Fluorinated Surfactants and Fluorinated Polymer Materials (i):General Review
Xiao Jinxin, Xing Hang, Dou Zengpei, Xiao Zibing
Beijing FLUOBON Surfactant Institute, China
Abstract Fluorinated surfactants and fluorinated functional materials were introduced. Their fundamental concept,classification and structure, major physicochemical properties, industrial production methods, as well as applications in industrial and high technological fields were summarized. Their current environmental problems and the countermeasures in this field were also briefed.
Key words fluorinated surfactant; fluorinated functional materials; fluoropolymer; PFOS; POPs
Fluorinated surfactants are a class of chemicals different from conventional surfactants (namely hydrocarbon surfactants or hydrogenated surfactants),in which hydrogen atoms are totally or partially substituted by fluorine atoms.[1-3]Fluorinated surfactants are also called fluorocarbon surfactants,fluorosurfactants, polyfluorinated surfactants,F-surfactants, etc. In addition, they can be called perfluorinated surfactants or perfluorocarbon surfactants if all hydrogen atoms on the hydrocarbon chain are substituted by fluorine atoms. In the case that some carbon atoms in the fluorinated carbon chain are bound to hydrogen atoms, such fluorinated surfactants can be further named as partially fluorinated surfactants or semi fluorinated surfactants.
The terminology of fluorinated surfactants is still under discussion.[1]Buck et al.[4]have put forward detailed suggestions on the naming and classification for fluorinated surfactants, however, up to now, a unified naming rule that is acknowledged has not been obtained. Surfactants, no matter fluorinated or hydrogenated, are classified in a similar way.
Owing to the specific performances of fluorinated surfactants, especially some of which are so outstanding that no parallel can be found among other surfactants, some people also call them “super surfactant”.[5]The role of conventional surfactants in industry is compared to that of aginomoto in cooking, which wins a good reputation of “industrial aginomoto”; therefore, fluorinated surfactants can be regarded as “special industrial aginomoto” or “king of industrial aginomoto”.
Fluoropolymers are the polymers in which the hydrogen atoms directly covalently bonded to the carbon atoms in the backbone or side chains are fully or partically substituted by fluorine atoms.[6]Fluoropolymer materials have many functions that ordinary polymers do not have, so they are usually called “ fluorinated functional materials”. Fluorinated functional materials have the best comprehensive performance among the polymer materials, which are often crowned with the good name of “king”. For example, fluoroplastics are called “king of plastics”,fluorine-containing rubber (fluororubber) is called“king of rubber”, and fluorine-containing coatings are called “king of coatings”.
Similarly, fluoropolymers / fluoropolymer functional materials are mainly named and classified in the same way as ordinary polymers / functional materials. In this paper, the properties, synthesis (production) and applications of fluorinated surfactants and fluorinated functional materials are reviewed.
Synthesis
The synthesis of fluorinated surfactants is relatively difficult, which is the main reason for their high prices. Generally, there are three steps in the synthesis of fluorinated surfactants: first, the compounds with fluoroalkyl groups containing 4-10 carbon atoms are synthesized, then the fluorinated intermediates that are easy to introduce various hydrophilic groups are prepared, and finally, various fluorinated surfactants are prepared by introducing various hydrophilic groups. The first step, i.e. the synthesis of fluoroalkyls is the key to the preparation of fluorinated surfactants and also the main factor that determines the cost. There are many ways to obtain fluoroalkyls in laboratory, but the methods used most widely in industrial production are electrochemical fluorination, telomerization and oligomerization (Figure 1-3). The first two methods,i.e. electrochemical fluorination and telomerization,have the largest production.
Three methods that are commonly used in industrial production are briefly described as follows.
Electrochemical fluorination
Electrochemical fluorination is to dissolve or disperse the substances being fluorinated in anhydrous hydrofluoric acid, and the electrolysis is conduct at a direct-current voltage lower than 8 V (generally 4-6 V). In the process of electrolysis, hydrogen is produced at the cathode, and organic substance is fluorinated at the anode where the hydrogen atoms in the organic substance are substituted by fluorine atoms but some other functional groups remained,such as acyl and sulfonyl. Typical examples of electrochemical fluorination are shown in Figure 1and. Starting from (2), a series of fluorinated surfactants, such as (3)~(21), can be obtained according to ordinary synthetic reactions or methods. Starting from (23), the compounds from (24) to (31) can also be obtained. In addition,starting from (26)~(30), derivatives similar to(9)~(21) can be obtained through reactions similar to (4)~(8).
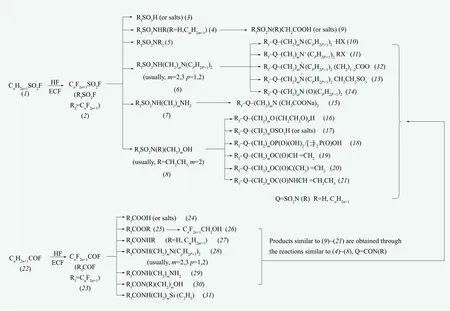
Figure 1. Production routes of principal fluorinated substances based on electrochemical fluorination (ECF) [7]
The main disadvantage of electrochemical fluorination is that its product is a mixture containing branched isomers. The longer chain in the raw material the more by-products will be obtained.Such branched isomers are difficult to be separated by ordinary separation methods. For example, a reagent company's reagent grade C8F17SO2F which is labeled as 98%, actually contains approximately 30% branched isomers (Reagent companies generally do not specify this fact, but consumers should pay attention during the purchase and application). To get products with completely linear chain, the method of telomerization needs to be used.
Telomerization
Fluorotelomers with low degree of polymerization are prepared by telomerization, using telogen (e.g.per fluoroalkyl iodide) and taxogen (e.g. tetra fluoroethylene).
Fluorotelomers can be classified into two categories according to the end groups. One is the telomerization using perfluoroalkyl iodide as telogen. The addition of different amounts of tetra fluoroethylene to telogen such as CF3I, CF3CF2I (the one most commonly used)and CF3CF2CF2I will produce longer perfluoroalkyl iodide (commonly 8-carbon). A typical route is shown in Figure 2
Due to the electron-withdrawing effect of fluoroalkyl groups, (33) is not easy to proceed nucleophilic substitution reaction directly, so it needs an addition reaction with alkene such as ethylene to produce(perfluoroalkyl)alkyl iodide, as shown in Figure 2. The most commonly seen (per fluoroalkyl)alkyl iodide is CF3CF2(CF2CF2)n-2CH2CH2I ((34),abbreviated as “n:2 FTI”). Starting from (34), various fluorinated substances, such as (35) and (38)~(45),can be prepared by using ordinary synthetic routes.Different from the products of electrochemical fluorination, the telomerization method will produce linear-chain products, which is its greatest advantage.
Another type of telomerization will generate fluorinated products with ω-H atom by using tetra fluoroethylene/hexa fluoropropylene and methanol.Typical routes are shown in Figure 2and. This method is convenient and commercializable for the synthesis of fluorinecontaining alcohols, because fluorinated alcohols are produced by the one-step telomerization using tetrafluoroethylene/hexafluoropropylene and methanol.
Although this type of reactions are relatively simple for the synthesis of fluoroalkyl groups,the surface activity of fluorinated surfactants thus prepared is poor, e.g. the values of γcmcfor the fluorinated surfactants with the terminal group HCF2(CF2)n— have generally been reported to be more than 25 mN/m. Although this kind of fluorinated surfactants are poor in surface activity,they may still meet the requirements of high thermal and chemical stability and both hydrophobicity and lipophobicity. Therefore, in the fileds where low surface tension is not required, the fluorinated surfactants with ω-H can still play the roles that are irreplaceable by hydrocarbon surfactants. For example, H(CF2CF2)4COOK is a good dispersing agent for dispersion polymerization of fluoroole fin.
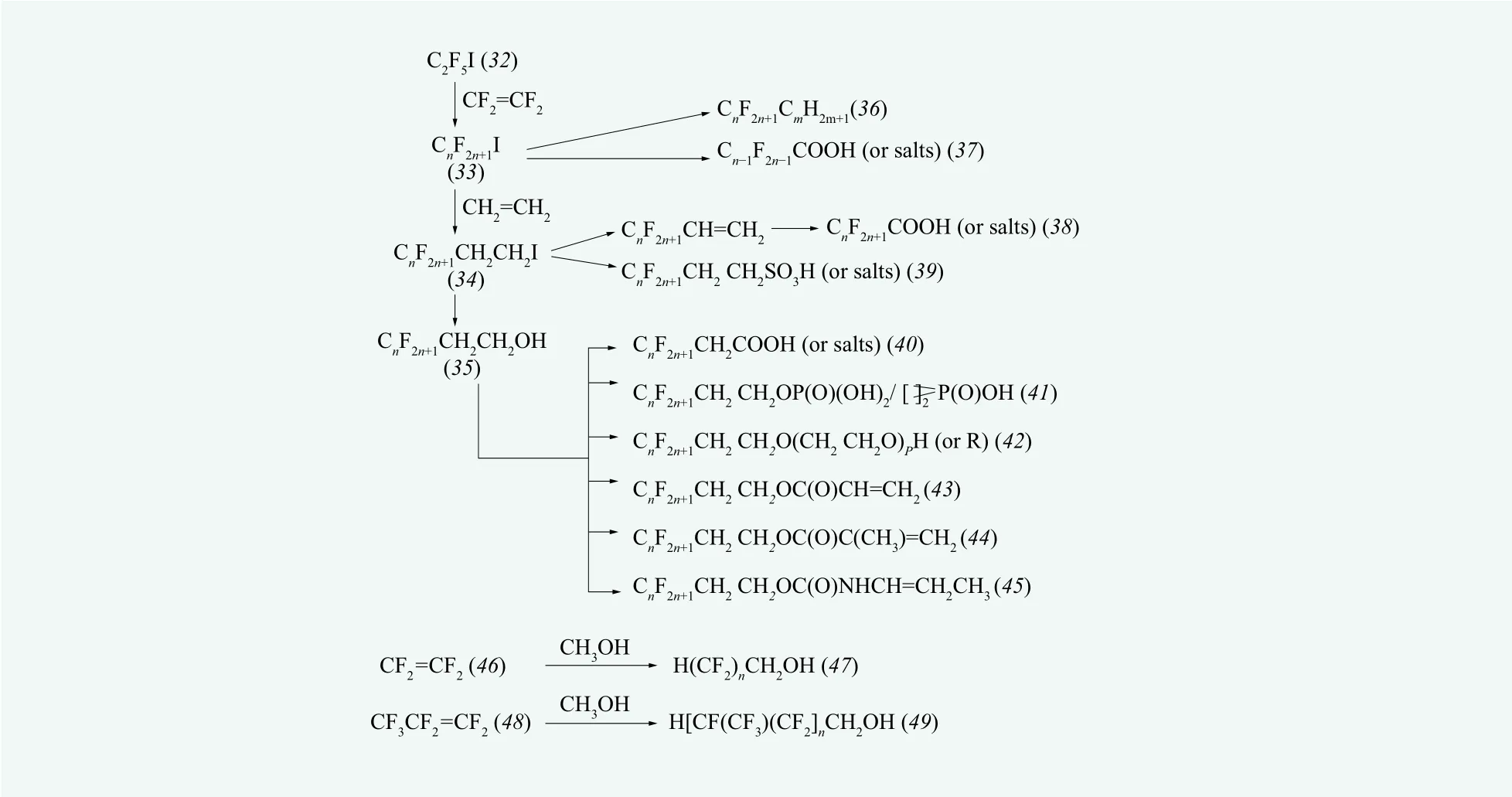
Figure 2. Production routes of principal fluorinated substances based on telomerisation [7]
Oligomerization
Highly branched fluorinated oligomers with low degree of polymerization are obtained by oligomerization of fluorolefin in aprotic solvent.Tetrafluoroethylene, hexafluoropropylene and hexafluoropropylene oxide are the most commonly used fluorolefins in oligomerization methods, as shown in Figure 3.
The main products of oligomerization of tetrafluoroethylene are tetramer, pentamer and hexamer of tetrafluoroethylene. The pentamer of tetrafluoroethylene, as shown in Figure 3 (50), is commonly used to synthesize fluorinated surfactants. The fluorine atom directly connected to double-bonded carbon atom in the molecule of (50) can be substituted with nucleophiles such as phenol in alkaline medium. An example of typical reaction is shown asA series of fluorinated surfactants can be synthesized by further introducing hydrophilic groups on the benzene ring (e.g.
The oligomerization of hexa fluoropropylene (HFP)generally produces a mixture of dimer (54) and trimer (55). The dimer of HFP has two isomers and the trimer of HFP has three isomers. Similar to the pentamer of tetrafluoroethylene, the oligomers of HFP can also react with phenol in a similar way to obtain various fluorinated surfactants similar to (52).
The oligomerization of hexa fluoropropylene oxide(HPFO) is shown in Figure 3. Starting from (57), a variety of substances can be obtained by the same routes as starting from (23).
Besides the reactions listed in Figure 1-3, the raw materials containing fluoroalkyls can also be used to prepare fluorinated surfactants of various structures by ordinary chemical reactions. This is beyond the scope of this review.
The fluorinated surfactants derived from the oligomers of HPFO are different from the common ones because they have relatively low Krafft points due to the branched structure of hydrophobic chain and the flexibility induced by ether oxygen. These fluorinated surfactants are highly surface active, and more importantly, the ether oxygen in hydrophobic chain tends to be attacked by bacteria and chemicals in the surroundings and can be degraded. Therefore, this kind of fluorinated surfactants are substitutes for C8-based ones that are being restricted by international community (in spite of the great controversies).
The preparation of fluoropolymers is similar to that of ordinary polymers. Most fluoropolymers are polymeric fluoroolefin, so the primary difference between fluoropolymers and ordinary polymers is the difference in monomers. The methods of preparing fluoropolymers are similar to that of ordinary polymers, such as bulk polymerization, solution polymerization, emulsion polymerization, dispersion polymerization, and polymerization in supercritical CO2rising in recent years. One obvious difference is that fluorine-containing monomers are usually insoluble in ordinary organic solvents. For solution polymerization, fluorine-containing solvents such as hydrofluoroalkanes and chlorofluorocarbons are generally required. For emulsion polymerization and dispersion polymerization, fluorinated surfactants are often needed as emulsifier or dispersing agent.However, the application of fluorine-containing solvents and long-chain fluorinated surfactants has been controversial in recent years due to the environmental problems. Another difference is the different initiators. Although the initiators for ordinary polymerization can also be used in the polymerization of fluoroolefins, hydrocarbon initiators are prone to introduce hydrocarbon end groups into the polymer, thus may degrade the performance of fluoropolymers. Therefore, perfluorinated initiators(e.g. [CnF2n+1C(=O)O]2) are more commonly used in the preparation of fluoropolymers. The preparation of fluoropolymers has been described in detail in literature,[6]so it won't be mentioned in this review.
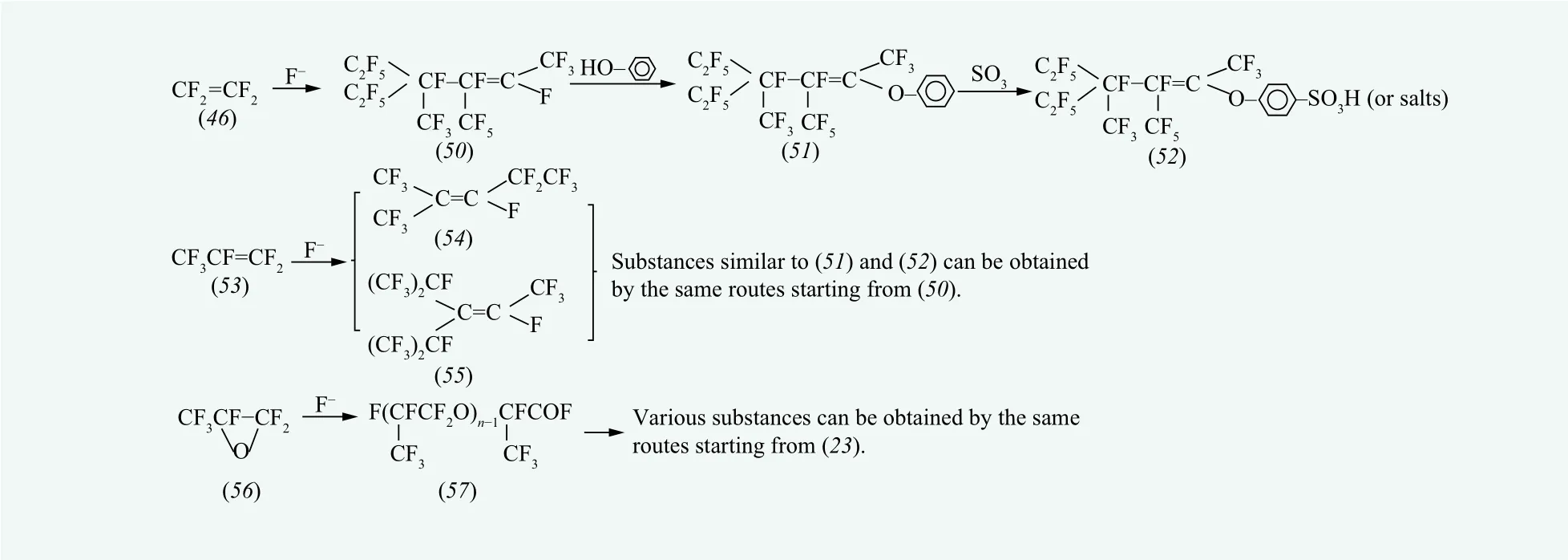
Figure 3. Production routes of principal fluorinated substances based on oligomerization [7]
Molecular structure
The type of fluoroalkyl groups makes one of the most important structural differences in fluorinated surfactants. The main types of fluoroalkyl groups are shown in Figure 1-3.
Different from hydrocarbon surfactants, the commonly used fluorinated surfactants have relatively short hydrophobic chains, and the main fluorocarbon chain generally involves no more than eight carbon atoms. The main fluorocarbon chain involving more than eight carbon atoms may lead to poor water solubility thus is usually less applied in aqueous solution. However, if the hydrophilicity is increased(e.g. increasing the number of hydrophilic groups or increasing the length of polyoxyethylene chain)so that the molecule reaches a balance between hydrophilicity and hydrophobicity, highly surface active fluorinated surfactants can also be obtained despite the long fluorocarbon chain involving more than eight carbon atoms. Especially in recent years, due to the restrictions from the international community on C8 fluorinated surfactants (Section 5 in this paper), the usage of fluorinated surfactants longer than C8 has significantly increased although there is still a great debate about this.
Most of fluorinated surfactants (except for some perfluorinated carboxylates and sulfonates) have a feature in their molecular structures, i.e. the hydrophilic group can not be directly connected with fluoroalkyl group. Usually, a linker is needed to connect hydrophilic group with fluoroalkyl group.A variety of linkers can be found, most of which contain CH2, N, S and O, etc. Typical examples of fluorinated surfactants with linkers are shown in Figure 1 ((11) and (12) which are cationic and zwitterionic ones, respectively). A large number of fluorinated surfactants, typically the ones synthesized by telomerization, have linkers which consist of one or more methylene groups between perfluoroalkyls and hydrophilic groups (Figure 2). Such kind of linkers (methylene groups) usually increase the hydrophobicity of the surfactant, but may decrease the overall lipophobicity, resulting in increasing solubilities in organic solvents and fat. Such linkers can also play a role in tuning of wetting properties of polymers. From a chemical reactivity standpoint,a -CH2CH2- linker can also be regarded as a spacer which acts as a screen between the fluorocarbon chain and the polar function, which essentially recovers its“normal” properties. A typical example is the product of telomerization (33): As mentioned above, owing to the strong electron-withdrawing effect of fluoroalkyl groups, (33) can not proceed nucleophilic substitution directly, however, if a -CH2CH2- group were inserted in the molecule, the molecule (i.e. (34)) would return to a “normal” alkyl halide, that is, the iodine atom would be available for substitution reaction to obtain various fluorinated surfactants.
The fluorinated surfactants mentioned above mainly refer to water-soluble ones. Another category is about oil-soluble ones. The molecule of the oil-soluble fluorinated surfactant consists of a fluorocarbon chain as oleophobic group and a hydrocarbon chain as oleophilic group, whose biggest characteristic is to reduce the surface tension of oil. They can be used in non-aqueous system. A typical example is semifluorinated alkanes ( (36) in Figure 2) (generally abbreviated as FnHm) which have a special name “primitive surfactant”.[8]Other examples for oil-soluble fluorinated surfactants are(4)~(8), (25)~(30), (33)~(35), and (51), etc. These products can be directly used as surfactants in nonaqueous systems (e.g., the pesticide “sulfluramid”C8F17SO2NHC2H5), and can also be precursors for the preparation of fluoropolymers (e.g., C8F17SO2N(C2H5)CH2CH2OH is the important precursor for the preparation of fluoropolymers (textile finishing agents)by 3M Co.).
Perfluorinated polymers and copolymers can be mainly classified into three categories according to the composition of backbone:
(1) Fluoropolymers and copolymers with a poly- or perfluorinated carbon-only backbone, e.g.poly(tetra fluoroethylene) (PTFE) and polyvinylidene fluoride (PVDF);
(2) F-alkylpolyethers with an oxygen-containing fluorinated backbone, possibly with side-chain CF3groups, as in those obtained from HFPO (e.g.HO[CF(CF3)CF2O]pH);

(3) Polymers with a hydrocarbon backbone and short appended fluoroalkyl side-chains such as those derived from acrylate, methacrylate or urethane monomers (as the (19)~(21) in Figure. 1 and (43)~(45)in Figure 2). This kind of fluoropolymers are widely used in water- and oil-repellents. The common water- and oil-repellents are emulsions of fluoroalkyl acrylate copolymers which are copolymerized from one or more kinds of fluorinated monomers and one or more kinds of non-fluorinated monomers by emulsion polymerization. Their structure is shown below (Figure 4).
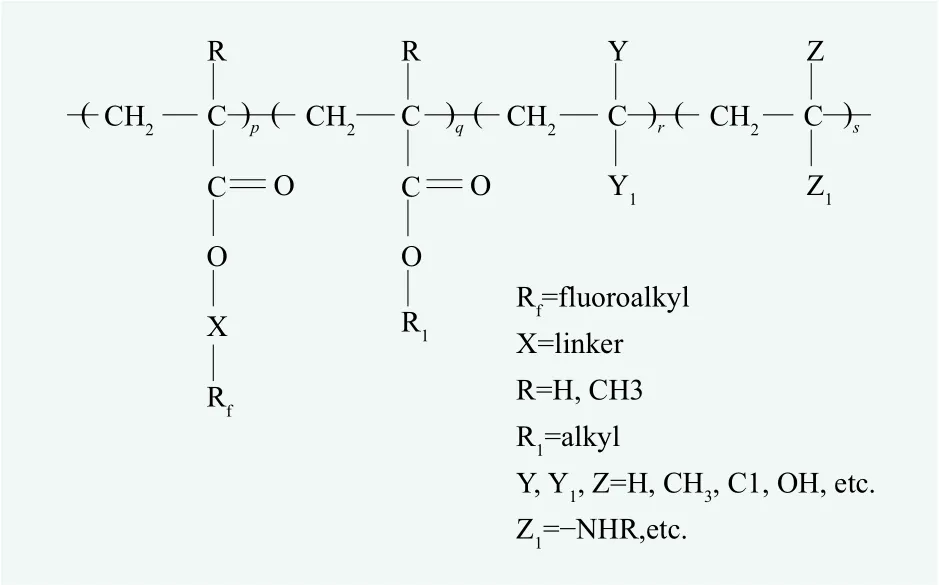
Figure 4. Molecular structure of water- and oil-repellents widely used in textile finishing agents
Water- and oil-repellents, if used for finishing the fabrics, are called fluorine-containing textile finishing agents, which can give the fabrics waterproof, oilrepellent, antifouling and stain release functions, and meanwhile can make the finished fabrics maintain the original hand feeling, air permeability, color and luster, comfortable wearing and other characteristics.Fluorine-containing textile finishing agents will be introduced as a special topic in our serial papers.
It is worth noting that some of fluorinated resins may contain ionic groups. The per fluorinated sulfonic acid resin and the per fluorinated carboxylic acid resin are quite representative. A typical structure is shown in Figure 5. This kind of fluoropolymers belong to solid superacid and they are mainly used as catalysts.A more important use is to serve as a proton exchange membrane (e.g. Na fion?film, Dupont Co.) in chloralkali industry and especially for fuel cells. These perfluorinated resins will be discussed as a special topic in our serial papers.
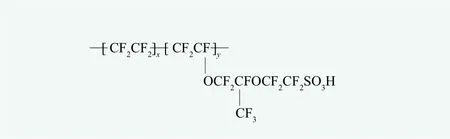
Figure 5. An example of per fluorosulfonic acid resin
Perfluoroalkanes (CnF2n+2) are both hydrophobic and oleophobic, which do not meet the definition of surfactants. However, its low-pressure vapor can significantly alter the surface tension of many liquids and produce some new surface phenomena,so it is also known as “gaseous surfactant”, “volatile surfactant” or “gas soap”. Therefore, on some occasions perfluoroalkanes are also classified as fluorinated surfactants, which subverts the traditional concept of surfactants together with the aforementioned “primitive surfactant”. Per fluoroalkanes and per fluorocarbon fluids will be introduced as a special topic in our serial papers.
Hybrid fluorinated surfactant is a variant of fluorinated surfactant which includes fluorocarbon chain and hydrocarbon chain (either on the same hydrophilic group or on the hydrophilic groups at different positions) in one molecule. Hybrid fluorinated surfactants may overcome some of the shortcomings of conventional fluorinated surfactants (e.g. poor ability in reducing oil-water interfacial tension).Since the rigidity of fluorocarbon chain leads to poor fabric softness when used in textile finishing agents,the fluorocarbon chain and siloxy group are both introduced into one molecule to obtain the fluorosiliconfluorinated surfactant or fluoropolymer.
Since the first appearance of commercially available fluorinated surfactants in 1950s, fluorinated surfactants of various structures have emerged one after another. In addition to the types of fluorinated surfactants mentioned above (i.e. hybrid surfactants,primitive surfactants, volatile surfactants and fluorosilicon surfactants), partially fluorinated surfactants,double chain fluorinated surfactants and fluorinated host-guest surfactants will be introduced as a special topic in our serial papers.
Performance
Fluorine is called an “enabler”. The fluorine atoms introduced give surfactants unparalleled performance.The properties of fluorinated surfactants can be generally summerized as: high surface activity, high thermal stability, high chemical stability, fluorocarbon chain being both hydrophobic and lipophobic.
High thermal stability
Fluorocarbon chain has high thermal stability and can be used in high temperature environments.Most importantly, fluorinated surfactants and fluoropolymers (such as fluororubber) can usually retain their properties at high and low temperatures and resist ultraviolet radiation, allowing them to perform at harsh conditions. It is worth noting that the thermal decomposition temperature of the shortchain per fluorocarboxylic acid (or salt) is lower, and the products of thermal decomposition are mainly perfluoro α-olefins.[9-11]This provides a laboratory method for the preparation of per fluoro α-ole fins (e.g.,tetra fluoroethylene, HFP, etc.).
High chemical stability
The fluorocarbon chains in fluorinated surfactants or fluoropolymers have excellent reaction tolerance for strong acid, strong base, oxidants and reductants(including at high temperatures). Thus in the extremely harsh environment they maintain their surface activity, film-forming ability, elasticity, etc., while other organic materials are unable to resist. Moreover,the presence of a number of fluorine atoms in carbon chain leads to the inactivation of some potential functional sites (such as oxygen, nitrogen, chlorine or bromine atoms, etc.) in the molecules. What is more peculiar, some fluorinated surfactants show higher surface activity in strong acidic medium or strong alkaline medium than in normal aqueous solution due to the increased solubility.
It should be pointed out that the stability of fluorinated surfactants mentioned here mainly refers to perfluorocarboxylic acid, perfluoroalkyl sulfonic acid and their salts. For other types of fluorinated surfactants, the “high stability” mainly refers to the fluoroalkyl segment while the stability of the nonfluoroalkyl segment depends on the stability of its structure. In most cases, even if the non- fluoroalkyl segment were destroyed, the products generated would be perfluorocarboxylic acid, perfluoroalkyl sulfonic acid and its salts, which are still fluorinated surfactants.
High surface activity
Fluorinated surfactants are by far the most surface active ones of all surfactants, some of which show the lowest surface tension (γcmc) and critical micelle concentration (cmc) in aqueous solution, which are much lower than that of corresponding hydrocarbon surfactants.
The minimum surface tension of fluorinated surfactants in aqueous solution can be below 20 mN/m or even ~15 mN/m. In contrast, the minimum surface tension for most of hydrocarbon surfactants can merely reach the level of 30~35 mN/m; the mixtures of caioinic-anionic hydrocarbon surfactants can reach 24~25 mN/m; while the γcmcof hyperbranched hydrocarbon surfactants have been reported to be as low as 23.8 mN/m.[12]The aqueous solution of silicone surfactants can reach γcmcvalues of ~20 mN/m.
By reducing surface tension and interfacial tension, fluorinated surfactants are useful in spreading,dispersion, emulsification, and adsorption on solid or certain liquid particles. It is worth noting that owing to the low surface tension of aqueous solution of fluorinated surfactants even at a very low concentration, an aqueous film can spread on the oil surface, which is the foundation of aqueous film-forming foam (AFFF), a highly effective extinguishing agent for oil fire.
In terms of the efficiency of reducing surface tension, generally fluorinated surfactants are used at the level of 1/10000, while silicone surfactants are used at the level of 1/1000 and hydrocarbon surfactants are used at the level of 1/100. The higher cost of fluorinated surfactants can be offset by their greater effectiveness and higher efficiency than other surfactants.
The oleophobicity of fluorocarbon chain
Unlike the hydrocarbon chains being hydrophobic but oleophilic, fluorocarbon chains are both hydrophobic and oleophobic. This makes fluorinated surfactants suitable for use in organic solvents (oils) to reduce surface tension, whereas hydrocarbon surfactants will not work. It is worth noting that fluorinated surfactants also have surface activity in supercritical carbon dioxide, which makes fluorinated surfactants widely used in this field (mainly used as emulsifiers).
Other features induced by fluorocarbon chain
In addition, the fluorocarbon chain has strong influence on the behaviors of hydrophilic terminal groups and the linking group between fluorocarbon chain and hydrophilic group, as listed below:
(1) Due to the high electronegativity of fluorine atoms, fluoroalkyl groups are strong electron with drawing groups, so per fluorinated carboxylic acids and per fluorinated sulfonic acids are both strong acids.
(2) The introduction of perfluoroalkyl group has also strengthen the acidity of aromatic acids and phenols, and made the substances containing active hydrogen in amides and alcohols being acidic.
(3) For substances containing basic groups, such as amines, the introduction of fluorine atoms or perfluoroalkyl groups in the molecules will significantly reduce their alkalinity due to the strong electron-withdrawing effect of fluorine atoms.
Moreover, the introduction of the fluorocarbon chain also leads to the change of a number of physical properties of compounds, as listed below:
(1) The intermolecular attraction of per fluoroalkanes is obviously weaker than that of alkanes. Therefore,perfluoroalkanes are as volatile as, or even more volatile than their counterparts of alkanes, although their molecular weights are more than twice as large as the alkanes'. Actually, some neutral fluorinated surfactants are more volatile than their corresponding hydrocarbon surfactants.
(2) Weak intermolecular interactions also enhance their ability to dissolve gases (such as Ar, O2and CO2), especially fluorocarbons' excellent ability to dissolve oxygen, making them candidates for artificial blood (blood substitutes).
(3) “Gas-like”, weak intermolecular cohesion also results in very low solubility in highly structured liquids such as water. Introducing a fluorocarbon chain in a molecule reduces not only its solubility in water but also in lipids. Consequently, fluorocarbon chains tend to separate from alkyl chains as well as from hydrophilic chains, moieties and functions, and tend to exclude both hydrophilic and lipophilic solutes.
(4) Fluorocarbon chain has low refractive index(close to or lower than water), which once makes it difficult to study fluorinated surfactants by light scattering method.
(5) The aptitude of fluorocarbon chains for ordered packing leads to higher melting points and thus to narrower liquid phase domains.
The “mutual phobicity” between fluorocarbon chain and hydrocarbon chain is noteworthy, as shown by the segregation in the micelles and monolayers formed by a mixture of fluorinated and hydrogenated surfactants. The hydrophobicity, lipophobicity, rodlike shape and rigidity of fluorocarbon chain concur to endow fluorinated surfactants with the power for segregation and self-assembly into discrete supramolecular constructs and thin interfacial films,as well as for compartmentation at the molecular level.[7]Fluorinated monolayers can be much thinner,yet more stable and less permeant than those obtained with nonfluorinated surfactants.[7]The thin film formed on the solid surface can be water and oil repellent. This will be covered in more detail in future as a special subject in our serial papers.
Application
Fluorinated surfactants and polymers / functional materials have outstanding, unique physicochemical properties. Being highly hydrophobic and oleophobic,they exhibit excellent surface properties. Their thermal stability and chemical inertness under extreme conditions enable them to perform under harsh conditions. The aptitude for segregation and self-assembly is able to obtain sturdy water and oil repellent film. Their unique spreading, dispersing,emulsifying, anti-adhesive and levelling, dielectric,piezoelectric and optical properties enable them to be widely used in numerous industrial and technical uses and consumer products. Especially in some special applications, their roles are irreplaceable by other surfactants. In future articles of this series,the applications of fluorinated surfactants and fluoropolymers, as well as some specific industrial applications, will be introduced in detail. In this paper, only the general application scope will be briefly introduced.
The application of fluorinated surfactants has the following significant characteristics:
(1) In the occasion where ordinary surfactants are used, the use of fluorinated surfactants can enhance the performance of the product. For example, the fracturing cleanup additives in oil field, wetting agents for inks and paints, additives for photographic film coating, detergents, and leveling agents for paints and coatings.
(2) Fluorinated surfactants can be used in some extreme conditions where ordinary hydrocarbon surfactants are not able to be used or poorly used.For example, the chromium mist inhibitor in electroplating, soldering flux, the fracturing cleanup additive in oil field, and the additive for alkaline battery electrolyte.
(3) The oleophobicity of fluorocarbon chain makes the fluorinated surfactant capable to be used in organic solvents (oils), e.g. the emulsifier in the emulsion polymerization of fluoroolefins,the emulsifier for artificial blood, the additives for paints, coatings and inks; fluorinated surfactants can also be used in oil recovery, extraction process,microemulsion, micellar catalysis, fluoroprotein foam(to reduce oil-carrying capacity of the solution or foam of extinguishing agent), etc.
(4) The hydrophobicity and lipophobicity of fluorocarbon chain lead to repellence to almost everything non-fluorinated, such as water, fat, oil,dirt and microorganisms, thus they erect effective protective barrier films. Therefore, they are applied in water- and oil-repellent and anti-fouling finishing agents for textile and paper, the antifoggant for plastic film, the fluorocarbon coatings, the fluororubber,and the surface modification agents for paper, metal,glass, ceramic, leather, plastics, etc.
(5) The lubrication of fluorocarbon chain (being anti-adhesive) enables it to be applied as the mold release agent in plastic processing, the lubricant for magnetic recording materials, etc.
The prices of fluorinated surfactants are relatively high, so that at present they are mainly applied in the fields where hydrocarbon surfactants are not competent or have poor performance; Most fluorinated compounds have been developed in response to the special requirements of military industry and high technology, and their applications in these fields are largely unknown to the public due to commercial and military confidential requirements. From another point of view, this has also seriously restricted the development of fluorinated compounds.
Research results have shown that the dosage of fluorinated surfactant can be reduced while its surface activity can be maintained by mixing fluorinated surfactant with hydrogenated surfactant. If oppositely charged hydrogenated and fluorinated surfactants are mixed, not only the dosage of the fluorinated surfactant can be greatly reduced, in some special circumstances,the mixture even has a higher ability to reduce the surface tension, that is, to achieve synergism.
Fluorinated functional materials are the materials with the most excellent comprehensive properties among polymer materials, which have outstanding heat resistance, chemical corrosion resistance,durability, weather resistance, solvent resistance,resistance to kinds of acid and alkali, low flammability,high transparency, low friction, low refractive index, low capacitance, low surface energy (neither lipophilic or hydrophilic), low hygroscopicity and strong oxidation resistance. Compared with common polymer materials, fluorine-containing materials show special properties and are often used in key positions to resist harsh environment and play a special role.They are indispensable functional materials in many fields and in modern science and technology.
At present, the application of fluorinated surfactants and fuoropolymers almost covers all fields of industry. In addition to conventional industrial/technological applications and consumer products,they are increasingly used in the fields of safety,energy and resources sparing, high-tech applications,and research tools; in recent years, they have also shown potential in the field of theranostics. Typical examples of applications are aqueous film-forming foam, fluoroprotein foam, chromium mist inhibitor,textile finishing agent, fluorocarbon coating, film release agent, artificial blood, supercritical carbon dioxide extraction, etc. It is worth mentioning that many of their important and unique mechanical,electrical, electronic, optical behaviors related to the “extreme” characteristics are achieved in hightech products. However, as mentioned above,many military and high-tech applications remain confidential. It can be said that the development level of fluorinated surfactants and fluoropolymers in a country basically represents the core competitiveness of the country.
Environmental and safety problems: the problems and countermeasures of PFOS
One of the most important characteristics of the fluorocarbon chain is its high thermal and chemical stability, but because of this, it is persistent and difficult to degrade in the environment. Long-chain fluorinated compounds are found bio-accumulative in food chains and have long half-life in human blood,which are considered as persistent organic pollutants(POPs). This brings about the environmental and safety problems of fluorinated compounds, i.e. the problem of PFOS.
The generic term of PFOS means a set of fluorinated compounds including C8F17SO2F, C8F17SO3H and their salts and derivatives. In recent years, substances that can degrade into perfluorooctane sulfonic acid or perfluorooctane sulfonate through biodegradation have also been classified as PFOS (regardless of the structure of the parent compound). Some literature and institution also call these fluorinated compounds“PFOS-related substances”. The per fluorinated alkyl chain in the molecule of PFOS contains eight carbon atoms. PFOS is a representative of “C8” compounds.Besides PFOS, the term of “C8 compounds” also includes the telomer such as C8F17CH2CH2I, C8F17CH2CH2OH and related derivatives.
PFOS have been in the list of Persistent Organic Pollutants of the Stockholm Convention since 2009.[4,7]Since then, the issue of PFOS raises related research and discussion (and even debate) globally.[5,13-17]The next in our serial papers will cover the problem of PFOS in detail.
Although the real harm of PFOS to the environment and human is still in the debate, if there is no clear positive conclusion, the international action for elimination of PFOS will become inevitable. This presents a dilemma for fluorinated surfactants and polymers: On the one hand, fluorinated surfactants are irreplaceable by other surfactants in many fields,e.g. aqueous film-forming foam (AFFF). Under current technical conditions, it is not possible for AFFF to completely exclude the use of fluorinated surfactants. On the other hand, the harm of PFOS to the environment has greatly limited their application.
The most urgent problem is to seek alternatives to PFOS, i.e. to develop fluorinated surfactants that do not have characteristics of POPs.
So far, to solve the environmental and safety problems caused by fluorinated surfactants, the following aspects can be started:
(1) Reducing the concentration of PFOS. Through the synthesis of fluorinated surfactants with high performance or the formulation with hydrocarbon surfactants, the working concentration of PFOS may be reduced to below the limit of possible risk to the environment, so that PFOS can still be used.
(2) Developing short-chain fluorinated products.According to the mechanism of surface activity of surfactants, the reduction of surface tension mainly depends on the structure of the outermost group of the surface adsorption layer. By changing the structure of fluorinated surfactants, shortening the length of fluorinated segments and attaching other groups,fluorinated surfactants with high surface activity and meanwhile with reduced harm to the environment may be obtained. It has been reported that the accumulation in human body can be neglectable when the fluoroalkyl group contains four or less fluorinated carbons.
(3) Replacing the perfluorinated segments with non-perfluorinated segments. It is also an effective way to avoid producing PFOA (perfluorooctanoic acid and derivatives) or PFOS by using a non-per fluorinated chain instead of a per fluorinated chain. Zaggia et al. have summarized the research progress related to this kind of products with non-perfluorinated chain.[5]
Future articles in our serial papers will introduce the research progress of PFOS substitutes in detail,especially the ones with short fluorocarbon chain.
It should be pointed out that China is now in the forefront of the problem of PFOS and has been blamed a lot. One of the main accusations is that American companies such as 3M stopped producing PFOS in 2002, while China took the opportunity to launch PFOS production projects on a large scale. Because China basically produces raw materials (perfluorooctane sulfonyl fluoride)which are exported to other countries and thereafter made into various terminal products, China not only makes little pro fit in the whole value chain, but also becomes the “scapegoat” for the problem of PFOS.This will be discussed in detail in our serial papers,and recommendations will be proposed therein.
Problems and challenges
Fluorochemical industry represents the chemical industry level of a country. Fluorinated surfactants and fluorinated functional materials are at the top of fluorochemical industry as well as the top of the whole product chain in chemical industry,which is one of the representatives of a country's core competitiveness. Fluorinated surfactants and fluorinated functional materials are high-end chemical products, priced in grams or kilograms,with extremely high profits. China's fluorochemical industry was developed in response to military needs.After more than 50 years of development, China's fluorochemical industry has made remarkable achievements. It can be said that China is a big country of fluorochemical industry, but unfortunately,China is a “weak” country of fluorochemical industry because China's fluorochemical industry is basically primary, and fine fluorine-containing chemicals such as fluorinated surfactants and fluorinated functional materials are very few, which have been monopolized by Europe, America and Japan. In general, China's fluorinated surfactant industry is still in the stage of tracking and imitation. In China, the proportion of independent research and development and original technology is low. There is still a big gap between China and the advanced foreign countries. Because the investment in scientific research is insufficient,high-quality products still need to be imported to meet special requirements.
One of the main problems for fluorinated surfactants and fluoropolymers is the lack of available data on physicochemical properties. On the one hand, they have strong application background in serving commercial and military purpose so that their informations are confidential; on the other hand, there is a lack of reagent grade raw materials. At present, most of the published reports on fluorinated surfactants and fluoropolymers are patents, and the research on physicochemical properties is much less. In contrast, the literature on fluorinated surfactants and fluoropolymers in environmental science and toxicology has grown explosively in recent years. Since the first report revealed that fluorocarbon compounds had been detected in locations all around the globe, the literature on the subject has become colossal, with over 400 papers published per year.[4,17]More than 2,500 relevant papers were published in 2001 alone,[14]but unfortunately, they were largely negative.
In China, the existing problems are embodied in the following aspects:
(1) Raw material production dominates, but deep processing has been ignored, and products of fluorinated surfactants are few;
(2) Lack of serial products. The product categories are few;
(3) Lack of reagent grade products;
(4) Low level repeated construction. Production technology is identical;
(5) Lack of end formula products. Application research lags;
(6) Low level of circular economy. The comprehensive utilization of by-products is poor;
(7) Talent shortage in the field of fluorinated materials. Up to now, there is no major of fluorinated materials or even relevant elective courses in Chinese universities. The research on fluorinated materials is only scattered in some national projects, without any systematic fundamental research, application research or engineering technology research.
We have discussed these problems in “Yearbook of China's Surfactant Industry (2012)”.[18]These problems will be analyzed in detail and corresponding solutions and suggestions will be proposed in our serial papers.
Problems are opportunities. The key is to weigh the pros and cons. We should not discard the huge bene fits for fear of slight risk that is debatable and can possibly be overcome. As a research worker on surfactants, we should further study the physicochemical properties of fluorinated surfactants and fluoropolymers, especially the relationship between structure and properties, in order to seek the ones with good performance but no harm to the environment.
 China Detergent & Cosmetics2019年1期
China Detergent & Cosmetics2019年1期
- China Detergent & Cosmetics的其它文章
- The Methodology of Cosmetics Efficacy Evaluation to Provide Scientific Support for Soothing Skin Efficacy Claims
- Determination of Bemegride and its Salts in Cosmetics by HPLC and Their Verification by HPLC-MS/MS
- Research on the Preparation of an Anti-Acne Cream and its Thermal Stability
- Research Progress on the Relationship between Skin Microbial Flora and Skin Health
- Determination of 24 Allergens in Perfume with High Performance Liquid Chromatography Tandem Mass Spectrometry
- Comparison of HPLC-DAD and GC-FiD Methods for Determination of 6-Methyl Coumarin in Cosmetics
