The effect of transition metal ions(M2+=Mn2+,Ni2+,Co2+,Cu2+)on the chemical synthesis polyaniline as counter electrodes in dye-sensitized solar cells☆
Kezhong Wu*,Lei Chen,Weizhen Cui,Bei Ruan,Mingxing Wu
Key Laboratory of Inorganic Nano-materials of Hebei Province,Department of Chemistry and Material Science,Hebei Normal University,Shijiazhuang 050024,China
1.Introduction
DSSCs have been attracted a lot of attention lately,due to their low manufacturing cost,high energy conversion efficiency,simplicity and environmentally benign preparation procedures[1,2].However,DSSCs still have serious issues that need to be resolved,such as high cost of Pt usually used in the counter electrodes(CEs),fast decay in the photo-to-electron conversion efficiency,susceptible to corrosion by the redox species,and frangibility to heat treatment[3,4].Among those challenges,many research groups have put focus on improving and extending platinum-free CEs of solar cells in different aspects.CEs not only serve to reduce triiodides to iodide species,which completes the cycle of electron transfer,but also have to enhance the efficiency of DSSCs while maintaining their stability.It is,therefore,crucial to develop alternative,scalable,structurally controllable,corrosion resistant and inexpensive CE materials with high electrocatalytic activity[5,6].Up to date,inorganic compounds[7,8],carbon[9],hybrid materials[10,11],and conductive polymers[12–14]etc.have been tested as counter electrode materials for DSSCs.
The feasibility of using flexible,electrochemically active,conductive polymers with high surface area to replace Pt CEs in DSSCs has been extensively studied in the recent years.Conductive polymers are promising candidates for catalytic materials used as CEs because of their low cost,remarkable conductivity,and obviously high electrocatalytic activity,not to mention the fact that conductive polymers synthesized by direct oxidative polymerization/electrochemical procedures have received much attention due to their potential for utilization in sensors,supercapacitors,and DSSCs[15,16].PANI occupies a significant position among the organic conductive polymers.Its unique properties,such as ease of preparation in aqueous medium,wide raw material sources,good chemical stability,remarkable dope ability,improved electrocatalytic activity,obvious electrochromic effects,and moderately high efficiency in the doped form have made this polymer a strong candidate with great technological promise for the modification of electrodes[17–19].Its electrical properties can be readily tuned by controlling its doping level.In the present work,the performance of platinum-free,PANI composite counter electrodes modified by transition metal ions(Ni2+,Mn2+,Co2+and Cu2+)in DSSCs was investigated.
2.Experimental
2.1.Materials
Aniline(Beijing Chemical Reagent Company,purity 99.5%),and hydrochloric acid solution(purity 98.0%),nickel(II)chloride hexahydrate(purity 98.0%),cobalt(II)chloride hexahydrate(purity 99.0%),manganese(II)chloride tetrahydrate(purity 99.0%),copper chloride dihydrate(AR)were purchased from Tianjin Damao Chemical Reagent Factory.Bichrome(purity 99.0%)was purchased from Beijing chemical factory.
2.2.Characterization
The surface morphology of PANI and PANI-transition metal ion doped composites was studied by scanning electron microscope(SEM)(S-4800 FEI,Hitachi).IR spectra were obtained by using a Fourier infrared spectroscope(FTIR-8900 SHIMADZU,Japan).The photocurrent–voltage was determined under simulated AM 1.5 illumination(I=100 mW·cm?2,PEC-L15,Peccell,Yokohama,Japan)with a digital source meter(Keithley 2601,Cleveland,OH)in nitrogen atmosphere.Electrochemical performance was evaluated by cyclic voltammetry measurements at a scan rate of 20 mV·s?1using electrochemical workstation(CHI660e,Chenhua,Shanghai).Electrochemical impedance spectra(EIS)and Tafel polarization curve of the DSSCs were measured in acetonitrile solution containing 0.1 mol·L?1of lithium iodide,0.6 mol·L?11-propyl-3-methylimidazolium iodide,0.07 mol· L?1iodine,0.5 mol·L?14-tert-butylpyridine,and 0.1 mol·L?1guanidinium thiocyanate in 3-methoxypropionitrile(MPN)with the CHI660e electrochemical workstation.The scan rate was 10 mV·s?1.
2.3.Synthesis of PANI
1 molaniline and 0.01 moltransition metalions(M2+=Co2+,Ni2+,Mn2+,Cu2+)were first dissolved in 20 ml of 2 mol·L?1HCl solution,and then another 30 ml of 2 mol·L?1HCl containing 0.6 mol potassium dichromate was added slowly.The mixture was stirred for 5 h.To remove the excess potassium dichromate,transition metal ions and byproducts,the as-prepared PANI nano fibers was washed with 2 mol·L?1HCl solution 3 times and collected by centrifugation.
2.4.Preparation of counter electrode
The electrode was prepared by following a series of procedures:the FTO glass was cleaned by washing with detergent,deionized water,and ethanol;0.1250 g electrode material was dispersed in 6 ml isopropyl alcohol;the solution was ball-milled for 50 min to achieve the spray paste.The last procedure was spraying the sample on the FTO glass with a spray gun;and finally the PANI-transition metal ion CEs dried under vacuum.
2.5.Cell fabrication
The DSSC had three parts:a TiO2photoanode with active area of 0.16 cm2,the transition metal ion doped PANI as CE,and an electrolyte solution containing the iodide/triiodide redox couple.Two identical pieces of the electrodes with the same area clipped with the electrolyte formed a symmetrical cell,which was sealed with a hot-melt surlyn film.The photoanode of 8 um thick TiO2film soaked in the N719 dye for 18 h.The electrolyte solution was composed of 0.1 mol·L?1of lithium iodide,0.6 mol·L?11-propyl-3-methylimidazolium iodide,0.07 mol·L?1iodine,0.5 mol·L?14-tert-butyl pyridine,and 0.1 mol·L?1guanidiniumthiocyanate in 3-methoxypropionitrile(MPN)[20].
3.Results and Discussion
The chemical structure of the PANI and PANI-transition metal ion doped composites were characterized by FTIR spectroscopy.Fig.1 shows the FTIR spectra,which are in good agreement with previously reported results.In terms of PANI,the peaks at 1564 cm?1and 1489 cm?1are attributed to C=C stretching vibrations of the quinoid and benzenoid rings,respectively[21].The C–N stretching of the benzenoid unit and C–N stretching of the quinoid unit are located at 1295 cm?1and 1238 cm?1.The contribution from C–H bending of the quinoid ring appears at 1127 cm?1.The spectrum of PANI-transition metal ion hybrid exhibits the same vibrational bands as pure PANI,suggesting that PANI-transition metal ion hybrid have characteristic absorption peaks of PANI,but in the presence of transition metal ions the peaks are slightly shifted.
Fig.2 shows the SEM picture of the surface of the CE with PANI and PANI-M2+(M2+=Co2+,Ni2+,Mn2+,Cu2+).Fig.2(b),(c),(d)show that the PANI-M2+(M2+=Co2+,Ni2+,Mn2+)forms uniform and regular nanorods with size of around 50 nm.It can be seen that the size of PANI-Ni2+nanorods is slightly larger than that of PANI-Mn2+.Fig.2(a),(e)show neither nanorods nor nanopaticles,and a certain degree of cross linking can be seen,which is unfavorable for the catalytic reduction of I3?with the PANI,PANI-Cu2+CE material.
Fig.3 demonstrates the photocurrent–voltage(J–V)curves based on PANI,PANI-Mn2+,PANI-Ni2+,PANICo2+and PANI-Cu2+as counter electrode in DSSCsforthe I?/I3?redox couple.The corresponding detailed photovoltaic electrochemical parameters from theJ-Vcurves are summarized in Table 1.The fill factor(FF)and power conversion efficiency(η)of the DSSCs can be obtained from the Eqs.(1)and(2),respectively[22].
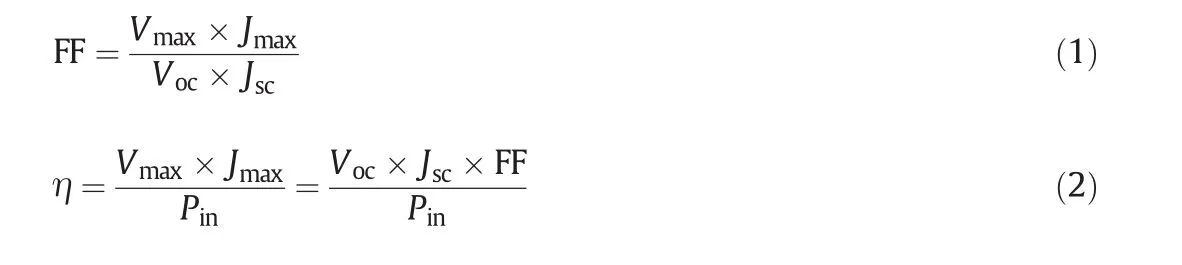
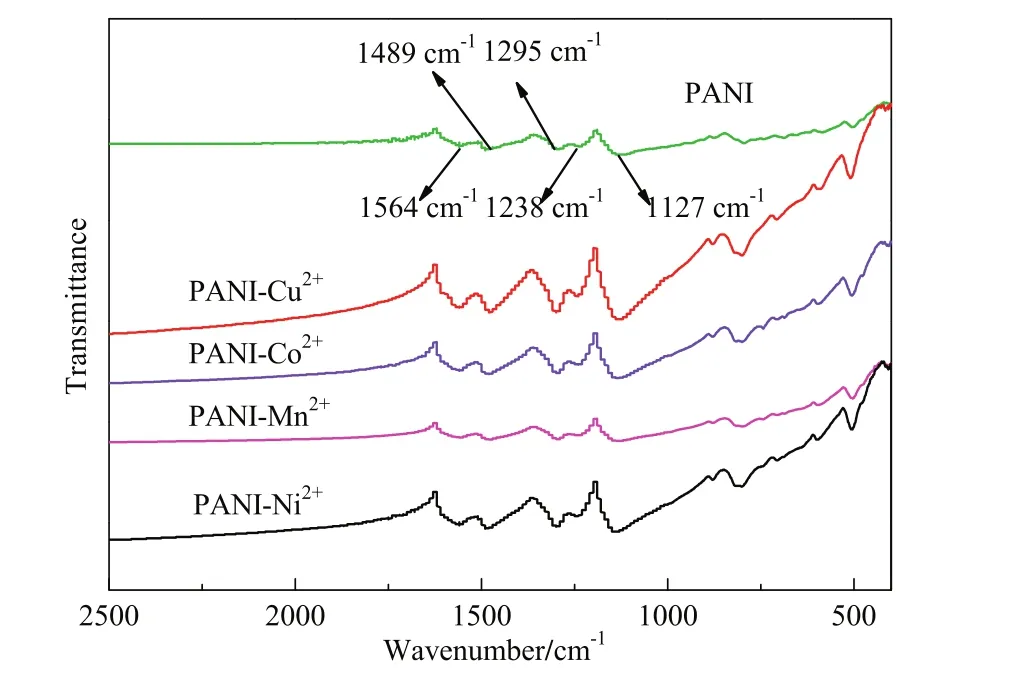
Fig.1.FTIR spectra of the PANI,PANI-Ni2+,PANI-Co2+,PANI-Mn2+and PANI-Cu2+.
whereJmax(mA·cm?2)is the current density andVmax(V)is the voltage at the point of maximum power output in theJ-Vcurves,Jscis the shortcircuit current density(mA·cm?2),Vocis the open-circuit voltage(V);Pinis the incident light power(mW·cm?2).Photovoltaic parameters of DSSCs using PANI,PANI-Mn2+,PANI-Ni2+,PANI-Co2+,PANI-Cu2+as CE are listed in Table 1.Power conversion efficiency(η)of DSSCs fabricated by using PANI-Mn2+,PANI-Ni2+and PANI-Co2+CEs were higher than the ones with undoped PANI CE,while the η of DSSCs containing PANICu2+CE was lower.
The electrocatalytic activity of PANI-Mn2+,PANI-Ni2+,PANI-Co2+,PANI,PANI-Cu2+CEs for the reduction of I?/I3?redox couple in the DSSCs was determined by using cyclic voltammetry(CV)as shown in Fig.4.In the case of chemically synthesized PANI CEs modified by transition metal ions of Ni2+,Mn2+,Co2+and Cu2+two typical redox peaks were clearly observed.The negative peak is associated with the reduction process of I3?+2e?→ 3I?,whereas the positive peak is attributed to the oxidation process of 3I?→ I3?+2e?[23].As it can be seen on Fig.4 the current density of the reduction peak at low potential for the PANI-Mn2+,PANI-Ni2+and PANI-Co2+electrode was?3.266 mA·cm?2,?2.574 mA·cm?2and?2.512 mA·cm?2.These absolute values were slightly higher than the value measured for the pure PANI electrode(?2.427 mA·cm?2),however,the current density of the reduction peak for the PANI-Cu2+(?2.067 mA·cm?2)was lower.The peak-to-peak separation(ΔEp)followed an order of PANIMn2+(0.297 V)<PANI-Ni2+(0.319 V)<PANI-Co2+(0.387 V)<PANI(0.429 V)<PANI-Cu2+(0.471 V).The increase in the current density of the reduction peak and decrease of ΔEpvalue indicates a charge transfer process and the electrocatalytic activity toward I3?/I?redox reaction increases in the following order:PANI-Mn2+>PANI-Ni2+>PANI-Co2+>PANI>PANI-Cu2+.This result is consistentwith the calculated power conversion efficiency(η)of DSSCs.
Fig.2.SEM images of the synthesized(a)PANI,(b)PANI-Co2+,(c)PANI-Ni2+,(d)PANI-Mn2+,(e)PANI-Cu2+.

Fig.3.J-V curves of PANI-Mn2+,PANI-Ni2+,PANI-Co2+,PANI,PANI-Cu2+.
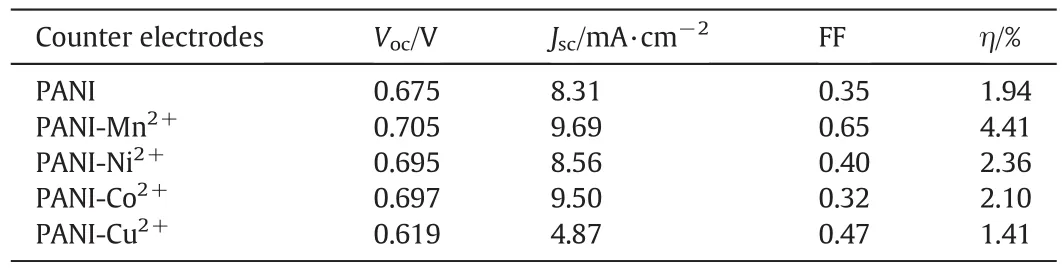
Table 1Photovoltaic parameters of DSSC using the chemical synthesis for PANI,PANI-Mn2+,PANI-Ni2+,PANI-Co2+,PANI-Cu2+as CEs
Fig.5 presents the Nyquist plots of the symmetrical PANI,PANIMn2+,PANI-Ni2+,PANI-Co2+and PANI-Cu2+CEs in the redox electrolyte.A Nyquist plot consists of two parts:a semicircle at high frequency region and a linear at low frequency range.The right of the semicircle at high frequency region is ascribed to the charge transfer resistance(Rct)atthe CE/electrolyte interface,while the intercept of the real axis for the semicircle athigh frequency represents the series resistance(Rs),which includes the resistance of the electrolyte and the electrode,and the contact resistance[24].There were no significant differences found for theRsvalues of the five CEs,it was about 45 Ω.TheRctvalues of the PANI-Mn2+,PANI-Ni2+,and PANI-Co2+were 77.5 Ω,128.9 Ω and 159.5 Ω,much lower than theRct165.2 Ω of PANI,resulting to higher catalytic activity of the electrodes in the redox process.TheRctvalues of the PANI-Cu2+was 325.5 Ω higher thanRctof PANI which explains its lower electrochemical catalytic activity for the redox reaction of the iodide/triiodide couple.The results show that doping of PANI with transition metal ions changes theRctof the material.(See Fig.5.)
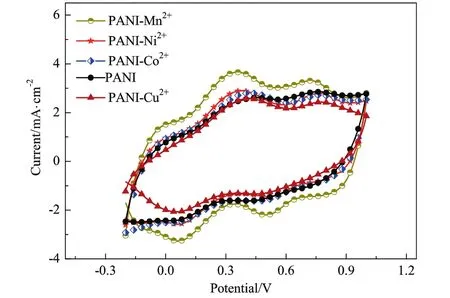
Fig.4.CV curves of PANI-Mn2+,PANI-Ni2+,PANI-Co2+,PANI,PANI-Cu2+.

Fig.5.EIS curves of PANI-Mn2+,PANI-Ni2+,PANI-Co2+,PANI,PANI-Cu2+.
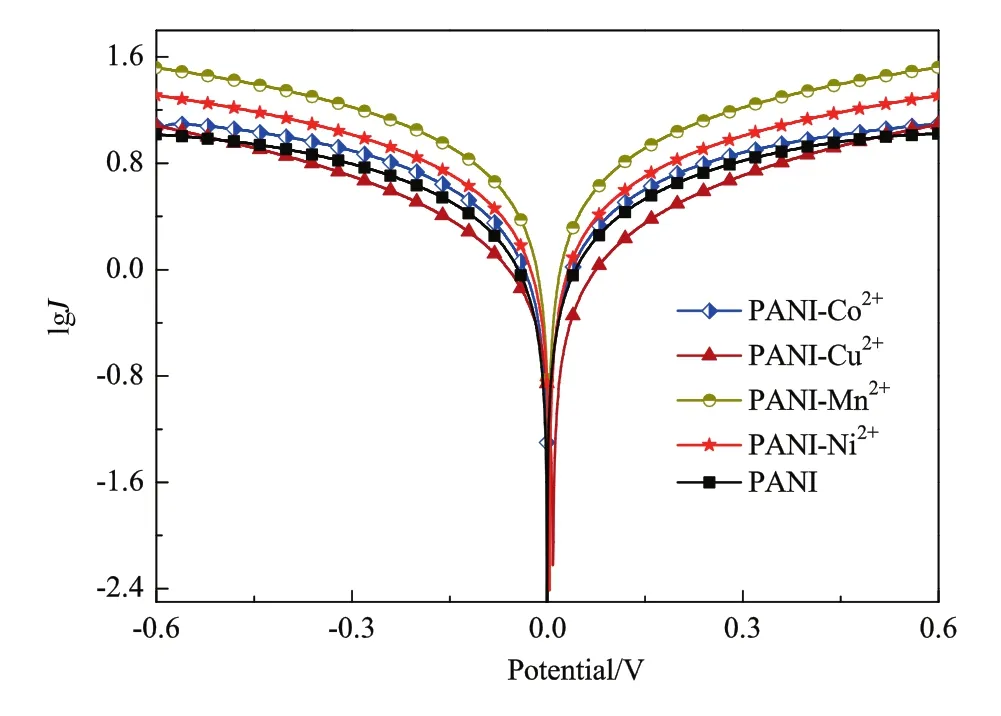
Fig.6.Tafel curves of PANI-Mn2+,PANI-Ni2+,PANI-Co2+,PANI,PANI-Cu2+.
The electrocatalytic performance of the PANI-Mn2+,PANI-Ni2+,PANI-Co2+,PANI,PANI-Cu2+CEs was further investigated by Tafel polarization using a symmetrical dummy cell(see fig.6).The Tafel curve consists of three parts:the limiting diffusion region,the Tafel region,and the polarization region[25].The sharper slope for an anode or the cathode branch means a higher exchange current density(J0)on the electrode and better electrocatalytic activity toward I3?reduction[26].The calculatedJ0followed the order of PANI-Mn2+(0.239/mA·cm?2)-PANI-Ni2+(0.037/mA·cm?2)> PANI-Co2+(?0.058/mA·cm?2)-PANI(?0.139/mA·cm?2)> PANI-Cu2+(?0.335/mA·cm?2).These exchange current density(J0)values were used to calculate the electrocatalytic activity of the electrodes.This result is consistent with the PCE(η)and the charge transfer resistance(Rct)of the different DSSCs.
4.Conclusions
A non-Pt material,more precisely a transition metal ion(M2+=Mn2+,Ni2+,Co2+,Cu2+)doped PANI prepared by chemical synthesis was used as CEs in DSSCs.An active PANI-M2+CE could be formed and the appropriate composition of PANI-M2+CE had a huge impact on the reduction of electrolyte leading to the increase of the overall efficiency.The PCE(η)on PANI-M(M=Mn2+,Ni2+,Co2+)CEs were 4.41%,2.36%,2.10%,respectively.These values were much higher than 1.94%,the value measured on the PANI electrode,while the PCE of PANI-Cu2+(η=1.41%)was lower.The outstanding electronic features of PANI-M2+CEs can elevate the comprehensive performance of DSSCs to the next level.
Nomenclature
FF the fill factor
Jmaxthe current density,mA·cm?2
Jscthe short-circuit current density,mA·cm?2
Pincthe incident light power,mW·cm?2
Rctthe charge transfer resistance Ω
Rsthe series resistance,Ω
Vmaxthe voltage at the point of maximum power output in theJ-Vcurves,V
Vocthe open-circuit voltage,V
η the power conversion efficiency
[1]H.H.Niu,S.X.Qin,X.L.Mao,S.W.Zhang,R.B.Wang,L.Wan,J.Z.Xu,S.D.Miao,Axlesleeve structured MWCNTs/polyaniline composite film as cost-effective counterelectrodes for high efficient dye-sensitized solar cells,Electrochim.Acta121(2014)285–293.
[2]M.X.Wu,Y.N.Lin,H.Y.Guo,K.Z.Wu,X.Lin,Highly efficient Mo2C nanotubes as a counter electrode catalyst for organic redox shuttles in dye-sensitized solar cells,Chem.Commun.50(2014)7625–7627.
[3]R.Chauhan,M.Shinde,A.Kumar,S.Gosavi,D.P.Amalnerkar,Microporous and mesoporous materials,Microporous Mesoporous Mater.226(2016)201–208.
[4]Y.M.Xiao,J.Y.Lin,W.Y.Wang,S.Y.Tai,G.T.Yue,J.H.Wu,Enhanced performance of low-cost dye-sensitized solar cells with pulse-electropolymerized polyaniline counter electrodes,Electrochim.Acta90(2013)468–474.
[5]M.Wang,Q.W.Tang,P.P.Xu,B.L.He,L.Lin,H.Y.Chen,Counter electrodes from polyaniline–graphene complex/graphene oxide multilayers for dye-sensitized solar cells,Electrochim.Acta137(2014)175–182.
[6]Y.M.Xiao,J.Y.Lin,J.H.Wu,S.Y.Tai,G.T.Yue,T.W.Lin,Dye-sensitized solar cells with high-performance polyaniline/multi-wall carbon nanotube counter electrodes electropolymerized by a pulse potentiostatic technique,J.Power Sources233(2013)320–325.
[7]Z.W.Shi,K.M.Deng,L.Li,Pt-free and efficient counter electrode with nanostructured CoNi2S4for dye-sensitized solar cells,Sci.Rep.5(2015)9317–9322.
[8]M.S.H.Choudhury,N.Kishi,T.Soga,Compression of ZnO nanoparticle films at elevated temperature for flexible dye-sensitized solar cells,J.Alloys Compd.656(2016)476–480.
[9]J.Z.Chen,C.Wang,C.C.Hsu,I.C.Cheng,Carbon,ultrafast synthesis of carbonnanotube counter electrodes for dye-sensitized solar cells using an atmosphericpressure plasma jet,Carbon98(2016)34–40.
[10]E.Bi,H.Chen,X.D.Yang,F.Ye,M.S.Yin,L.Y.Han,Fullerene-structured MoSe2hollow spheres anchored on highly nitrogen-doped graphene as a conductive catalyst for photovoltaic applications,Sci.Rep.5(2015)13214–13223.
[11]S.H.Hsu,C.T.Li,H.T.Chien,R.R.Salunkhe,N.Suzuki,Y.Yamauchi,K.C.Ho,K.C.W.Wu,Platinum-free counter electrode comprised of metal–organic-framework(MOF)-derived cobalt sulfide nanoparticles for efficient dye-sensitized solar cells(DSSCs),Sci.Rep.4(2014)6983–6988.
[12]J.H.Wu,Y.Li,Q.W.Tang,G.T.Yue,J.M.Lin,M.L.Huang,L.J.Meng,Bifacial dyesensitized solar cells:A strategy to enhance overall efficiency based on transparent polyaniline electrode,Sci.Rep.4(2014)4028–4034.
[13]Y.Qiu,S.Lu,S.S.Wang,X.H.Zhang,S.T.He,High-performance polyaniline counter electrode electropolymerized in presence of sodium dodecyl sulfate for dyesensitized solar cells,J.Power Sources253(2014)300–304.
[14]S.H.Park,K.H.Shin,J.Y.Kim,S.J.Yoo,K.J.Lee,J.J.Shin,J.W.Choi,J.Jang,Y.E.Sung,The application of camphorsulfonic acid doped polyaniline films prepared on TCO-free glass for counter electrode of bifacial dye-sensitized solar cells,Photochem.Photobiol.A Chem.245(2012)1–8.
[15]R.S.Chen,F.Formenti,H.McPeak,A.N.Obeid,C.Hahn,A.Farmery,Experimental investigation of the effect of polymer matrices on polymer fibre optic oxygen sensors and their time response characteristics using a vacuum testing chamber and a liquid flow apparatus,Sensors Actuators B Chem.222(2016)531–535.
[16]I.Fratoddi,A.Bearzotti,I.Venditti,C.Cametti,M.V.Russo,Role of nanostructured polymers on the improvement of electrical response-based relative humidity sensors,Sensors Actuators B Chem.225(2016)96–108.
[17]F.M.Wisser,J.Grothe,S.Kaskel,Nanoporous polymers as highly sensitive functional material in chemiresistive gas sensors,Sensors Actuators B Chem.223(2016)166–171.
[18]R.R.Salunkhe,M.B.Zakaria,Y.Kamachi,S.M.Alshehri,T.Ahamad,N.L.Torad,S.X.Dou,J.H.Kim,Y.Yamauchi,Fabrication of asymmetric supercapacitors based on coordination polymer derived nanoporous materials,Electrochim.Acta183(2015)94–99.
[19]W.A.Christinelli,R.Gon?alves,E.C.Pereira,A new generation of electrochemical supercapacitors based on layer-by-layer polymer films,J.Power Sources303(2016)73–80.
[20]M.X.Wu,Y.N.Lin,H.Y.Guo,T.L.Ma,A.Hagfeldt,Highly effective Pt/MoSi2composite counter electrode catalyst for dye-sensitized solar cell,J.Power Sources263(2014)154–157.
[21]G.Q.Wang,W.Xing,S.P.Zhou,The production of polyaniline/graphene hybrids for use as a counter electrode in dye-sensitized solar cells,Electrochim.Acta66(2012)151–157.
[22]S.P.Lim,A.Pandikumar,Y.S.Lim,N.M.Huang,H.N.Lim,In-situ electrochemically deposited polypyrrole nanoparticles incorporated reduced graphene oxide as an efficient counter electrode for platinum-free dye-sensitized solar cells,Sci.Rep.4(2014)5305–5311.
[23]G.T.Yue,F.R.Tan,F.M.Li,C.Chen,W.F.Zhang,J.H.Wu,Enhanced performance of flexible dye-sensitized solar cell based on nickel sulfide/polyaniline/titanium counter electrode,Electrochim.Acta149(2014)117–125.
[24]K.H.S.Lessa,Y.Zhang,G.A.Zhang,F.Xiao,S.Wang,Conductive porous sponge-like ionic liquid-graphene assembly decorated with nanosized polyaniline as active electrode material for supercapacitor,J.Power Sources302(2016)92–97.
[25]Y.D.Wang,C.Y.Zhao,M.X.Wu,W.Liu,T.L.Ma,Highly efficient and low cost Pt-based binary and ternary composite catalysts as counter electrode for dye-sensitized solar cells,Electrochim.Acta105(2013)671–676.
[26]B.L.He,X.Meng,Q.W.Tang,P.J.Li,S.S.Yuan,P.Z.Yang,Low-cost CoPt alloy counter electrodes forefficientdye-sensitized solarcells,J.PowerSources260(2014)180–185.
 Chinese Journal of Chemical Engineering2017年5期
Chinese Journal of Chemical Engineering2017年5期
- Chinese Journal of Chemical Engineering的其它文章
- Application of response surface methodology for optimization of purge gas recycling to an industrial reactor for conversion of CO2 to methanol
- Catalytic ozonation of thymol in reverse osmosis concentrate with core/shell Fe3O4@SiO2@Yb2O3 catalyst:Parameter optimization and degradation pathway☆
- Influence of Na+,K+,Mg2+,Ca2+,and Fe3+on filterability and settleability of drilling sludge☆
- Partition coefficient prediction of Baker's yeast invertase in aqueous two phase systems using hybrid group method data handling neural network
- Solubility and metastable zone width measurement of 3,4-bis(3-nitrofurazan-4-yl)furoxan(DNTF)in ethanol+water
- Measurement and calculation of solubility of quinine in supercritical carbon dioxide☆
