Suppression of gold nanoparticle agglomeration and its separation via nylon membranes☆
Ayyavoo Jayalakshmi,In-Chul Kim ,Young-Nam Kwon ,2,*
1 School of Urban and Environmental Engineering,Ulsan National Institute of Science and Technology,Ulsan 689-798,Korea
2 KIST-UNIST Ulsan Center for Convergent Materials,UNIST(Ulsan National Institute of Science and Technology),Korea
3 Environment and Resources Research Center,Korea Research Institute Chemical Technology,Daejeon 305-606,Korea
1.Introduction
Particle contamination in the semiconductor manufacturing can cause low-throughput,high defects and minified executions through micro-lithographic processes[1-4],and thus the control of the contamination has become a critical issue,especially,in integrated circuits and lab on chips[5,6].Various micro-lithographic stages have higher complications to accomplish several layer circuits in the fabrication of compact semiconductor chips[7,8].Generally,the contamination in micro-lithographic process by soft nanoparticles on the wafer surface leads to the microbridge defects of integrated circuits[9,10],and this has been addressed by preventing the deleterious particulate contamination with application of polymeric membranes[11-13].
For evaluation of polymer membranes use of gold nanoparticles has several bene fits due to their highly detectable characteristics using optical method, fine particle size distribution,high stability,and innocuous property[14,15].Virtually,all nanoparticles can agglomerate or precipitate and the degree of agglomeration or precipitation is determined by several factors such as pH,concentration and temperature[16,17].According to the DLVO(Derjaguin-Landau-Verwey-Overbeek)theory,the agglomeration of nanosized colloidal particles occurs due to the decrease of surface potentials[18].In this scenario,the electric double layer plays a fundamental role in the stabilization of colloidal particles,where the higher ionic strength of the solution compresses the double layer and shrinks the electrostatic repulsion,extending an irreversible cluster of nanoparticles[19,20].Nylon membranes have the remarkable property being more solvent permeable and selective to the nanoparticles.However,the gold nanoparticles,which have been used for the evaluation of the membranes,can easily aggregate atthe nylon membrane surface and making itdifficultto make a precise evaluation.Using a preventive ligand,the gold colloid aggregation can be reduced[19,21].These ligands such as thiomalic acid,thioglycolic acid,thiosalicylic acid,1-thioglycerol,2-sulfanylethanol,1-sulfanyl-2-propanol,isobutanol-2-amine,and serinol can be used[22,23].Burgesset al.also showed that citric acid acted as a reductant and stabilizer for gold colloids[19].They demonstrated thatthe thermodynamic description required for single gold crystals(two-dimensional gold surface)could be extended to nanosized three-dimensional gold sols.Kimmuraet al.proposed mercaptosuccinic acid(MSA)to disperse gold nanoparticles[24].The function of ligand was shown to modify the surface of gold colloid(change of zeta potential and ionic strength)and to reduce the interaction between the particle and surface medium.
The aim of this research is to understand the fundamental behavior of 2-amino-2-hydroxymethyl-1,3-propanediol(ADP)ligand in gold nanoparticle solution for a better evaluation of the nylon membranes and to utilize the ligand to prevent gold colloid clusters(i.e.between two particles and among the gold colloid and membrane surface).For the study,the nylon ultra filtration membranes were fabricated by the non-solvent induced phase separation(NIPS)method.The surface characteristics were investigated using various analytical tools,and the prepared nylon membrane was used to separate gold nanoparticles(20 and 50 nm).The effectofADP and pHvalues on the rejection ofgold colloid was investigated,and the interparticle interaction energy was also calculated.The manipulation of gold colloids accumulating on the membrane surface was correlated with a microscopic study using scanning electron microscopy(SEM),transmission electron microscopy(TEM),and confocal laser scanning microscopy(CLSM).The impedance study of the prepared membrane,particle size and zeta potential of the gold colloids was also demonstrated.
2.Experimental
2.1.Materials
Nylon 6,6 polymer was purchased from BASF and used without any pretreatment.Formic acid,lithium chloride(LiCl),and ethanol were purchased from Sigma-Aldrich(MO,USA)and were allused as received.Gold nanoparticle(~20 nm and~50 nm)solutions were procured from PBI solution.2-Amino-2-hydroxymethyl-1,3-propanediol(ADP)was purchased from Sigma-Aldrich,Korea and used as ligand for the gold nanoparticle.Deionized water(DI water)(Milli-Q Advantage A10,Millipore Corporation)was used to prepare all the aqueous solutions.
2.2.Membrane preparation
The dope solution formembrane preparation was formulated by dissolving(28 wt%)nylon 6,6 polymer in the solvent mixture containing formic acid(61 wt%),LiCl(3 wt%)and ethanol(8 wt%).The nylon membrane was prepared by non-solvent induced phase separation(NIPS)method(80%of relative humidity)and named Nylon A(NA).After solvent evaporation,the membrane was immersed in distilled water.
2.3.Characterization
Hydrophilicity of the membrane was evaluated by contact angle measurements(Phoenix300 Plus,Surface and Electro Optics Co.Ltd.,Korea)using the sessile drop method.The wetting energy(We),work of adhesion(Wa)and spreading co-efficient(Sc)were calculated using measured contact angle θ values and γ is surface tension of water(Eqs.(1)-(3))

The cross-sectionalimages ofthe membranes were observed by field emission scanning electron microscopy(FESEM Nano230,FEI,USA).To reduce image artifacts caused by the electrostatic charge,allthe samples were Pt-coated at20 mAand 0.2 Pa for 60 s using a Turbo Pumped High-Resolution Chromium Sputter Coater(K575X,EMITECH,Germany).The surface morphologies of the nylon membranes were examined using a Multimode V(Veeco,USA)atomic force microscope(AFM)capable of imaging at vertical lateral resolutions of 0.01 nm.High resolutiontransmission electron microscopy(TEM)of gold nanoparticles and goldfiltered membranes was performed in bright field mode at 80 kV using a Hitachi H-7650 TEM,and the images were acquired with an Olympus Cantega 11 megapixel digital camera.CLSM was used to analyze the surface morphology and roughness of the membrane(CLSM,OLS-2000,Olympus,Japan),and the membrane surfaces were imaged at a scan size of 10 μm × 10 μm and the surface roughness was measured.
Particle size and zeta potentialofgold nanoparticle were measured by a sub-micron size and zeta potential measuring system(Malvern,UK).
2.4.Gold nanoparticle separation
The permeation experiments were performed using membrane cells with an exposed membrane area of 5.30 cm2under dead end filtration mode.An applied pressure of 0.1 MPa for the particle separation was generated by nitrogen gas,and the flow rate of feed solution was 1.0 L·min-1.The valve located at the end of the cellwas used to pressurize the feed solutions and control the feed pressure.Gold nanoparticle(20 and 50 nm)solutions were used for the separation studies.Filtration through each membrane was carried out independently,and the concentration of the feed solution was kept constant during the membrane filtration.The rejection was estimated using an inductively coupled plasma-optical emission spectrometer(ICP-OES).The percentage of the solute rejection was calculated using Eq.(4).
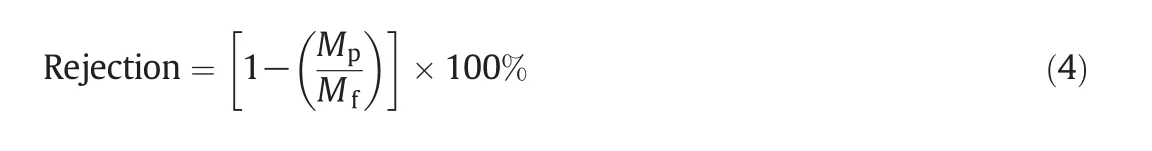
For Eq.(4),MfandMpare the concentration of solute in the feed and permeate solution,respectively.
Interparticle interaction energy of gold nanoparticles(assuming spherical shape)was calculated by using van der Waals attraction(DLVO theory)between two particles[18].
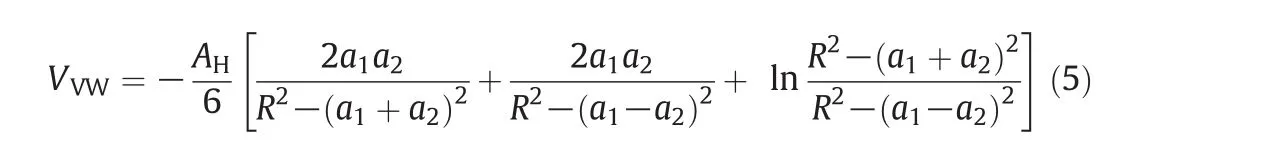
For Eq.(5),AHis the Hamaker constant(2.5×10-19J where an average was used),a1anda2are the radii of the particles,andRis the distance between the centers of two particles.
3.Results and Discussion
3.1.Nylon membrane preparation
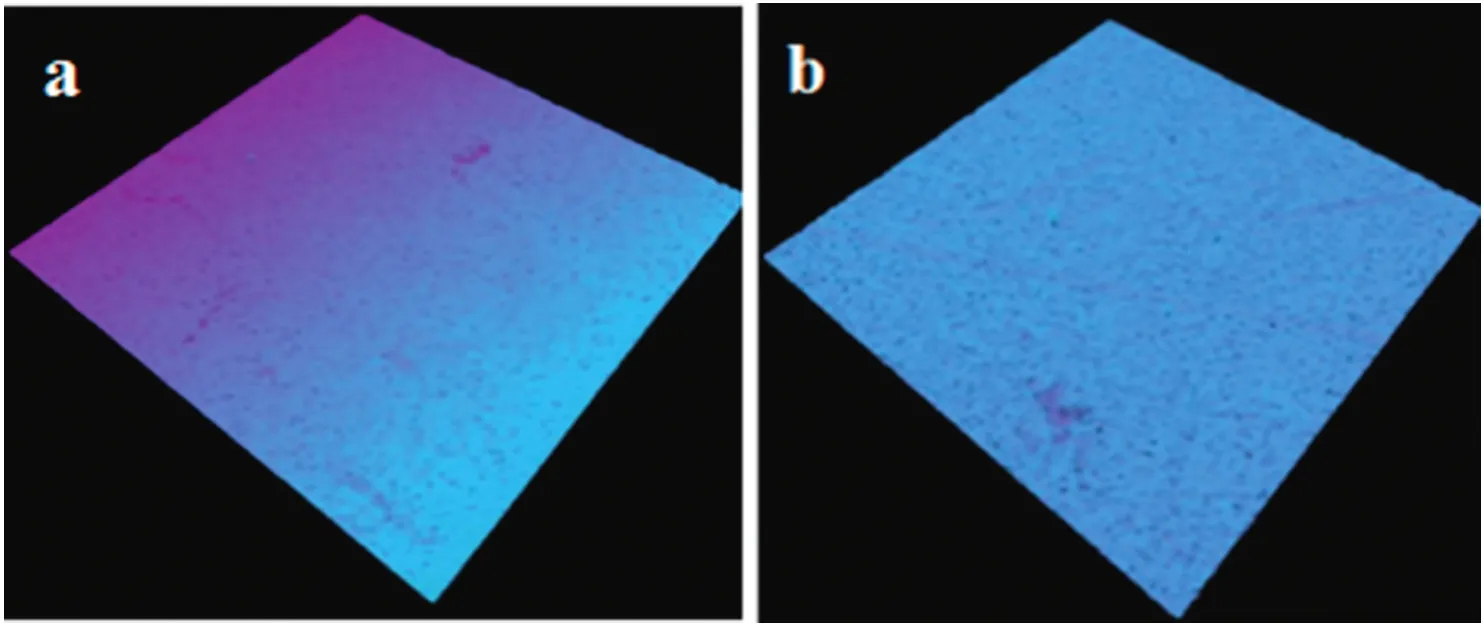
The nylon membrane(NA)was fabricated by immersion precipitation method with~0.05 mm thickness.SEM micrographs of nylon membranes are shown in Fig.1.The dense layer was observed in the cross sectional SEM images,and the microporous support layer present in middle part of the membrane showed a partially anisotropic structure.Spongy-like structure was visible in the nylon membrane on the exterior side in lower magnification,whereas Darcy structure was observed athighermagnification.The thread-like structure and multiform of ovoid structure were noticed in the back side of the membrane.Fig.2a shows 3-D CLSM fluorescent images of NA.The homogeneity structure was exhibited on the skin layer of membrane surface in NA membrane.In AFM,two-dimensional(2-D)and three-dimensional(3-D)micrographs were included to show the membrane valley and nodules(Fig.2b and c).Surface morphology of NA membrane showed surface roughness and membrane nodules.Surface roughness calculated from AMF showed slightly higher values compared to CLSM surface roughness(Table 1).Various surface factors(wetting energy,work ofadhesion,and spreading co-efficient),determining the performance/fouling of the prepared NA membrane,were calculated by contact angle values as shown in Table 2.
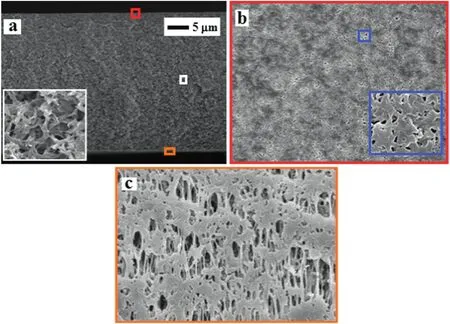
Fig.1.Scanning electron micrographs of nylon membrane(NA):(a)the cross-section images of NA with thickness(~50 μm)in the magnification of 1.0 k,(b)top surface of NA with magnification of 3.0 k,(c)the bottom structure of membrane support with magnification of 10.0 k.
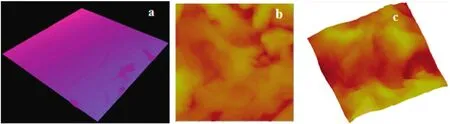
Fig.2.Confocal laser scanning micrographs and atomic force micrographs of NA membrane:(a)3-D display of CLSM images of NA membrane,(b)and(c)2-D and 3-D display of AFM images of NA membrane.All membranes were scanned at 1 μm(scan size).
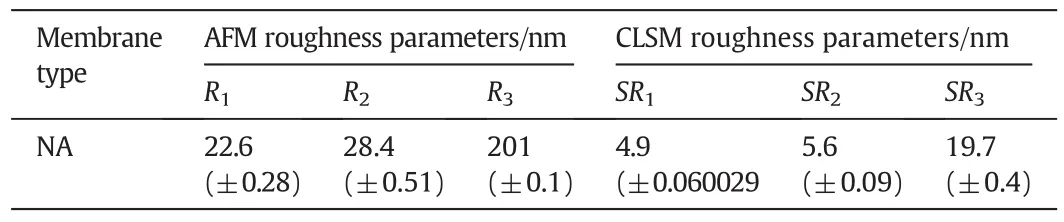
Table 1Comparison of surface roughness parameters from AFM and CLSM
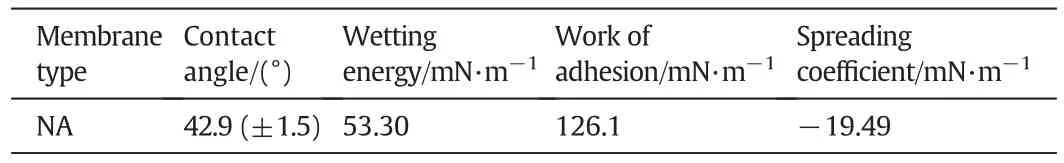
Table 2Various surface factors calculated from contact angle values
3.2.Rejection study of gold nanoparticle
The feed of gold nanoparticle solution was prepared by adding ADP as ligand.Incorporation of ADP in feed solution was substantial because nylon membrane had a highly positive polarity group(amide linkage)and easily absorbed several colloids dispersed in liquid with a high negative charge.The amine group present in the ligand can bind the gold surface particle and the hydroxyl groups in the ligand can form hydrogen bonds with water molecules,hindering the agglomeration and dispersing the gold nanoparticles in the solution.The behavior of ADP ligand in gold nanoparticle solution was schematically represented in Fig.3.The particle size of the gold nanoparticle solution with ADP was observed using a zeta-sizer analyzer.The particle size of the gold nanoparticles is shown in Fig.4.The addition of ADP solution slightly increased the size ofgold nanoparticles by 2.5-2.6 nmdue to the hydration of ADP bound to the nanoparticles.
The separation of gold nanoparticle using nylon membrane was analyzed using inductively coupled plasma with optical emission spectroscopy(ICP-OES).The percentage rejection values of the gold colloid(20 and 50 nm without ADP)were 88.6%and 94.7%,respectively,for NA membrane at neutral pH(Fig.5).For the gold nanoparticles of 20 and 50 nm with 1.0 mmol·L-1ADP solution,the rejection percentages were 93.5%and 99.8%,respectively.Nylon membrane exhibited higher separation of gold nanoparticles in the presence of ADP due to higher complexation of gold nanoparticle with ADP.The ADP in gold solution had sufficient energy barrier to prevent the aggregation of gold particles.The gold colloid separation efficiency of nylon membrane showed higher capability at neutral pH.
3.3.Effect of pH on gold rejection
The rejection of gold nanoparticles was observed in various pH values from 4 to 14 using the NA membranes,and the data are shown in Fig.6.At pH 4,the percentage rejection of gold nanoparticle(20 and 50 nm)with ADP was showed 98.16%,and 99.45%,respectively.For increasing pH,the rejection percentage slightly decreased at pH 6,then increased at pH 8,and slightly decreased at yet a higher pH.The prepared nylon membrane shows relatively stable rejection in all pH range.The change in rejection percentage was likely due to change in the zeta potential and ionic strength of the gold solution(Table 3).With increasing pH,the surface of gold nanoparticles became more negative and also the ionic strength of the solution changed due to the acid or base added as they reached the specific pH values.The ionic strength of gold nanoparticle solution at pH 6 was lowest and thus the decline of ionic strength might have decreased the rejection at a given pH.This con firmed that the gold nanoparticles were stably dispersed since the ADP energy barrier was highly sufficientto prevent agglomeration of gold colloids.
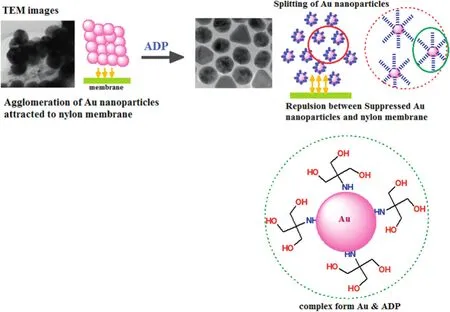
Fig.3.Schematic representation ofsuppression ofgold colloids cluster using ADP during membrane filtration.Agglomeration ofgold nanoparticles adsorbed in nylon membrane surface in TEM images.The ADP amino group provides a strong electro-attractive force to the gold colloids and gold-ADP complex is repulsed by the nylon membrane.
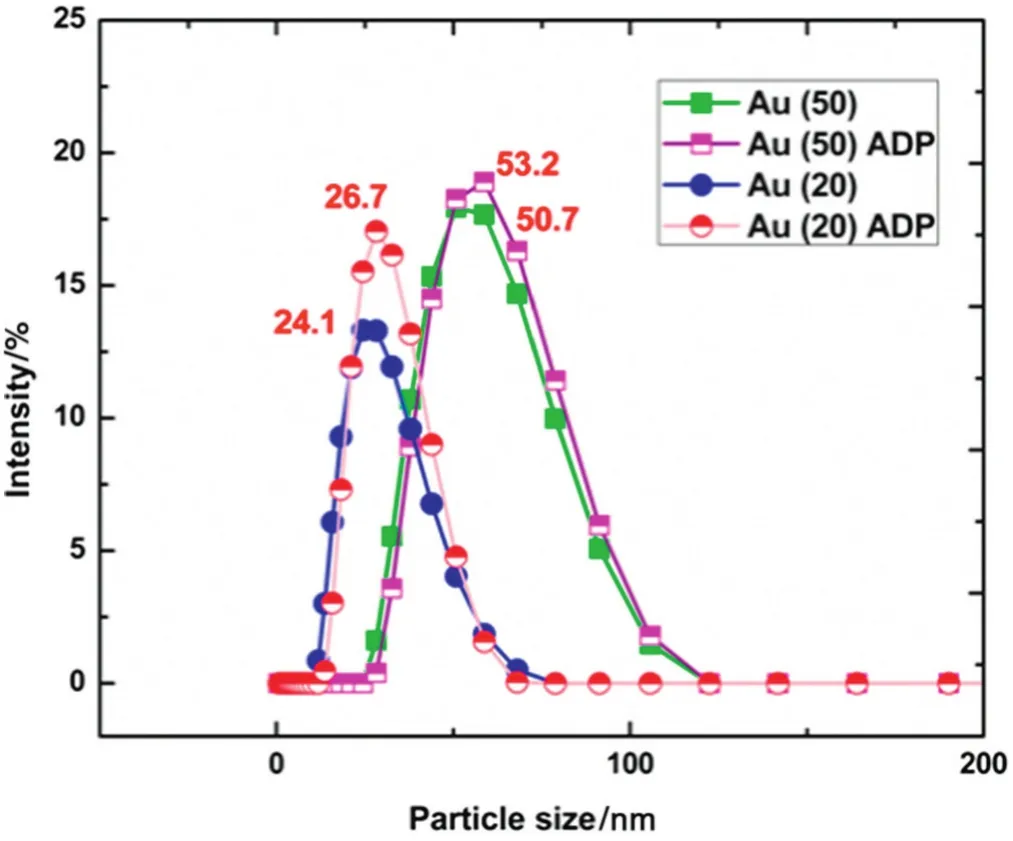
Fig.4.The particle size of gold nanoparticle with and without addition of ADP solution(pH 7.5).
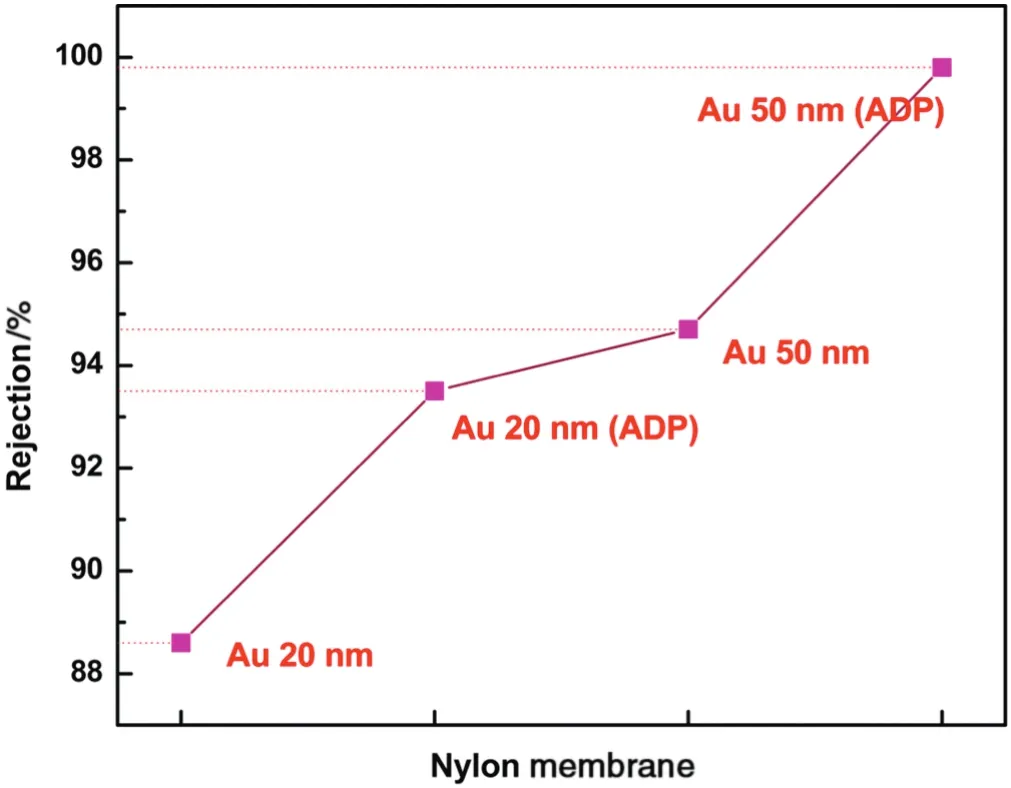
Fig.5.Rejection of gold nanoparticles with and without ADP for NA membrane atpH 7.5.All the membranes were compacted for 30 min before gold nanoparticle separation at 0.1 MPa with DIwater.The feed solution ofgold particle(20 and 50 nm)(concentration~50×10-6)with and without ADP at 1.0 mmol·L-1 concentration was used.
The stabilization of gold nanoparticles and its agglomeration mainly depend on solution pH,cross linkage of ligands and the ionic strength[18].The ADP in the gold solution made the nanoparticles more stable without any clusters forming and also increased the rejection capability of the nylon membrane.The larger-sized of gold nanoparticles at50 nm showed higherrejection than smaller-sized gold nanoparticles at20 nm due to the size effect.The incorporation of ADP into the gold nanoparticle solution suppressed the agglomeration among particles and reduced the adsorption of gold particles on the membrane surface.Therefore,ADPfunctionality contributed to the properties ofboth the gold solution and the nylon membrane.The order of gold colloid rejection capability at various pH was pH 8~pH 4>pH 10>pH 12~pH 14>pH 6.
The interaction energy values of gold nanoparticles are given in Table 4.The interaction energy of particles was reduced after adding the ADP to the gold solution.Thus the reduction of interaction energy between the gold nanoparticles con firms the depression of agglomeration of gold clusters.This interaction energy is also known as van der Waals interaction energy[25].The Hamaker constant(AH)of gold nanoparticles was used in estimating the interaction and the range ofvalues is(1-4)×10-19J was utilized[26]for the above calculation.
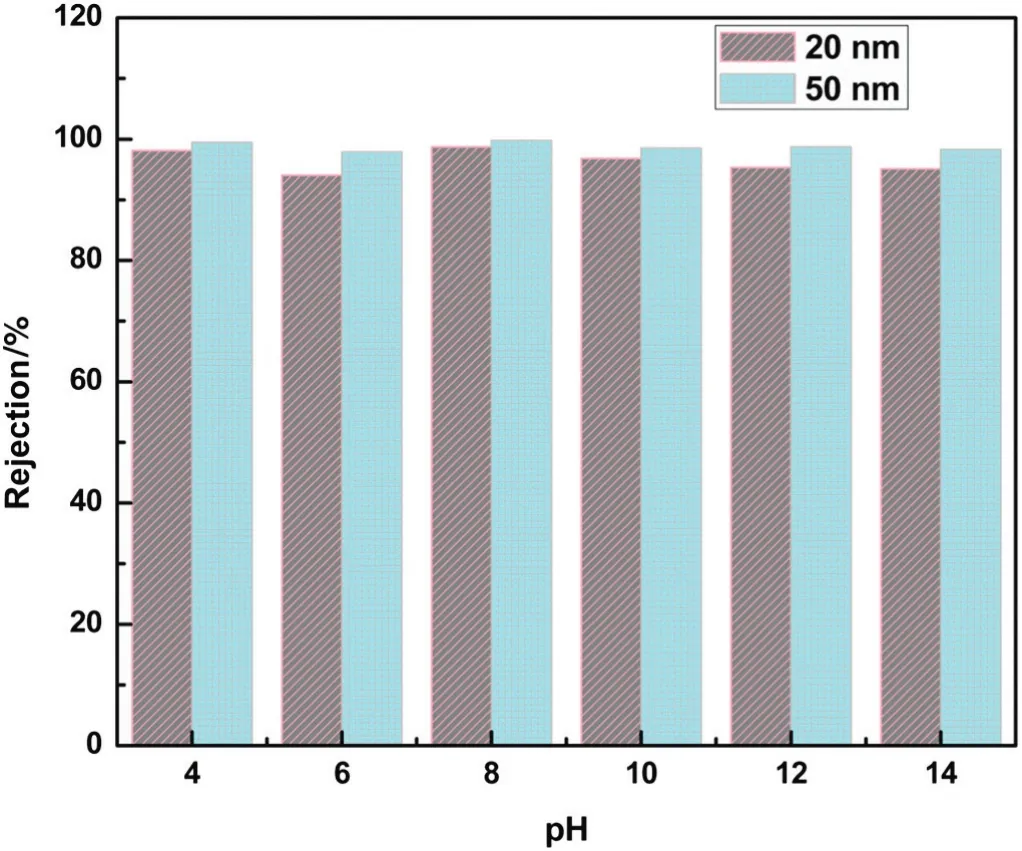
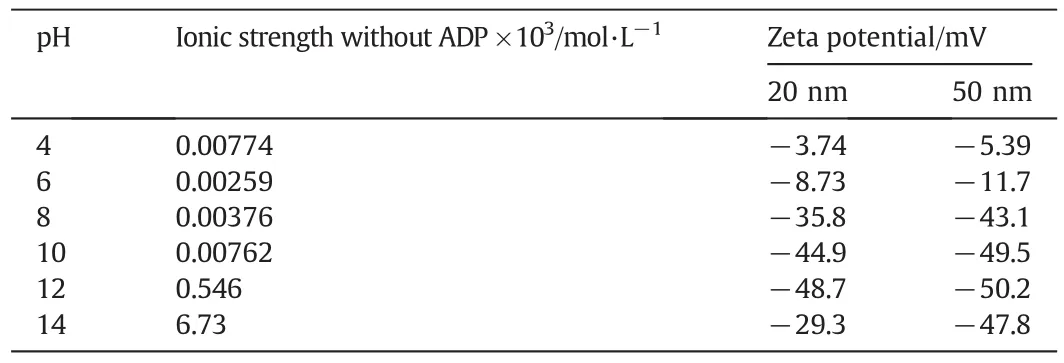
Table 3Ionic strength and zeta potential of gold solution with and without ADP at different pH values
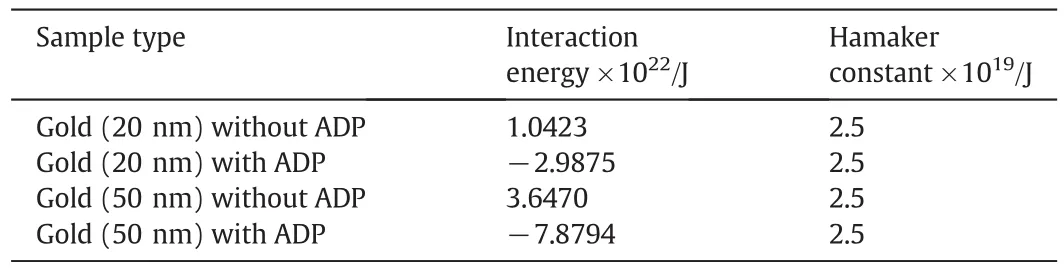
Table 4Interparticle interaction energy of gold solution with and without ADP
3.4.Correlated with microscopic analysis
The gold nanoparticle agglomeration during me mbrane filtration and the suppression of gold colloid clusters with the addition of ADP were also con firmed by TEM images.The binding of gold nanoparticles was also evidenced by SEM,AFM and CLSM(explained below).TEM images of gold nanoparticle- filtered membrane in the presence of ADP during membrane filtration of nylon membrane are shown in Fig.7.Sphere-shaped nanoparticles were observed in TEM and the splitting of gold colloids deposition using ADP was verified.The particle size of gold nanoparticle was précised at20.3 nmand 50.1 nmand itresembled the monodispersity of the gold particle.The EDX mapping image also evidenced the particle size.The above data con firmed that the particle size remained the same as after the rejection study and demonstrated the reduction of interacting force between the gold nanoparticles and the nylon membrane.
The rejected gold nanoparticles in the presence of ADP on the nylon membrane surface were analyzed with SEM as shown in Fig.8.The gold nanoparticles were randomly deposited with uniform shapes on the membrane surface,and the de-structuring of the gold colloid flocks in the prepared NA membrane was observed with both particle sizes(20 and 50 nm).Fig.9 shows the EDAX imagesof the gold nanoparticles deposited on nylon membrane surface.The deposited nylon membranes were cleaned by water without any chemicals and sonicated for 2 min.Gold nanoparticles were loosely adsorbed on membrane surface in the presence ofADP ligand and those could be easily removed by the cleaning process.The majority of the gold particles were washed off and 12%of gold particles remained and were left scattered on the membrane surface.In comparison,the clustered particles formed during the operation without ADP could not be easily removed,and the 22%of gold remaining post-wash was tightly bound to the membrane surface in the form ofgroupsofgold clusters.Thus,the ADP ligand suppressed formation of gold clusters on the nylon membrane surface.The gold colloids deposited on nylon membrane in 3-D CLSM are shown in Fig.10.In this instance,the gold nanoparticles were deposited homogeneously without colloidal clusters on the nylon membrane.The deposited particles on membrane surface of NA in CLSM images and the formation looked like quantum dots.
4.Conclusions and Future Prospects
The present investigation depicts the successful fabrication of nylon membrane using a simple phase inversion technique and utilization of ADP ligand to de-structure gold colloid flocks(both within the particles themselves and between the gold and membrane surface).The prepared membranes achieved relatively high gold nanoparticle separation efficiencies from gold colloids(20 and 50 nm)with the ADP solution at 1.0 mmol·L-1.Ultrathin dense layer,microporous Darcy-like structure and thread-like ovoid structures were seen in NA membrane with SEM.The deep depressions(membrane pores)and nodules were ascertained in AFM micrographs.In laser micrographs,smoother surface with less prominent grains was seen.The surface roughness features of NA membranes were compared with AFM and CLSM.
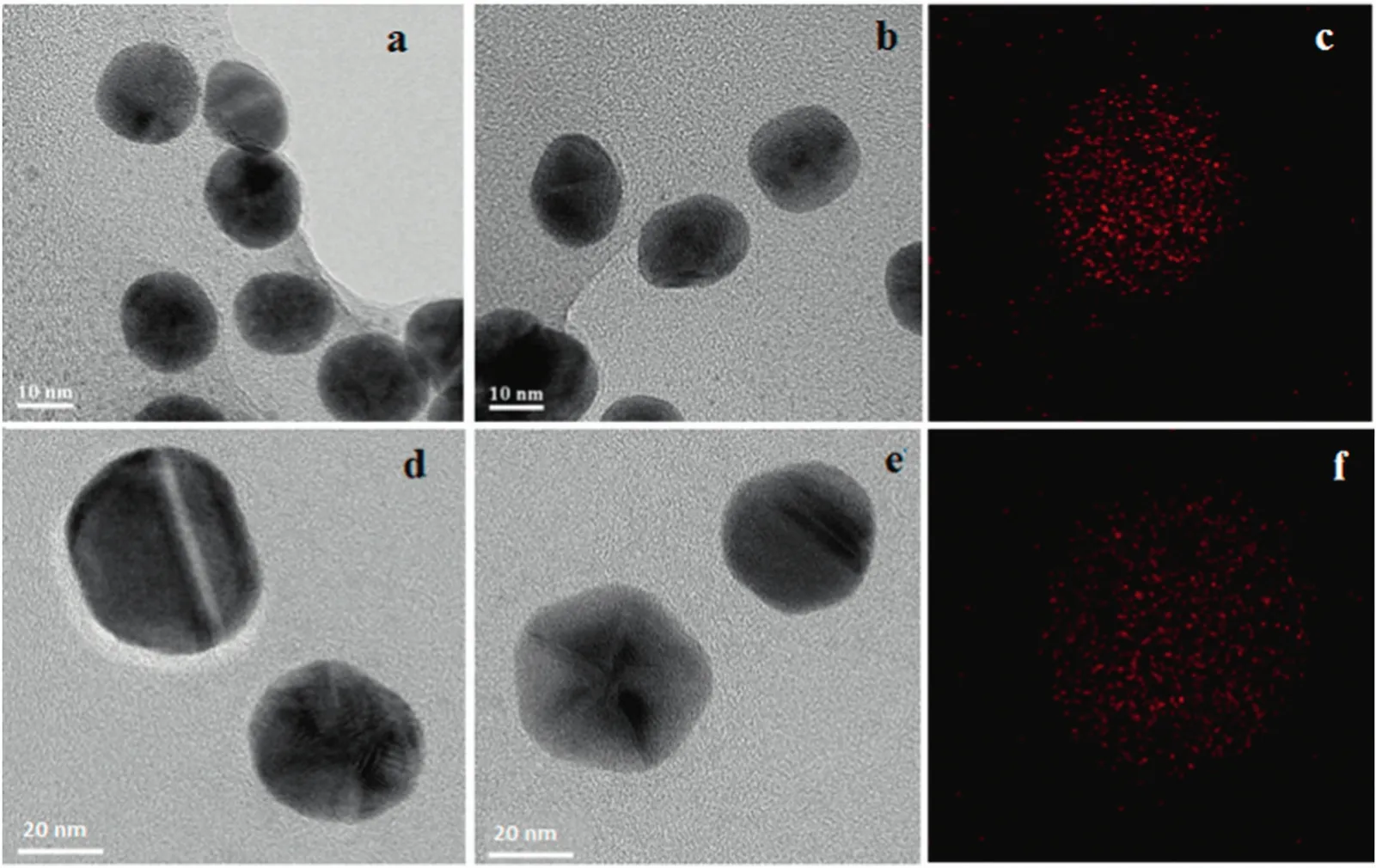
Fig.7.Transmission electron micrographs of separated gold nanoparticle with 1.0 mmol·L-1 ADP during membrane filtration:(a)and(b)the separated gold nanoparticles(20 nm)with TEM images of the NA membrane,(d)and(e)the separated gold nanoparticles(50 nm)with TEM images of the NA membrane,(c)and(f)EDX mapping TEM images of separated gold nanoparticle(20 and 50 nm,respectively)for the membranes.
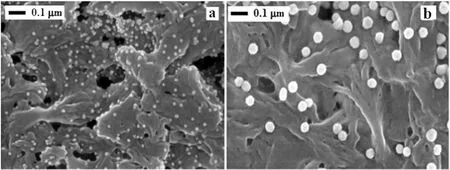
Fig.8.Scanning electron micrographs of separated gold nanoparticle in the presence of ADP during membrane filtration:(a)and(b)the separated gold nanoparticle(20 and 50 nm,respectively)with SEM images of NA membrane with magnification of 70 k&100 k.

Fig.9.EDAX(SEM)images:(a)and(c)membranes filtered with gold nanoparticles(in presence and absence ofADP),(b)and(d)after cleaning,the separated gold nanoparticle remaining on the nylon membrane.The membranes were cleaned and sonicated for 2 min.
The separation efficiency ofthe gold nanoparticles(20 and 50 nm)in the presence of ADP with the nylon membrane was highest at pH 4 and 8.The gold colloid agglomeration was suppressed using ADP and the monodispersity of gold nanoparticles was evidenced by TEM,AFM,CLSM and SEM.The interparticle interaction energy was reduced in the gold solution with the ADP ligand.The accurate particle size of gold nanoparticles was con firmed by TEM and zeta-sizer.This study showed that the addition of ADP prevented the agglomeration of gold nanoparticles,which deviates the evaluation of precise pore size of the nylon membranes.The fabrication of nylon membrane with various pore sizes(less than 5 nm,10 nm,20 nm)for a separation study is required and the findings may translate to additional applications.
Nomenclature
AHHamaker constant(=2.5×10-19J)
a1anda2Radii of the particles,nm
MpConcentration of solute in the permeate,mg·L-1
MfConcentration of solute in the feed,mg·L-1
RDistance between the centers of two particles,nm
ScSpreading co-efficient,mN·m-1
WeWetting energy,mN·m-1
WaWork of adhesion,mN·m-1
θ Contact angle values,°
γ Surface tension of water,mN·m-1
[1]D.Gentili,M.Cavallini,Wet-lithographic processing of coordination compounds,Coord.Chem.Rev.257(2013)2456-2467.
[2]P.M.Harrey,B.J.Ramsey,P.S.A.Evans,D.J.Harrison,Capacitive-type humidity sensors fabricated using the offset lithographic printing process,Sensors Actuators B Chem.87(2002)226-232.
[3]J.Lauria,R.Albright,O.Vladimirsky,M.Hoeks,R.Vanneer,B.v.Drieenhuizen,L.Chen,L.Haspeslagh,A.Witvrouw,SLM device for 193 nm lithographic applications,Microelectron.Eng.86(2009)569-572.
[4]N.S.Leyland,J.R.G.Evans,D.J.Harrison,Lithographic printing of ceramics,J.Eur.Ceram.Soc.22(2002)1-13.
[5]K.Jiang,C.H.Lee,P.Jin,An ultrathick SU-8 UV lithographic process and sidewall characterization A2,in:Wolfgang Menz,Stefan Dimov,Bertrand Fillon(Eds.),4M 2006-Second International Conference on Multi-Material Micro Manufacture,Elsevier,Oxford 2006,pp.211-216.
[6]W.Y.Kim,H.C.Lee,Developmentof manipulation technology of ferroelectric polymer film:Photo-lithographic patterning and multilayer formation,Microelectron.Eng.88(2011)1576-1581.
[7]A.Singh,S.K.Kulkarni,C.Khan-Malek,Patterning of SiO2nanoparticle-PMMA polymer composite microstructures based on softlithographic techniques,Microelectron.Eng.88(2011)939-944.
[8]T.Vandeweyer,C.Baerts,N.Horiguchi,M.Ercken,New lithographic requirements for the implant levels in scaled devices,Microelectron.Eng.88(2011)2171-2173.
[9]J.Marques-Hueso,R.Abargues,J.Canet-Ferrer,J.L.Valdes,J.Martinez-Pastor,Resistbased silver nanocomposites synthesized by lithographic methods,Microelectron.Eng.87(2010)1147-1149.
[10]A.P.Oost,P.L.De Boer,Tectonic and climatic setting of lithographic limestone basins,Geobios27(Suppl.1)(1994)321-330.
[11]M.E.Anderson,C.Srinivasan,R.Jayaraman,P.S.Weiss,M.W.Horn,Utilizing self-assembled multilayers in lithographic processing for nanostructure fabrication:Initial evaluation of the electrical integrity of nanogaps,Microelectron.Eng.78-79(2005)248-252.
[12]I.S.Chronakis,Chapter 22—Micro-and Nano- fibers by Electrospinning Technology:Processing,Properties,and Applications A2—Qin,Yi,Micromanufacturing Engineering and Technology,second ed.William Andrew Publishing,Boston,2015 513-548.
[13]J.E.Krzanowski,Fabrication and tribological properties of composite coatings produced by lithographic and microbeading methods,Surf.Coat.Technol.204(2009)955-961.
[14]M.-C.Daniel,D.Astruc,Gold nanoparticles:assembly,supramolecular chemistry,quantum-size-related properties,and applications toward biology,catalysis,and nanotechnology,Chem.Rev.104(2004)293-346.
[15]A.N.Shipway,E.Katz,I.Willner,Nanoparticle arrays on surfaces for electronic,optical,and sensor applications,ChemPhysChem1(2000)18-52.
[16]C.S.Weisbecker,M.V.Merritt,G.M.Whitesides,Molecular self-assembly of aliphatic thiols on gold colloids,Langmuir12(1996)3763-3772.
[17]T.Yonezawa,K.Yasui,N.Kimizuka,Controlled formation ofsmaller gold nanoparticles by the use of four-chained disul fide stabilizer,Langmuir17(2001)271-273.
[18]T.Kim,K.Lee,M.-S.Gong,S.-W.Joo,Control of gold nanoparticle aggregates by manipulation of interparticle interaction,Langmuir21(2005)9524-9528.
[19]J.Kunze,I.Burgess,R.Nichols,C.Buess-Herman,J.Lipkowski,Electrochemical evaluation of citrate adsorption on Au(1 1 1)and the stability of citrate-reduced gold colloids,J.Electroanal.Chem.599(2007)147-159.
[20]J.B.Schlenoff,M.Li,H.Ly,Stability and self-exchange in alkanethiol monolayers,J.Am.Chem.Soc.117(1995)12528-12536.
[21]A.N.Takehito Mizuna,Shuichi Tsuzuki,A novel filter rating method using less than 30-nm gold nanoparticle and protective ligand,IEEE Trans.Semicond.Manuf.22(2009).
[22]S.I.Stoeva,A.B.Smetana,C.M.Sorensen,K.J.Klabunde,Gram-scale synthesis of aqueous gold colloids stabilized by various ligands,J.Colloid Interface Sci.309(2007)94-98.
[23]V.J.Gandubert,R.B.Lennox,Assessment of 4-(dimethylamino)pyridine as a capping agent for gold nanoparticles,Langmuir21(2005)6532-6539.
[24]S.Chen,K.Kimura,Synthesis and characterization of carboxylate-modified gold nanoparticle powders dispersible in water,Langmuir15(1999)1075-1082.
[25]E.J.W.Verwey,J.Th.G.Overbeek,Theory of the Stability of Lyophobic Colloids,Dover,Mineola,NY,1999.
[26]S.Biggs,M.K.Chow,C.F.Zukoski,F.Grieser,The role of colloidal stability in the formation of gold sols,J.Colloid Interface Sci.160(1993)511-513.
 Chinese Journal of Chemical Engineering2017年7期
Chinese Journal of Chemical Engineering2017年7期
- Chinese Journal of Chemical Engineering的其它文章
- Elevating the flexibility and operability of dividing-wall distillation columns via feed thermal condition adjustment☆
- Hydrodynamic dispersion ofreactive solute in a Hagen-Poiseuille flow of a layered liquid
- Interpenetrating polymers supported on microporous polypropylene membranes for the transport of chromium ions☆
- Synthesis of clay-supported nanoscale zero-valent iron using green tea extract for the removal of phosphorus from aqueous solutions
- Horizontal gas mixing in rectangular fluidized bed:A novel method for gas dispersion coefficients in various conditions and distributor designs
- Experimental detection of bubble-wall interactions in a vertical gas-liquid flow☆
