Post-self-repair process of neuron cells under the influence of neutral and cationic nanoparticles
Ting Wng,Gunwen Qu,Yu Deng,Jing Shng,Zhngqi Feng,Fengyu Yng,Nongyue He,Jie Zheng
a State Key Laboratory of Bioelectronics, National Demonstration Centre for Experimental Biomedical Engineering Education, School of Biological Science and Medical Engineering, Southeast University, Nanjing 210096, China
b State Key Laboratory of Natural Medicines, Department of Pharmacology, China Pharmaceutical University, Nanjing 210002, China
c Department of Chemical and Biomolecular Engineering, The University of Akron, Akron, OH 44325, United States
d School of Chemical Engineering, Nanjing University of Science and Technology, Nanjing 210094, China
e Nanjing Daniel New Mstar Technology Ltd., Nanjing 211200, China
Keywords:
Lysosome
S phase
Cyclin E
p21
Fbw7
ABSTRACT
The prevalence of functionalized nanoparticles in biological and clinical fields attracts intensive toxicology investigations.Minimizing the nanoparticles' biohazard remains a challenge due to the insufficient understanding on the nanoparticle-induced cell death mechanism.In the presented study,we observed the lysosome and genome injuries and so caused cell cycle changes and regulations of retinal ganglion neuron cell 5 (RGC-5) induced by aminated and alkylated nanoparticles.Alkylated nanoparticles induced malignant lysosome and genome damages followed by severe post-self-repair responses.RGC-5 treated with alkylated nanoparticles presented dramatic S phase prolongation resulted from cyclin E accumulation mediated by Fbw7 downregulation, which assisted DNA replication after failed self-repair of the malignantly damaged DNA caused by alkylated nanoparticles.Differently,aminated nanoparticles in RGC-5 induced moderate lysosome and genome injuries and these damages could be repaired in the p21-involved pathway, so that cells did not induce apparent cyclin E accumulation nor Fbw7 downregulation as post-self-repair response.These results helped us to understand the toxicity of analogous nanoparticles on retinal ganglions such as glaucoma treatment.This work provides new insights into nanoparticle functionalization and toxicity in relation to the research on the toxicology and pathology of nerve cells.
Nanoparticle-based products possess various commercial use in human life from medicine to cosmetics.When nerve cells are exposed to nanoparticles, unhealable metabolism disorders happen and present as nerve system diseases, such as neurodegeneration disease,since neurons will undergo several different death pathways such as apoptosis and autophagy [1].As is reported, several nanoparticles, such as silica nanoparticles, will impact neuron survival, proliferation, differentiation, mutation,impair and repair [2-5].However, the underlying mechanism is less explored.
Cell death processes can be switched on by cell cycle deregulation which related to the nanoparticle surface charge determined by different surface functional groups [6].Surface charge, such as positive and neutral charge, is crucial design criteria of nanoparticles because it affects the toxin mode of nanoparticles by altering their cellular uptake, intracellular targeting and intracellular behaviors [7].Generally, cationic nanoparticles are taken up by cells with higher rates and amounts than the neutrally charged nanoparticles via clathrin mediated machinery [8,9].Also, the surface charge affects the cell metabolism especially cell cycle regulation.For example, cationic nanoparticles caused G1 phase arrest by rupturing lysosome and producing more ROS (Reactive oxygen species) [10,11].However,molecular biology of cell cycle changes and underlying regulations after nanoparticle exposure were rarely investigated.
Retinal ganglion cell 5(RGC-5)is a type of neuron that located near the inner surface of retina (the ganglion cell layer).RGC-5 maintains typical features of neurons and they are not protected by the blood-brain barrier, so they could become a novel model for direct interaction between neurons and various nanoparticles.When this type of cells interacts with various nanoparticles in the physiological condition it will present their typical features as neurons.In this case, we used RGC-5 cell line to study central neuron interacting directly with different surface charged nanoparticles.The up-taken processes of positively and neutrally charged nanoparticles were explored and neuron survival was investigated.After that, proliferation, impair and repair of RGC-5 were investigated in detail based on ROS release and the early and late neuron apoptosis.Further, we analyzed the self-repairing process of RGC-5 by testing the cyclin-dependent kinase inhibitor p21.Based on cell cycle analysis of RGC-5,we explored the S phase arrest and underlying mechanism on molecular level.In this sense,we illuminated the cell cycle changes based on molecular biology and analysis the cell cycle regulations after p21 involved cell repair.
Therefore, we used aminated and alkylated silicon dioxide(SiO2) nanoparticles (Nanjing CaiNa Biological Technology Co.,Ltd.)with diameter of 200 nm to test their cytotoxicity to neurons.Mouse retinal ganglion cell 5 (RGC-5) was the model of neurons.The sterile aminated and alkylated nanoparticles were suspended in phosphate-buffered saline (PBS) and the final working concentration was 50 μg/mL (2 μg/1000 cells).The cells were cultured with high glucose DMEM and with 10% fetal bovine serum.To test the endocytosis when clathrin activity was inhibited, cells were treated with 10 μg/mL chlorpromazine hydrochloride (CPZ-HCl) for 1 h.CPZ-HCl was washed out and cells were used for further experiments.
SiO2nanoparticles with modification were dried on the foil for Scanning Electron Microscope (SEM, JSM-6700 F, JEOL) imaging.The images were recorded at an acceleration voltage of~10 kV and a magnification of 3000-5000.Size distributions and zeta potential in PBS of SiO2NPs, NH2-SiO2NPs and CH3-SiO2NPs were analyzed using Zetasizer Nano ZS,Malvern Panalytical.X-ray photoelectron spectrometry (XPS Thermo Scientific) was used to examine the surface element compositions of the two particle samples.Infrared spectroscopy (NICOLET iS50 FT-IR) was used to distinguish the functional groups on the SiO2particle samples.For IR examination, aminated and alkylated nanoparticles were dehydrated under an infrared lamp, and then the dried particles were embedded with potassium bromide (KBr) and tableted to~500 nm thick films for further infrared spectroscopy.Infrared spectra were acquired at 2 cm-1resolution.
For confocal microscopy observation, RGC-5 cells were seeded in the 8-well plates at a concentration of 5000 cell/well and coincubated the cells with 50 μg/mL aminated and alkylated nanoparticles for 1 h, 5 h and 24 h.Cell membrane was labelled with Cholestrol-PEG-FITC(488 nm,NanoCS,USA),lysosomes were labelled with LysoTracker?Deep Red (647 nm, Thermo Fisher Scientific, USA) and nanoparticles were visualized by scattering light,RT(15/85)gate[12].Cells were observed under confocal laser scanning microscope (TCS SP8, Leica, Germany).
The endocytosis of aminated or alkylated nanoparticles under the normal condition and clathrin inhibition was also semiquantified via flow cytometry.Nanoparticles could scatter light because their diameters are similar to the laser wavelength,while healthy cells are generally transparent to light.Therefore, cells endocytosing more nanoparticles presented higher side scattered light intensity (SSC-H).Cell proportion with high SSC-H (>106)(Fig.S2 in Supporting information) was selected to demonstrate the endocytosis of nanoparticles[13].After coculturing cells with 50 μg/mL aminated or alkylated SiO2nanoparticles for 1 h,5 h and 24 h, the residual nanoparticles were washed out thoroughly to eliminate false positive signals.Then cells were trypsinized and suspended in culture medium.The cells were then centrifuged to cleanse the residue nanoparticles.The gathered cells were then resuspended in DMEM for flowcytometry analysis and 50,000 cells were counted in each analysis.The cells with SSC intensities higher than 106were quantified to represent large quantity endocytosis of aminated or alkylated nanoparticles.In this process, died cells were washed out, and only the cells with relatively high viability could be detected by flow cytometer.
For cell apoptosis RGC-5 cells were tested using Cell Apoptosis Kit(Beyotime Institute of Biotechnology,China).Cells were plated in 6-well plates and treated with aminated and alkylated nanoparticles for 24 h, and then the nanoparticles were washed out with PBS for three times to eliminate noises from nanoparticles.For cell apoptosis assay,105cells were gathered and suspended in binding buffer.The cell aliquots were then stained with 5 μL Annexin-V-FITC and 5 μL propidium iodide(PI)and incubated for 10 min in darkness.Then,the apoptotic induction was assessed by flow cytometer.
ROS release under the influence of aminated and alkylated nanoparticles was detected with ROS Detection Kit (Beyotime Institute of Biotechnology,China).Cells and nanoparticle were cocultured.Then, cells were trypsinized and suspended in DMEM culture medium without serum.Cells were labelled with DCFH-DA at concentration of 105cells/10 μg DCFH-DA for 20 min at 37°C,and then cells were washed with culture medium without serum to eliminate unbound dyes.The dyed cells were gathered to detect DCFH-DA fluorescence (488-552 nm) using flow cytometry.Cell with fluorescence intensity at 102-103were counted representing cells with high ROS release
For cell cycle assay, RGC-5 cells were seeded in 6-well plates and treated with aminated or alkylated nanoparticles for 24 h,and then the nanoparticles were washed out.For cell cycle assay,105cells were gathered and resuspended in PBS.The cell suspension was then mixed with cell cycle fluid (Rnase:PI=1:9,Beyotime Institute of Biotechnology, China) in darkness under room temperature for 30-60 min for cell cycle assay.Then the PI intensity was measured on flow cytometry for cell cycle analysis.Cell cycle was fitted using Novoexpress (ACEA Bioscience, Inc.)with CV% controlled under 5%.
For western blot assay,106cells were seeded in 6-well plates and treated with aminated and alkylated nanoparticles for 24 h.After washing out the nanoparticles, cells were gathered,washed and suspended in cold PBS and then lysed in Cytobuster TM Protein Extraction Reagent (Novagen, USA) at 4°C for 30 min.After centrifugation at 12,000 rpm under 4°C for 20 min,the protein was extracted in the supernate.The protein concentration was measured via the BCA method, and then protein (≤40 μg) were resolved by 10% SDS-polyacrylamide gel electrophoresis and transferred electrophoretically to polyvinylidene difluoride(PVDF)membranes and then were blocked overnight in 5% skim milk in TBST(containing 20 mmol/L Tris-HCl pH 7.4,100 mmol/L NaCl,and 0.1% Tween 20) buffer at 4°C.After washed in TBST buffer, they were then incubated with anti-Cyclin E1-cdk 2 polyclonal antibody[ab71535](Abcam,USA),anti-Fbw7 antibody[ab192328](Abcam,USA)and anti-p21 antibody[ab109199](Abcam,USA)respectively as the primary antibody overnight, and then incubated with the HRP*Goat anti-Rabbit IgG secondary antibody(ImmunoWay,USA)for 3 h.After incubation, the bands were then detected using the ECL Prime Western Blotting Detection System (BioRaD, USA).
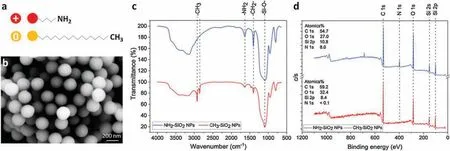
Fig.1.(a)Scheme of the modified nanoparticles.(b)SEM image of SiO2 NPs.(c)IR analysis of SiO2 NPs,NH2-SiO2 NPs as well as CH3-SiO2.(d)XPS analysis of SiO2 NPS,NH2-SiO2 NPs as well as CH3-SiO2.
For the characterization of aminated and alkylated nanoparticles schemed in Fig.1a, we detected the size and surface functional groups of the two types of nanoparticles.As is shown in Fig.1b and Fig.S1(Supporting information),with SEM analysis and dynamic light scattering (DLS) size measurement the results showed that SiO2nanoparticles had an average particle size range of 200±30 nm.Zeta potential of SiO2NPs in PBS (0.1 mol/L) was-19.3 mV, which changed to 3.8 mV and -5.8 mV after modified with-NH2and-CH3,respectively.X-ray photoelectron spectrometry (XPS, Thermo Scientific) and infrared spectroscopy (NICOLET iS50 FT-IR) were used to examine the surface element compositions and functional groups of aminated and alkylated SiO2NPs.According to IR spectra(Fig.1c),signals at 1650 cm-1representing amino groups from aminated nanoparticles.And signals at 1470 cm-1, 2850 cm-1and 2925 cm-1representing alkyl groups that on alkylated nanoparticles.The IR results confirmed that the amino groups and alkyl groups were functionalized on the nanoparticles successfully.Also,XPS spectra(Fig.1d)showed that aminated nanoparticles owned only peaks representing element of C, N, O and Si, while alkylated nanoparticles owned only peaks representing element of C, O and Si.The results indicated that aminated and alkylated nanoparticles were not contaminated by other functional groups.
First of the interactions between aminated/alkylated nanoparticles and RGC-5 cells, the endocytosis of aminated and alkylated nanoparticles in RGC-5 cells were analyzed by confocal microscopy and flow cytometry.As is shown in Figs.2a and b,after 1 h co-incubation, small amounts of nanoparticles (red) entered cells and they stayed adjunct to cell membrane(green).From 5 h to 24 h, large quantities of nanoparticles were internalized continuously,but few particles transported to the center of cells.After 24 h,the nanoparticle amount in cells did not increase much and the nanoparticles distributed mainly near the nucleus of cells(Fig.S3 in Supporting information).

Fig.2.The cellular uptake of(a)aminated and(b)alkylated nanoparticles at different time points in the absence and presence of clathrin inhibitor(scale bar=10 μm).(c)Flow cytometry analysis of cellular uptake amounts of aminated and alkylated nanoparticles with and without clathrin inhibitor.(n=5;50,000 cells per group).(d)The subcellular localization of aminated and alkylated nanoparticles (red) in comparison with lysosomes (green) after 1 h, 5 h, and 24 h co-incubation (scale bar =10 μm).
Referring to flow cytometry analysis results(Fig.2c and Fig.S2),side scattered light intensity(SSC-H) was selected as an indicator presenting the endocytosis quantity of the nanoparticles.As is shown in Fig.2c and Fig.S2,the cell proportion with large amounts of nanoparticles internalized increased from 1 h to 5 h and reached amplitude at 5 h(56%)but decreased from 5 h to 24 h.The results revealed that the endocytosis of both aminated and alkylated is a time-depending process within 5 h.However, after 5 h, the proportion of cell endocytosing large amounts of nanoparticles decreased because the dilution effect of cell division as well as the cell death caused by too much nanoparticle endocytosis.Compared with aminated ones,alkylated nanoparticles were less internalized by cells.Moreover,the endocytosis of alkylated particles decreased more rapidly after 12 h.Since the dilution rate is relatively unchanged, we speculated that cells with large quantities of the alkylated particles turn to unhealthy and SSC-H results will be influenced by alkylated particles.
Clathrin is an important mediator for the endocytosis of nanoparticles sized 100-200 nm[14].Thus,we explored the role of clathrin in the endocytosis of aminated and alkylated nanoparticles by inhibiting clathrin activity with chlorpromazine hydrochloride (CPZ HCl).
The cellular-uptake amount of aminated nanoparticles under clathrin inhibition were lower than the normal endocytosis within 12 h but overwhelmed the uninhibited group at 24 h(Figs.2a and c,Fig.S2).The ultra-high SSC intensity at 24 h could also attribute to the scattered light from swelled organelles such as lysosomes(Fig.2d)[15].Organelle swell usually happens after cell damages,indicating the cytotoxicity of the aminated nanoparticles.The results revealed that the inhibition of clathrin retarded the endocytosis of aminated nanoparticles effectively but did not prevent it totally.Thus, the clathrin mediated endocytosis should be a main part of the endocytosis of aminated nanoparticles, and some other endocytosis mechanisms such actin also involved in their transportation [16].Otherwise, the cellular uptake amounts of alkylated nanoparticles after clathrin inhibition were significantly lower than that in the non-inhibition group(Figs.2b and c, Fig.S2).And cell proportion with large amounts of internalized nanoparticles decreased with time.After 24 h, the continuous decrease of uptake quantity of alkylated nanoparticle manifested the potent inhibition induced by CPZ treatment.Additionally,CPZ is not only a clathrin inhibitor but also a clinical-used antipsychotic medication.Researches have described that CPZ could retard the endocytosis process of neurons as well as decrease the viability of neurons especially under environmental stress[17].CPZ exhibited dual-inhibition effects on the endocytosis of alkylated nanoparticles because of its inhibition on clathrin activity and cell metabolism.When cells were exposed to alkylated nanoparticles after CPZ treatment, cell swelling could be observed in 24 h.At 24 h, most of the cells with large quantities of alkylated nanoparticles would die from the synergistic cytotoxicity of CPZ and a small proportion of these cells were in healthy conditions.Cytotoxicity effects were not obviously seen in the aminated nanoparticle treated groups,because aminated nanoparticles were less potent than the alkylated ones and could not induce the synergistic effects.
Therefore,both aminated and alkylated nanoparticles could be endocytosed via the clathrin-mediated pathway, while the endocytosis of alkylated nanoparticles mostly depended on clathrin pathway.The endocytosis mediated by reticulin follows a pathway that ultimately fuses late endocytosis with lysosome[18].Nevertheless, the different charged nanoparticles would interact differently with lysosomes.For example, cationic nanoparticles could escape from lysosomes, but the neutral ones accumulated in lysosomes and caused lysosome dysfunction[19].Therefore,we observed the intracellular trafficking into lysosomes of the two kinds of nanoparticles and studied their potential mechanism of their cytotoxicity.
Further from the endocytosis of the two types of nanoparticles,the subcellular localization of aminated and alkylated nanoparticles in RGC-5 cells were observed via confocal microscopy(Fig.2d and Fig.S3).Fig.2d showed co-localization of nanoparticles and lysosomes.After 1 h co-incubation, nanoparticles(red) can be found distributed in the cytoplasm, and some aminated nanoparticles transferred into lysosomes (green), while rare alkylated nanoparticles appeared in lysosomes.In 5 h, most aminated nanoparticles could be observed to escape from lysosomes while more alkylated nanoparticles enter lysosomes.The residual nanoparticles moved near to the center of the cells.After 24 h, most aminated nanoparticles were found to escape from lysosomes and only a few particles stay in lysosomes.Otherwise, more alkylated nanoparticles were internalized by lysosomes.Moreover, lysosomes swelled and deformed since 1 h after aminated nanoparticles treatment, indicating the acute damages on lysosomes or H+leakage caused by the particles.Comparably,swollen and deformed lysosomes did not appear until 5 h after alkylated nanoparticle exposure(Fig.2d).However,with incubation time extending, the lysosome swell and deformation deteriorated.And lysosomes were much more severely deformed under the effects of alkylated nanoparticles.
According to the observations, aminated nanoparticles could transfer to lysosomes within 1 h but after 1 h they escape slowly from lysosomes (Fig.2d).This special intracellular trafficking attributes to their amino groups and positive surface charge,which could assist them to escape from late endosomes and lysosomes.Their unprotonated amides could absorb H+in acidic organelles such as late endosomes (pH 5.0) and lysosomes (pH 4.5) and resulted in more H+pumped into the organelles leading to an increased influx of Cl-and water,which is also known as“protonsponge effect” [20,21].Thus, the osmotic swelling ruptured the lysosome membrane and provided channels for the particles to escape.In accordance to our observations,aminated nanoparticles hurt lysosomes and caused lysosome content leakage but did not induce deteriorating lysosome injury and dysfunction by rupturing lysosome membrane.
The endocytosed alkylated nanoparticles did not appear in lysosomes but can be observed in cytoplasm after a short period after co-incubation (Fig.2d,1 h).However,as foreign matter,the alkylated nanoparticles were finally transferred to lysosomes(Fig.2d, 24 h).Since they were undegradable and could not escape from lysosomes, the particles accumulated in lysosomes can be observed to increase with time extending.The accumulation of these undigested substrate is an important reason of lysosome storage disorders (LSD), which further cause the dysfunction of lysosomes and cellular damages [22].In the meanwhile, the accumulated nanoparticles in lysosomes could also cause lysosome membrane permeability (LMP) [23].Finally,lysosomes burst, collapsed and lost function with lysosome contents leakage.
Lysosome injury is widely described as a precursor of cell fate change, including cell cycle change and cell apoptosis.Therefore,cell apoptosis, ROS release, cell cycle change, and cell cycle regulation under the effects of neutral and cationic nanoparticles were further examined (Fig.3).
Figs.3a and b showed that alkylated nanoparticles induced obvious early apoptosis and ROS release, while aminated nanoparticles caused only moderate early apoptosis and ROS release.Simultaneously, both kinds of nanoparticles caused G1 phase prolongation after 12 h coincubation, though, alkylated nanoparticles induced much slighter G1 phase arrest than the aminated ones (Fig.3c).Along with the G1 phase arrest, p21 abundance increased dramatically in the cells treated with aminated nanoparticles,but only slight p21 increase was observed in cells treated with alkylated nanoparticles ( Figs.3d and e).

Fig.3.(a)Cell apoptosis analysis and(b)ROS release of cells exposed to aminated and alkylated nanoparticles for 24 h.The cells in control groups were cultured without any of the particles(*P <0.05,**P <0.01;n=5;50,000 cells per group for ROS,100,000 cells per group for cell apoptosis).(c)Cell cycle assay after 12 h and 24 h aminated and alkylated nanoparticle incubation (*P <0.05, ** P <0.01; n=5; 100,000 cells per group).(d) Quantitative data of p21, cyclin E and Fbw7 abundance after cells exposed to aminated and alkylated nanoparticles(n=3).(e)p21,cyclin E and Fbw7 abundance after cells exposed to aminated and alkylated nanoparticles.Cells in control groups were cultured without any of the particles.
As is described,lysosome damage can be found in Fig.2d,24 h.Damaged lysosomes will release massive hydrogen ions and various enzymes, which will trigger ROS release.And these released lysosome contents will attack proteins and nucleic acid,causing DNA damages and intrinsic cell apoptosis [24-27].With the cell injuries, cells tend to initiate self-repairing process to repair the broken DNA by prolonging G1 phase.Combining with Figs.2 and 3, we deduced that different lysosome damages will stimulate cells into different DNA-repairing process.Regarding the two kinds of nanoparticles, aminated ones merely opened channels on lysosome membrane via proton-sponge effect, while these channels could not release lysosome contents obviously nor trigger ROS release (Figs.2d and 3b).Thus, the so-caused DNA damages are so moderate that could be repaired in the p21-invovled pathway.The vigorous p21 upregulation of RGC-5 prolonged the G1 phase after cells were treat with aminated nanoparticles for 12 h in order to initiated effective self-repairing process.Therefore, the cells escaped from severe early apoptosis.Otherwise, the alkylated nanoparticles induced lysosome storage disorder and finally induced violent lysosome burst and collapse,which caused lysosome content eruption and immediate ROS release(Figs.2d and 3b).Following the serious lysosome damages and ROS release, the so-induced malignant DNA damage could hardly be repaired.So RGC-5 affected by alkylated nanoparticles did not overexpress much p21 and failed induce effective selfrepair since they were too malignantly damaged by the nanoparticles to repair themselves.Consistently,cells could not escape from apoptosis pathway.
After 24 h co-incubation, both aminated and alkylated nanoparticles induced G1 phase shortening and S phase arrest(Fig.3c).Simultaneously, cyclin E accumulated but Fbw7 abundance decreased in cells treated with aminated and alkylated nanoparticles ( Figs.3c-e).Compared with aminated ones, alkylated nanoparticles were found to cause more obvious cell cycle changes,more apparent cyclin E accumulation and more significant Fbw7 decrease in cells ( Figs.3c-e).
Cyclin E serves in S phase to assist DNA synthesis[28].It begins to be synthesized in late G1 phase and reaches peak in S phase around G1/S checkpoint.In late S phase cyclin E will be degraded mediated by ubiquitin, and cells enter G2 phase.Fbw7 is considered to be an important participator in the ubiquitinmediated degradation of cyclin E by binding directly to cyclin E and initiate the degradation process [29].However, unnormal accumulation of cyclin E could result in undesired G1/S transition and S phase prolongation [28].
According to the results, we deduced DNA damages from the NPs results in ROS release, early apoptosis and increasing of p21 abundance of the RGC-5.Also, p21 upgrade suggested DNA selfrepairs processed after lysosome damages caused by nanoparticles.Cyclin E accumulation in the experiments were observed in S phase illuminated that under the influence of NPs, DNA selfrepair failure happened in G1 phase severely disrupted DNA synthesis.
Regarding alkylated nanoparticles, the induced DNA damages were too serious to be fully repaired before G1/S checkpoint.Once the injured DNA chains began to be replicated, they recruited more cyclin E to aid stabilizing the replication fork.Thus, cells extended their S phases and downregulated the Fbw-7, so that cyclin E tended not to be degraded in the Fbw-7 pathway and maintained its high abundance in S phase to assist DNA replication.Moreover, with the assistance of p21, late G1 phase progressed relatively smoothly, which required normal cyclin E synthesis.Thus, cyclin E was accumulated in consistent with S phase arrest.Furthermore,the accumulated cyclin E also affected G1/S checkpoint and disturbed the revolve of cell cycle, which finally resulted in cell apoptosis, too.By contrast, aminated nanoparticles did not induce strong ROS release nor serious DNA damages.These mild DNA damages induced very active p21-involved self-repair( Figs.3d and e)and were better healed before the G1/S checkpoint.These less injured DNA chains needed but not much cyclin E to assist their replication in the S phase.Therefore, cells retained some cyclin E in the S phase to stabilize the newly repaired DNA chains and allowed the other to be degraded through the Fbw-7 mediated pathway.In this sense,aminated nanoparticles induced less cyclin E accumulation and Fbw-7 downregulation following weaker lysosome damages.
In conception, aminated and alkylated nanoparticles caused lysosome damages and DNA injures in different extents so that induced different post-self-repair responses in RGC5 cells(Fig.4).For alkylated nanoparticles, because they are neutral charged and undegradable, when they entered lysosomes, they accumulated in lysosomes and caused lysosome storage disorders,which finally resulted in lysosome burst and collapse.The rapid and violent lysosome damages caused lysosome content release and ROS release, which induced DNA damages and early apoptosis.Though too quick and serious to be repaired,the so-caused DNA damages still went through p21-involved self-repair before the cells enter cell cycle.Due to the severity of these DNA damages, the DNA chains in S phase were not thoroughly repaired and required adequate cyclin E to assist in the replication process.So that Fbw-7,the important mediator of cyclin E degradation, was downregulated to maintain more cyclin E for DNA replication.Differently,aminated nanoparticles caused lysosome membrane rupture via proton-sponge effect with their positive charge.This mild lysosome injury induced moderate ROS release and DNA injuries but slight early apoptosis,because the injured DNA was repaired by the active and effective p21-involved self-repair process.Following, the cells entered cell cycle and then S phase when some cyclin E were required to assist the replication of the newly repaired DNA while the others were degraded smoothly via Fbw-7 mediation.Therefore, RGC5 cells exhibited a post-self-repair response presented by cyclin E accumulation and Fbw-7 downregulation after lysosome damages,DNA damages, and cell apoptosis resulted from aminated and alkylated nanoparticles.
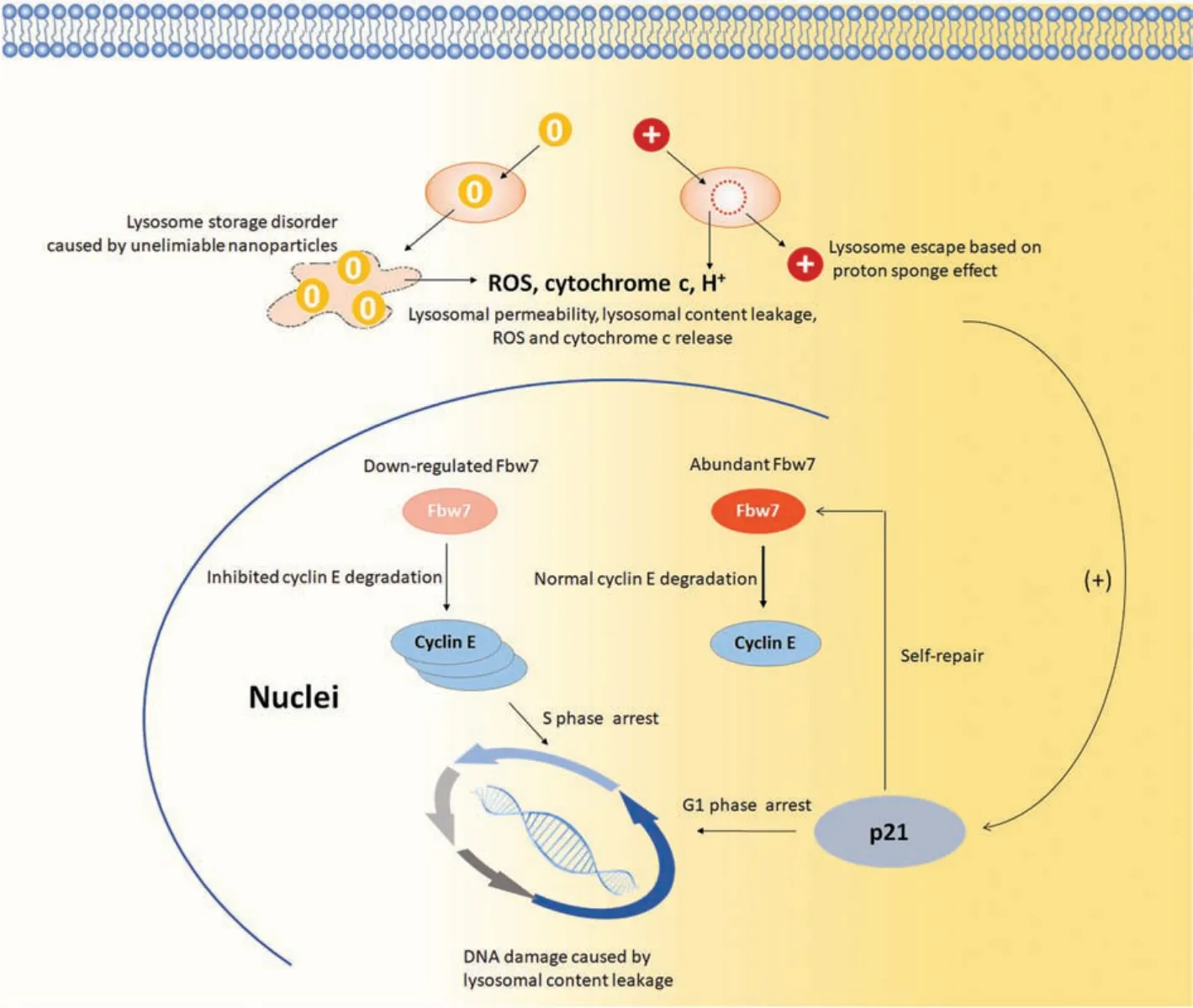
Fig.4.Different metabolism pathway and cell cycle regulation induced by aminated and alkylated nanoparticles.
Therefore, we concluded that both aminated and alkylated nanoparticles, regardless of the surface charge, can both be internalized by RGC5 via the clathrin mediated endocytosis.However, the different surface charge resulted in different cell damages and different post-self-repair response.The alkylated nanoparticles caused rapid and serious lysosome damages and following early cell apoptosis with DNA damages.The rather serious DNA damages could not be perfectly repaired in the p21-involved pathway, so that cells initiated active post-self-repair response by downregulating Fbw-7 and accumulating cyclin E in the S phase in order to stabilize the replication of these damaged DNA.The overwhelmed cyclin E also deteriorated cell apoptosis.Otherwise, aminated nanoparticles did not harm lysosome severely and induced moderate ROS release and DNA injuries but no obvious early cell apoptosis.These mild DNA damages were repaired by p21-involved self-repair process, allowing cells to enter cell cycle and replicate DNA.So, the cells presented merely slight cyclin E accumulation and Fbw-7 downregulation as postself-repair response.Conclusively, though neutral and cationic nanoparticles could both enter cells via clathrin-dependent way,the neutral nanoparticles caused more significant lysosome and DNA damages than the cationic ones and less successful self-repair.Consistently, cells initiated more active post-self-repair in cell cycle processing.This work depicted new insights on how the surface functionalization contribute to the neural cell toxicity and neuron response of nanoparticles.And it will bring new illumination on the nanoparticle-involved pathology and pharmacy in neural system.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This research is supported by the National Natural Science Foundation of China (Nos.11204033, 51773093), the Natural Science Foundation of Jiangsu Province (No.BK20141397), the Research Fund for the Doctoral Program of Higher Education of China (No.20120092120042); the CMA L'Oréal China Skin Grant 2015(No.S2015121421)and the Open Research Fund of State Key Laboratory of Natural Medicines,China Pharmaceutical University(No.SKLNMKF201803),Southeast University and Nanjing Medical University Cooperation Project (No.2242018K3DN14).We would like to thank Prof.Zhao and his group in Zhongda Hospital,Southeast University for their assistance during cell culture.
Appendix A.Supplementary data
Supplementary material related to this article can be found,in the online version,at doi:https://doi.org/10.1016/j.cclet.2019.11.021.
 Chinese Chemical Letters2019年12期
Chinese Chemical Letters2019年12期
- Chinese Chemical Letters的其它文章
- CdS nanocrystallites sensitized ZnO nanorods with plasmon enhanced photoelectrochemical performance
- A simple visual method for DNA detection based on the formation of gold nanoparticles
- Self-assembly of L-tryptophan on Cu(111)studied by low-temperature scanning tunneling microscopy
- Functional delivery vehicle of organic nanoparticles in inorganic crystals
- Facile assembly of mesoporous silica nanoparticles with hierarchical pore structure for CO2 capture
- A chlorinated non-fullerene acceptor for efficient polymer solar cells
