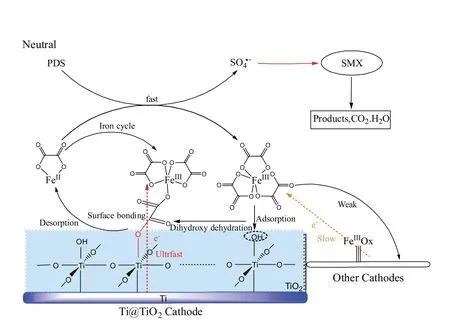
Fig.4.Scheme of the proposed reaction mechanisms in the Fered-Fenton like-Ox system.
Acknowledgments
This study is financially supported by the National Natural Science Foundation of China (Nos.21677055 and 21407052), and the Fundamental Research Funds for the Central Universities,HUST(Nos.2017KFXKJC004 and 2016YXMS287).Huazhong University of Science & Technology Analytic and Testing Centre is thanked for the advanced analytic operations.
 Chinese Chemical Letters2019年12期
Chinese Chemical Letters2019年12期
- Chinese Chemical Letters的其它文章
- Post-self-repair process of neuron cells under the influence of neutral and cationic nanoparticles
- CdS nanocrystallites sensitized ZnO nanorods with plasmon enhanced photoelectrochemical performance
- A simple visual method for DNA detection based on the formation of gold nanoparticles
- Self-assembly of L-tryptophan on Cu(111)studied by low-temperature scanning tunneling microscopy
- Functional delivery vehicle of organic nanoparticles in inorganic crystals
- Facile assembly of mesoporous silica nanoparticles with hierarchical pore structure for CO2 capture
