ZD7288, a selective hyperpolarization-activated cyclic nucleotide-gated channel blocker, inhibits hippocampal synaptic plasticity
Xiao-xue Zhang, Xiao-chun Min, Xu-lin Xu, Min Zheng, Lian-jun Guo,
1 Department of Laboratory Medicine, Affiliated Pu'ai Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei Province, China
2 Department of Pharmacology, School of Basic Medical Sciences, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei Province, China
3 School of Biomedical Engineering, Hubei University of Science and Technology, Xianning, Hubei Province, China
RESEARCH ARTICLE
ZD7288, a selective hyperpolarization-activated cyclic nucleotide-gated channel blocker, inhibits hippocampal synaptic plasticity
Xiao-xue Zhang1,#, Xiao-chun Min1,#, Xu-lin Xu2, Min Zheng3,*, Lian-jun Guo2,*
1 Department of Laboratory Medicine, Affiliated Pu'ai Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei Province, China
2 Department of Pharmacology, School of Basic Medical Sciences, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei Province, China
3 School of Biomedical Engineering, Hubei University of Science and Technology, Xianning, Hubei Province, China
Graphical Abstract

#These authors contributed equally to this study.
orcid: 0000-0001-9708-6442 (Lian-jun Guo)
The selective hyperpolarization-activated cyclic nucleotide-gated (HCN) channel blocker 4-(N-ethyl-N-phenylamino)-1,2-dimethyl-6-(methylamino) pyrimidinium chloride (ZD7288) blocks the induction of long-term potentiation in the perforant path—CA3 region in rat hippocampus in vivo. To explore the mechanisms underlying the action of ZD7288, we recorded excitatory postsynaptic potentials in perforant path—CA3 synapses in male Sprague-Dawley rats. We measured glutamate content in the hippocampus and in cultured hippocampal neurons using high performance liquid chromatography, and determined intracellular Ca2+concentration ([Ca2+]i) using Fura-2. ZD7288 inhibited the induction and maintenance of long-term potentiation, and these effects were mirrored by the nonspecific HCN channel blocker cesium. ZD7288 also decreased glutamate release in hippocampal tissue and in cultured hippocampal neurons. Furthermore, ZD7288 attenuated glutamate-induced rises in [Ca2+]iin a concentration-dependent manner and reversed 8-Br-cAMP-mediated facilitation of these glutamate-induced [Ca2+]irises. Our results suggest that ZD7288 inhibits hippocampal synaptic plasticity both glutamate release and resultant [Ca2+]iincreases in rat hippocampal neurons.
nerve regeneration; ZD7288; Ihchannels; perforant path—CA3 synapse; long-term potentiation; field excitatory postsynaptic potentials; glutamate release; neural regeneration
Introduction
Hyperpolarization-activated current (Ih) is mediated by hyperpolarization-activated cyclic nucleotide-gated (HCN) channels, also known as Ihchannels. These channels are widely distributed throughout the heart and the nervous system (DiFrancesco, 1993; Pape, 1996; Chen, 1997; Notomi and Shigemoto, 2004). Ihis very important in the control of cardiac and neuronal rhythmicity (DiFrancesco, 1993; Dickson et al., 2000). In addition, Ihcontributes to the determination of resting membrane potential, synaptic integration and transmission (Siegelbaum, 2000; Nolan et al., 2004). 4-(N-ethyl-N-phenylamino)-1, 2-dimethyl-6-(methylamino) pyrimidinium chloride (ZD7288) specifically blocks Ihchannels in various configurations (Gasparini and DiFrancesco, 1997; Satoh and Yamada, 2000; Gonzalez-Iglesias et al., 2006; Inaba et al., 2006; Matsuda et al., 2008a), and is often used to assess the physiological and pathophysiological roles of Ihchannels. In particular, the involvement of ZD7288 in synaptic modulation and plasticity is attracting increasing attention. ZD7288 inhibits long-term potentiation (LTP) at synapses between the perforant path and granule cells, mossy fibers and CA3 region, and Schaffer collateral pathway and CA1 region (Chevaleyre and Castillo, 2002; Mellor et al., 2002; Chen, 2004; He et al., 2010). However, its mechanism of action at the hippocampal perforant path—CA3 synapse is incompletely understood.
The hippocampal CA3 is a brain region that is involved in autoassociative memory. The entorhinal cortex sends a major projection to the hippocampus (Steward, 1976). The direct perforant path projection from the entorhinal cortex, terminating on pyramidal cells in the CA3, is a major route of cortical input to the hippocampal CA3, and memory retrieval is mediated by the associative plasticity of perforant path—CA3 synapse(O'Reilly and McClelland, 1994). In a previous study, we showed that ZD7288 suppresses basal synaptic transmission at the perforant path—CA3 synapse (Zheng et al., 2006). However, to date, no evidences have demonstrated that whether and how ZD7288 contributes to longterm plasticity at these synapses. We therefore examined the effects and mechanisms of ZD7288 on LTP induction and maintenance in the perforant path—CA3 pathway in adult rats.
Materials and Methods
Ethics statement and experimental animals
Animal studies were approved by the Animal Care and Use Committee of Tongji Medical College, Huazhong University of Science and Technology, China, and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Precautions were taken to minimize suffering and the number of animals used in each experiment. Specific-pathogen-free adult male Sprague-Dawley rats, aged 10 weeks and weighing 200 ± 20 g, were provided by the Experimental Animal Center of Tongji Medical College (License No. SCXK (E) 2010-0009) and housed under controlled temperature (20—24°C) and humidity (40—70%) conditions, with a 12-hour day/night cycle. The animals were acclimated to the environment for at least 7 days before experiments.
Electrophysiological recordings in vivo
Animals were anesthetized intraperitoneally with urethane 1.2 g/kg and fixed in a stereotaxic frame (SN-3, Narishige, Tokyo, Japan). Body temperature was kept at 37 ± 0.5°C during the experiment, using a constant temperature water cycling system. The skull landmark bregma was chosen as the stereotaxic reference point. Small holes were made in the skull and a stimulating electrode (bipolar stainless steel, 140 μm diameter) was placed at the perforant path (6.8—7.0 mm anteroposterior, 4.3—4.5 mm rostrolateral, depth 3.0—4.0 mm) and a recording electrode (monopolar stainless steel, 140 μm diameter) was positioned in ipsilateral hippocampal CA3 (3.3—3.5 mm anteroposterior, 3.3—3.5 mm rostrolateral). The depth of the recording electrode was determined by the maximal response. Test stimuli were given every 2 seconds (0.5 Hz, 0.15 ms duration) with a programmable electric stimulator (SEN-3201, Nihon Kohden, Tokyo, Japan) using an isolation unit (SS102J, Nihon Kohden). Field excitatory postsynaptic potentials (fEPSPs) were acquired, amplified, monitored and analyzed with a SMUP-PC biology signal processing system (Second Military Medical University, Shanghai, China). Baseline fEPSPs were recorded at 50—60% of the maximal response. LTP was then induced by a series of high-frequency stimuli (4 trains of 50 pulses at 100 Hz, 150 μs duration, intertrain interval of 20 seconds). fEPSPs were recorded 90 minutes after high-frequency stimulation. Baseline values were calculated by taking the mean EPSP amplitude at 5 different time points within 30 minutes before high-frequency stimulation. The ratio of absolute fEPSP amplitude to baseline value was used to describe the amplitude level.
For hippocampal administration of saline or drugs, a cannula was carefully inserted into the CA3 area with an introductory tube fixed parallel to the recording electrode, reaching 0.1—0.2 mm higher than the electrode tip. To test the effects of blockers on the induction of LTP, we applied 0.1 μM ZD7288 (Tocris Cookson, Avonmouth, UK) or the monovalent cation cesium (Cs+), a known nonspecific Ihantagonist (5 μM CsCl; Sigma-Aldrich, St. Louis, MO, USA; Matsuda et al., 2008b) 5 minutes before high-frequency stimulation. To test the effects of blockers on the maintenance of LTP, ZD7288/Cs+was slowly administered using an infusion/withdrawal pump 30 minutes after the high-frequency stimulation.
Neuronal culture
Primary hippocampal neurons were obtained from neonatal (1—2 day-old) Sprague-Dawley rats, provided by the Experimental Animal Center of Tongji Medical College (He et al., 2009; Huang et al., 2009). Rats were decapitated and brains were rapidly removed and placed in ice-cold phosphate buffered saline. The hippocampus was dissected out and digested with 0.125% trypsin for 20 minutes at 37°C, followed by mechanical dissociation and centrifugation at 1,000 ×g for 8 minutes. Cells were resuspended and plated on 96-well plates (for amino acid analysis) or poly-D-lysine-coated coverslips (for measurement of internal calcium concentration, [Ca2+]i). Neurons were cultured in Dulbecco's modified Eagle's medium/Ham's Nutrient Mixture F12 (DMEM/F12; Hyclone, Logan, UT, USA) with 10% fetal bovine serum (Lanzhou National Hyclone Bio-Engineering Co., Ltd., China), 100 U/L penicillin, 100 mg/L streptomycin and 0.5 mM glutamine, and kept at 37°C in a 5% CO2incubator (SHEL LAB, Cornelius, OR, USA). 10 mg/L arabinosylcytosine was added to the medium at 72 hours to reduce the number of non-neuronal cells. Half of the medium was changed every 2 days. Experiments were performed on days 8—11. The cells were incubated for 24 hours with ZD7288 (1, 5, or 50 μM), 8-bromoadenosine cyclic adenosine monophosphate (8-BrcAMP, 5 or 50 μM; Sigma-Aldrich), or forskolin (1 or 5 μM; Sigma-Aldrich), and the culture medium was collected for glutamate measurement.
Glutamate measurement
After LTP recording, the ipsilateral hippocampus was carefully dissociated from the brain, washed with ice-cold normal saline, and frozen at -80°C until processing. The thawed tissue was homogenized in 0.4 M perchloric acid. After aliquots were taken for protein determination, the homogenate was centrifuged at 10,000 × g for 15 minutes at 4°C. The homogenate was then neutralized with 2 M KHCO3and centrifuged at 3,000 × g for 5 minutes. The supernatant was frozen at -80°C for analysis. Protein levels were measured using Coomassie Brilliant Blue (DiFrancesco, 1993; Dickson et al., 2000; Zhu et al., 2014). The neuronal culture medium was collected after 24 hours of drug incubation and centrifuged at 3,000 × g for 5 minutes. The supernatant was stored at -80°C for analysis. Following automatic precolumn derivatization with O-phthaldialdehyde (Sigma-Aldrich) as previously described (McMahon and Barrionuevo, 2002), glutamate analysis was performed using high performance liquid chromatography (Kromasil ODS2 C18 column, Kromasil, Akzonobel, Zurich, Switzerland) with fluorescence detection using a spectrophotometer (excitation wavelength 330 nm, emission wavelength 420 nm; Prostar 370, Varian, Amsterdam, Holland). The external standard method was used to quantify the concentration of glutamate (Sigma-Aldrich) according to each peak area. Data were normalized against baseline glutamate values obtained from hippocampal tissue infused with saline and neurons treated with DMEM/F12. Glutamate level in each sample was expressed as the ratio of glutamate in the sample to the baseline value.
Measurement of [Ca2+]i
[Ca2+]imeasurements were performed according to our previous study in neurons (Huang et al., 2009). Cultured hippocampal neurons were incubated with 1 μM Fura-2 acetoxy-methylol ester (Biotium, Sunnyvale, CA, USA) for 30 minutes at 37°C, washed three times with artificial cerebrospinal fluid (containing 140 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM glucose and 10 mM hydroxyethyl piperazine ethanesulfonic acid, pH 7.3), and then incubated at room temperature in the dark for 30 minutes. Fura-2 fluorescence was observed by a Ratio Vision digital fluorescence microscopy system (TILL Photonics GmbH, Freiburg, Germany). Fluorescence signals were evoked by 340 and 380 nm excitation wavelengths and collected at 510 nm by TILLvisION 4.0 software. The 340:380 nm fluorescence ratio was used to represent [Ca2+]i. Peak calcium change was represented as the percentage increase from baseline. Neurons were incubated in ZD7288 (25, 50 or 100 μM) or 8-Br-cAMP (5 or 50 μM) for 15 minutes prior to stimulation with 50 μM glutamate. All experiments were repeated in triplicate, using different batches of cells across 4—5 dishes.
Statistical analysis
All data are presented as the mean ± SEM, and were analyzed using SPSS 12.0 software (SPSS, Chicago, IL, USA). Statistical significance between groups was determined using oneway analysis of variance followed by post hoc comparisons. P< 0.05 was considered statistically significant.
Results
Effects of ZD7288 on the induction of LTP at the perforant path—CA3 synapse in rats
Our previous study in vivo showed that ZD7288 depressed basal synaptic transmission at the perforant path—CA3 synapse in a concentration-dependent manner (Zheng et al., 2006). Here, we have shown that ZD7288 and CsCl blocked the induction of LTP at the perforant path—CA3 synapse. High-frequency stimulation caused a marked increase in amplitude of the fEPSP in rats that received normal saline over the 90-minute recording period, and the mean magnitude of fEPSP was 281.8 ± 6.6% of the baseline value (P <0.05; Figure 1). LTP was induced in the perforant path—CA3 synapse by high-frequency stimulation of perforant path fibers. However, application of ZD7288 0.1 μM at 5 minutes before high-frequency stimulation significantly decreased the amplitude of fEPSP, and this inhibitory effect was maintained throughout the recording period. At 30, 60 and 90 minutes after high-frequency stimulation, fEPSP amplitudes were 92.4 ± 10.1%, 85.6 ± 12.0% and 85.2 ± 11.8% of baseline respectively (P < 0.01, vs. normal saline group). The induction of LTP was markedly suppressed by ZD7288.
To confirm that the ZD7288-induced reduction of LTP was due to its role of blocking Ihchannels, we used another known Ihblocker, Cs+, commonly used as a diagnostic tool for Ih(Wickenden et al., 2009), to test its effect on LTP induction. Cs+(1 mM) significantly inhibited the induction of LTP at the perforant path—CA3 synpase. fEPSP amplitudes were significantly lower at each time point after application of CsCl (1 mM) 5 minutes before high-frequency stimulation. At 30, 60 and 90 minutes after high-frequency stimulation, fEPSP amplitudes were 41.6 ± 12.8%, 75.6 ± 11.6% and 78.1 ± 5.5% of baseline, respectively (P < 0.01, vs. normal saline group). The inhibitory effect of Cs+on LTP was attenuated over time. Our results indicate that Ihchannels are involved in the induction of LTP at perforant path—CA3 synapses.
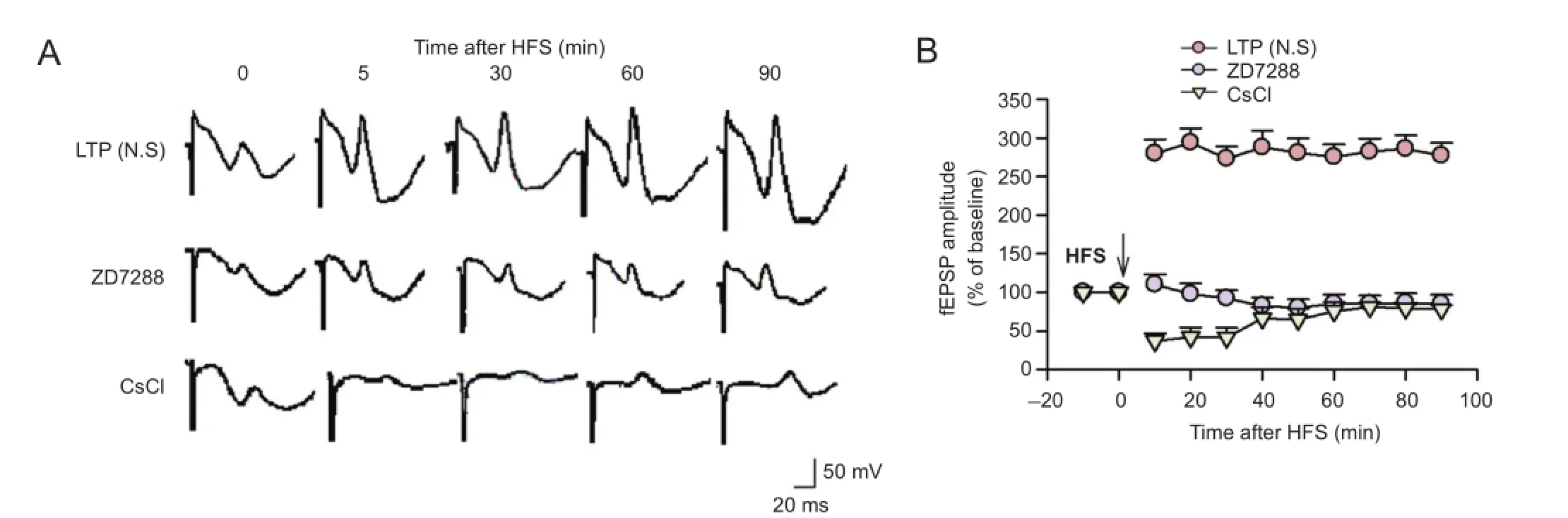

Figure 1 Effects of ZD7288 and CsCl on the induction of LTP at the PP-CA3 synapse in anesthetized rats.
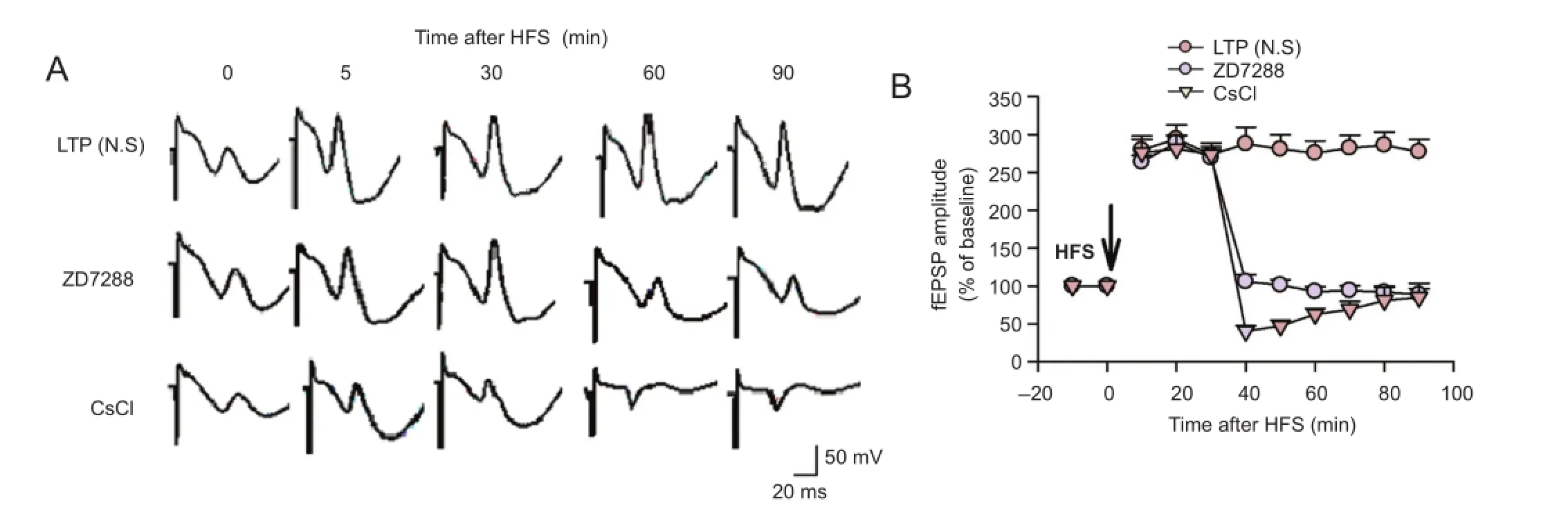

Figure 2 Effect of ZD7288 and CsCl on the maintenance of LTP at PP-CA3 synapses in anesthetized rats.
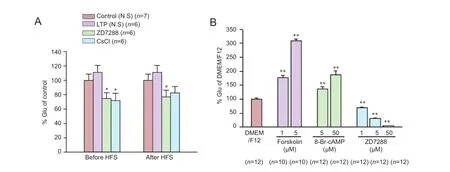
Figure 3 Effects of ZD7288, CsCl and cAMP on the release of glutamate in vivo and in vitro.
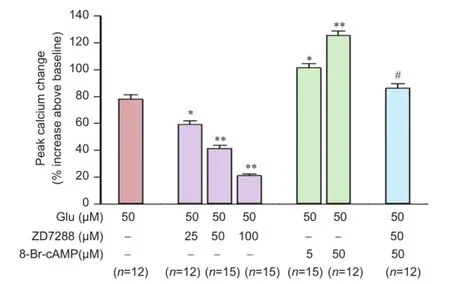
Figure 4 Effects of ZD7288 and 8-Br-cAMP on Glu-induced [Ca2+]irise in rat hippocampal neurons.
Effect of ZD7288 on LTP maintenance at perforant path—CA3 synapses in rats
To further study the role of Ihchannels in the maintenance of LTP, the effect of ZD7288 on previously-established LTP was examined. Application of 0.1 μM ZD7288 30 minutes after high-frequency stimulation almost completely reversed the established LTP (Figure 2). Amplitudes were 92.6 ± 6.4% and 88.9 ± 7.6% of baseline at 60 and 90 minutes after high-frequency stimulation, respectively (P < 0.01, vs. normal saline group). Application of 1 mM CsCl 30 minutes after high-frequency stimulation produced similar inhibitory effects. fEPSP amplitudes were 62.6 ± 7.6% and 84.8 ± 18.8% of baseline at 60 and 90 minutes after high-frequency stimulation, respectively (P < 0.01, vs. normal saline group). Moreover, the inhibitory effect of CsCl decreased with time. These results demonstrate that ZD7288 and Cs+block LTP maintenance at perforant path—CA3 synapses in rats.
Effect of ZD7288 on glutamate release in the hippocampus
In some neurons, presynaptic Ihchannels regulate synaptic transmission by controlling transmitter release. Glutamate plays an important role in LTP formation at the perforant path—CA3 synapse, of which the LTP induction is N-methyl-D-aspartate receptor-dependent (McMahon and Barrionuevo, 2002). We examined the effect of ZD7288 on glutamate release in hippocampal tissues. Application of high-frequency stimuli resulted in a slight increase of glutamate levels in rats that received normal saline (Figure 3A). Glutamate levels were increased to 111.1 ± 9.6% (P > 0.05, vs. control rats receiving normal saline and no high-frequency stimulation [138.4 ± 34.3 μmol/g protein, normalized as 100 ± 8.8%]). Following application of ZD7288 (0.1 μM) 5 minutes before high-frequency stimulation, glutamate content was reduced to 74.9 ± 8.0% (P < 0.05, vs. normal saline group). CsCl (1 mM) application before high-frequency stimulation produced the same effect as ZD7288; glutamate content was decreased to 71.9 ± 10.0% (P < 0.05, vs. normalsaline group). Furthermore, application of 0.1 μM ZD7288 30 minutes after high-frequency stimulation markedly decreased the glutamate content to 77.0% ± 9.4% (P < 0.05, vs. normal saline group). The glutamate content was reduced to 82.5% ± 9.1% when application with 1 mM CsCl after high-frequency stimulation. However, there was no significant difference compared with normal saline group (P > 0.05, vs. normal saline group).
Next, we examined the effect of ZD7288 on glutamate release in cultured hippocampal neurons. ZD7288 inhibited glutamate release in a concentration-dependent manner (Figure 3B). After incubation with 1, 5 and 50 μM ZD7288 for 24 hours, glutamate content in extracellular fluid was decreased to 69.0 ± 2.8%, 31.4 ± 2.0% and 4.4 ± 0.3%, respectively (P < 0.01, vs. DMEM/F12 group [100.2 ± 4.2%]).
It has been reported that the activity of Ihchannels can be enhanced by elevating cAMP levels. To confirm that the inhibitory effect of ZD7288 on glutamate release is Ihchannel-dependent, we explored the effects of pharmacological elevation of cAMP levels on glutamate release, using forskolin and 8-Br-cAMP. After incubation with 5 and 50 μM 8-BrcAMP, glutamate content in extracellular fluid increased to 136.1 ± 7.4% and 188.1 ± 13.8% respectively (P < 0.01, vs. DMEM/F12 group). Moreover, after incubation with forskolin (1 and 5 μM), extracellular glutamate content was increased to 177.6 ± 6.8% and 308.7 ± 6.9%, respectively (P< 0.01, vs. DMEM/F12 group).
Effect of ZD7288 on glutamate-induced [Ca2+]irise in rat hippocampal neurons
Intracellular calcium plays an important role in transmitter release. We therefore also measured the effect of ZD7288 on intracellular calcium levels. In cultured hippocampal neurons, glutamate (50 μM) evoked a significant increase of [Ca2+]i78.0 ± 3.3% increase above baseline. After incubation with ZD7288 (25, 50, or 100μM) for 20 minutes, 50 μM glutamate-induced [Ca2+]irises were attenuated to 59.2 ± 2.7%, 41.4 ± 2.3% and 21.0 ± 1.4%, respectively glutamate (P < 0.01, vs. 50 μM glutamate group; Figure 4). ZD7288 attenuated the glutamate-induced [Ca2+]irise in a concentration-dependent manner. We also explored the effect of 8-Br-cAMP on glutamate-induced [Ca2+]irise. 8-Br-cAMP facilitated the glutamate-induced rise in [Ca2+]i(Figure 4). After incubation with 5 and 50 μM 8-Br-cAMP for 5 minutes, glutamate-induced [Ca2+]iincreased by 101.3 ± 3.1% and 125.4 ± 3.4%, respectively (P < 0.01, vs. 50 μM glutamate group). After incubation with ZD7288 for 20 minutes, 50 μM 8-Br-cAMP increased the glutamate-induced rise in [Ca2+]iby 86.2 ± 3.3% (P < 0.01, vs. 50 μM 8-Br-cAMP group). Treatment with 50 μM ZD7288 almost completely reversed the facilitation of 8-Br-cAMP in [Ca2+]i.
Discussion
Ihchannels play important roles in regulating excitability and rhythmic activity of neurons. Ihchannels are also important in synaptic modulation and plasticity. In a wellknown trisynaptic model of hippocampal circuitry, ZD7288 depressed synaptic transmission at perforant path—granule cell synapses by inhibiting postsynaptic glutamate receptors (Chen, 2004). ZD7288-induced reduction of mossy fiber LTP is due to its inhibition of neurotransmitter release (Mellor et al., 2002). Our previous study demonstrated that Ihchannels were also involved in Schaffer—CA1 pathway LTP via inhibiting N-methyl-D-aspartate receptor function (He et al., 2010). Here, we investigated the role of Ihin synaptic plasticity of the direct cortical projection to the hippocampus. We focused on the perforant path—CA3 synapse, the major route of cortical projection to the hippocampal CA3 area, which mediates memory retrieval. In agreement with previous studies indicating that perforant path inputs might be capable of driving CA3 pyramidal cells to fire (Urban et al., 1998; McMahon and Barrionuevo, 2002), our data demonstrated that perforant path fiber stimulation induces LTP, the average fEPSP amplitude being sustained at 282% of baseline for over 1 hour. Furthermore, treatment with ZD7288 inhibited LTP induction and completely reversed the established LTP, and the inhibitory effects were maintained for at least 1 hour.
In addition to blocking Ihchannels, which may result in nonspecific inhibition of the postsynaptic glutamate receptor, ZD7288 depresses LTP by inhibition of postsynaptic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid and N-methyl-D-aspartate receptor-mediated responses at the perforant path—granule cell synapse (Chen, 2004). McMahon and Barrionuevo (2002) showed that perforant path—CA3 LTP is N-methyl-D-aspartate receptor-dependent. To investigate whether ZD7288-induced synaptic depression is a specific consequence of Ihchannel blockade, we investigated whether its effects were similar to another Ihchannel blocker, CsCl. Indeed, 1 mM CsCl produced a comparable LTP-inhibiting effect to ZD7288, blocking LTP induction when applied before high-frequency stimulation, and reversing it when applied after high-frequency stimulation. These data demonstrate that the induction and maintenance of LTP are both suppressed when Ihchannels are blocked, suggesting that Ihchannels might directly participate in the process of induction and maintenance of LTP. However, the inhibitory effect of CsCl gradually attenuated over time. As Cs+is a nonselective Ihchannel blocker, inhibition of potassium channels may have contributed to this attenuation.
Ihchannels are widely distributed in the nervous system, and have been identified in mammalian presynaptic terminals (Southan et al., 2000; Cuttle et al., 2001). The channels are assembled as HCN1—HCN4subunits. HCN1and HCN2are presynaptic and localized in the CA3 pyramidal cell layer (Notomi and Shigemoto, 2004). Many previous studies have revealed that functional presynaptic Ihchannels play significant roles in synaptic transmission and long-term plasticity by controlling transmitter release (Beaumont and Zucker, 2000; Mellor et al., 2002; Huang and Hsu, 2003). To explore the possibility that ZD7288 depressed perforant path—CA3 pathway synaptic plasticity via a presynaptic mechanism, we examined the effects of ZD7288 on glutamate release. Wefound that treated with ZD7288 before and after high-frequency stimulation the glutamate content of hippocampal cells was lower than that of the saline group, and CsCl (1 mM) also inhibited glutamate release. The increase in the level of glutamate after high-frequency stimulation indicated that the stimulation activated glutamatergic neurons. However, the glutamate increase was not significantly different from control. It is probably because that the whole hippocampus was used in this study to detect glutamate content. Therefore, we further studied the effects of ZD7288 on glutamate release using cultured hippocampal neurons. As predicted, ZD7288 markedly inhibited glutamate release in the cultured cells. Ihchannels are activated not only by hyperpolarization, but also by the gating of intracellular cAMP levels. cAMP enhances the activity of Ihchannels by directly binding to the channel or by indirect activation of protein kinase A (Lüthi and McCormick, 1998; Abi-Gerges et al., 2000; Mellor et al., 2002; Genlain et al., 2007). Increased intracellular cAMP and activation of protein kinase A are essential for the generation of LTP (Pape, 1996; Mellor et al., 2002). ZD7288 inhibits cAMP-triggered increases of miniature excitatory postsynaptic current frequency by blockade of Ihchannels (Genlain et al., 2007). Here, cAMP level was elevated by applying the cAMP analog 8-Br-cAMP and the adenylyl-cyclase activator forskolin. We found that both 8-Br-cAMP and forskolin increased glutamate release in cultured hippocampal neurons. These results suggest that Ihchannels are involved in glutamate release. ZD7288 inhibited LTP formation by depressing glutamate release and by blocking Ihchannels.
Two mechanisms have been proposed to underlie Ihchannel modulation of glutamate release. One is associated with Ca2+-induced Ca2+release from the store and suggests that the activation of Ihchannels accompanied by Ca2+influx triggers the process of modulation (Yu et al., 2004). The other proposed mechanism is that Ihchannels directly couple to the release machinery in a calcium-independent manner (Beaumont and Zucker, 2000). In the present study, to test whether the ZD7288-induced inhibition of glutamate release was associated with intracellular calcium, glutamate was used as an activator to induce a rise in [Ca2+]i. ZD7288 inhibited glutamate-induced [Ca2+]iincreases in a concentration-dependent manner and reversed the 8-Br-cAMP-evoked rise in [Ca2+]i. Our data suggest that the inhibitory effect of ZD7288 on glutamate release results from its attenuation of [Ca2+]i. The activation of Ihchannels may enhance Ca2+influx into the presynaptic terminal by depolarization; the opening of voltage-dependent calcium channels would, in turn, lead to persistent enhancement of glutamate release. More research is needed to explore the details of this proposed mechanism.
Ihchannels are involved in many diseases, including epilepsy, vascular dementia and peripheral neuralgia (Li et al., 2010; Takasu et al., 2010; DiFrancesco et al., 2011). Ischemia is one of the commonest damaging factors in the nervous system. The excessive release of glutamate and the overload of intracellular Ca2+play key roles in ischemic neuronal death (Mori et al., 2004; Zhao et al., 2006). Neuronal hyperexcitability enhances calcium influx, which subsequently triggers the release of excitatory neurotransmitters, especially glutamate. The excessive release of glutamate can lead to extra Ca2+influx during ischemia. Ihchannels are involved in ischemic lesions. Our previous studies showed that HCN1mRNA and protein were decreased in chronic incomplete global cerebral ischemia (Li et al., 2010). We propose that the Ihchannel blocker ZD7288 inhibits both glutamate release and the glutamate-induced rise in [Ca2+]i, which might contribute to its neuroprotective effects under conditions of cerebral ischemia.
In conclusion, the Ihchannel blocker ZD7288 can markedly suppress LTP at perforant path—CA3 synapses. The inhibitory effect is likely due to attenuating release of glutamate and glutamate-induced [Ca2+]irise in rat hippocampal neurons.
Author contributions: XXZ and XCM performed the research, analyzed the data and wrote the paper. MZ participated in the study and revised the paper. XLX and LJG designed the research and revised the paper. All authors approved the final version of the paper.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Abi-Gerges N, Ji GJ, Lu ZJ, Fischmeister R, Hescheler J, Fleischmann BK (2000) Functional expression and regulation of the hyperpolarization activated non-selective cation current in embryonic stem cell-derived cardiomyocytes. J Physiol 523:377-389.
Beaumont V, Zucker RS (2000) Enhancement of synaptic transmission by cyclic AMP modulation of presynaptic Ih channels. Nat Neurosci 3:133-141.
Chen C (1997) Hyperpolarization-activated current (Ih) in primary auditory neurons. Hearing Res 110:179-190.
Chen C (2004) ZD7288 inhibits postsynaptic glutamate receptor-mediated responses at hippocampal perforant path-granule cell synapses. Eur J Neurosci 19:643-649.
Chevaleyre V, Castillo PE (2002) Assessing the role of Ih channels in synaptic transmission and mossy fiber LTP. Proc Natl Acad Sci U S A 99:9538-9543.
Cuttle MF, Rusznák Z, Wong AYC, Owens S, Forsythe ID (2001) Modulation of a presynaptic hyperpolarization-activated cationic current (I(h)) at an excitatory synaptic terminal in the rat auditory brainstem. J Physiol 534:733-744.
Dickson CT, Magistretti J, Shalinsky MH, Fransén E, Hasselmo ME, Alonso A (2000) Properties and role of I(h) in the pacing of subthreshold oscillations in entorhinal cortex layer II neurons. J Neurophysiol 83:2562-2579.
DiFrancesco D (1993) Pacemaker mechanisms in cardiac tissue. Annu Rev Physiol 55:455-472.
DiFrancesco JC, Barbuti A, Milanesi R, Coco S, Bucchi A, Bottelli G, Ferrarese C, Franceschetti S, Terragni B, Baruscotti M, DiFrancesco D (2011) Recessive loss-of-function mutation in the pacemaker HCN2 channel causing increased neuronal excitability in a patient with idiopathic generalized epilepsy. J Neurosci 31:17327-17337.
Gasparini S, DiFrancesco D (1997) Action of the hyperpolarization-activated current (Ih) blocker ZD 7288 in hippocampal CA1 neurons. Pflugers Arch 435:99-106.
Genlain M, Godaux E, Ris L (2007) Involvement of hyperpolarization-activated cation channels in synaptic modulation. Neuroreport 18:1231-1235.
Gonzalez-Iglesias AE, Kretschmannova K, Tomic M, Stojilkovic SS (2006) ZD7288 inhibits exocytosis in an HCN-independent manner and downstream of voltage-gated calcium influx in pituitary lactotrophs. Biochem Biophys Res Commun 346:845-850.
He W, Cheng Z, Fu G, Xu X, Lu Q, Guo L (2010) ZD7288-induced suppression of long-term potentiation was attenuated by exogenous NMDA at the Schaffer collateral-CA1 synapse in the rat in vivo. Eur J Pharmacol 631:10-16.
He Z, Lu Q, Xu X, Huang L, Chen J, Guo L (2009) DDPH ameliorated oxygen and glucose deprivation-induced injury in rat hippocampal neurons via interrupting Ca2+overload and glutamate release. Eur J Pharmacol 603:50-55.
Huang CC, Hsu KS (2003) Reexamination of the role of hyperpolarization-activated cation channels in short- and long-term plasticity at hippocampal mossy fiber synapses. Neuropharmacology 44:968-981.
Huang L, Li Q, Li H, He Z, Cheng Z, Chen J, Guo L (2009) Inhibition of intracellular Ca2+release by a Rho-kinase inhibitor for the treatment of ischemic damage in primary cultured rat hippocampal neurons. Eur J Pharmacol 602:238-244.
Inaba Y, Biagini G, Avoli M (2006) The H current blocker ZD7288 decreases epileptiform hyperexcitability in the rat neocortex by depressing synaptic transmission. Neuropharmacology 51:681-691.
Lüthi A, McCormick DA (1998) H-current: properties of a neuronal and network pacemaker. Neuron 21:9-12.
Li S, He Z, Guo L, Huang L, Wang J, He W (2010) Behavioral alterations associated with a down regulation of HCN1 mRNA in hippocampal cornus ammon 1 region and neocortex after chronic incomplete global cerebral ischemia in rats. Neuroscience 165:654-661.
Matsuda Y, Ang FY, Nakajima K, Kogure S (2008a) Effects of hyperpolarization-activated channel blocker ZD7288 on polar excitations of frog sciatic nerve. J Physiol Sci 58:99-104.
Matsuda Y, Saito N, Yamamoto K, Niitsu T, Kogure S (2008b) Effects of the Ih blockers CsCl and ZD7288 on inherited epilepsy in Mongolian gerbils. Exp Anim 57:377-384.
McMahon DB, Barrionuevo G (2002) Short- and long-term plasticity of the perforant path synapse in hippocampal area CA3. J Neurophysiol 88:528-533.
Mellor J, Nicoll RA, Schmitz D (2002) Mediation of hippocampal mossy fiber long-term potentiation by presynaptic Ih channels. Science 295:143-147.
Mori T, Tateishi N, Kagamiishi Y, Shimoda T, Satoh S, Ono S, Katsube N, Asano T (2004) Attenuation of a delayed increase in the extracellular glutamate level in the peri-infarct area following focal cerebral ischemia by a novel agent ONO-2506. Neurochem Int 45:381-387.
Nolan MF, Malleret G, Dudman JT, Buhl DL, Santoro B, Gibbs E, Vronskaya S, Buzsáki G, Siegelbaum SA, Kandel ER, Morozov A (2004) A behavioral role for dendritic integration: HCN1 channels constrain spatial memory and plasticity at inputs to distal dendrites of CA1 pyramidal neurons. Cell 119:719-732.
Notomi T, Shigemoto R (2004) Immunohistochemical localization of Ih channel subunits, HCN1—4, in the rat brain. J Comp Neurol 471:241-276.
O'Reilly RC, McClelland JL (1994) Hippocampal conjunctive encoding, storage, and recall: avoiding a trade-off. Hippocampus 4:661-682.
Pape HC (1996) Queer current and pacemaker: the hyperpolarization-activated cation current in neurons. Annu Rev Physiol 58:299-327.
Satoh TO, Yamada M (2000) A bradycardiac agent ZD7288 blocks the hyperpolarization-activated current (Ih) in retinal rod photoreceptors. Neuropharmacology 39:1284-1291.
Siegelbaum SA (2000) Presynaptic facilitation by hyperpolarization-activated pacemaker channels. Nat Neurosci 3:101-102.
Southan AP, Morris NP, Stephens GJ, Robertson B (2000) Hyperpolarization-activated currents in presynaptic terminals of mouse cerebellar basket cells. J Physiol 526:91-97.
Steward O (1976) Topographic organization of the projections from the entorhinal area to the hippocampal formation of the rat. J Comp Neurol 167:284-314.
Takasu K, Ono H, Tanabe M (2010) Spinal hyperpolarization-activated cyclic nucleotide-gated cation channels at primary afferent terminals contribute to chronic pain. Pain 151:87-96.
Urban NN, Henze DA, Barrionuevo G (1998) Amplification of perforant-path EPSPs in CA3 pyramidal cells by LVA calcium and sodium channels. J Neurophysiol 80:1558-1561.
Wickenden AD, Maher MP, Chaplan SR (2009) HCN pacemaker channels and pain: a drug discovery perspective. Curr Pharm Des 15:2149-2168.
Yu X, Duan KL, Shang CF, Yu HG, Zhou Z (2004) Calcium influx through hyperpolarization-activated cation channels (I(h) channels) contributes to activity-evoked neuronal secretion. Proc Natl Acad Sci U S A 101:1051-1056.
Zhao YM, Sun LN, Zhou HY, Wang XL (2006) Voltage-dependent potassium channels are involved in glutamate-induced apoptosis of rat hippocampal neurons. Neurosci Lett 398:22-27.
Zheng M, Guo LJ, Xu XL, Hu HZ, Zong XG (2006) ZD7288 inhibits the synaptic transmission in the pathway from perforant pathway fibers to CA3 region in rat hippocampus. Yao Xue Xue Bao 41:565-571.
Zhu L, Qu XH, Sun YL, Qian YM, Zhao XH (2014) Novel method for extracting exosomes of hepatocellular carcinoma cells. World J Gastroenterol 20:6651-6657.
Copyedited by Slone-Murphy J, Pack M, Yu J, Qiu Y, Li CH, Song LP, Zhao M
10.4103/1673-5374.182705 http://www.nrronline.org/
How to cite this article: Zhang XX, Min XC, Xu XL, Zheng M, Guo LJ (2016) ZD7288, a selective hyperpolarization-activated cyclic nucleotide-gated channel blocker, inhibits hippocampal synaptic plasticity. Neural Regen Res 11(5)∶779-786.
Funding: This work was supported by grants from the National Natural Science Foundation of China, No. 81173038, 81001425.
Accepted: 2015-12-22
*Correspondence to: Min Zheng or Lian-jun Guo, ljguo@hust.edu.cn.
 中國(guó)神經(jīng)再生研究(英文版)2016年5期
中國(guó)神經(jīng)再生研究(英文版)2016年5期
- 中國(guó)神經(jīng)再生研究(英文版)的其它文章
- Recovery of injured fornical crura following neurosurgical operation of a brain tumor: a case report
- Gender difference in the neuroprotective effect of rat bone marrow mesenchymal cells against hypoxiainduced apoptosis of retinal ganglion cells
- Vitamin B complex and vitamin B12levels after peripheral nerve injury
- Methylprednisolone microsphere sustained-release membrane inhibits scar formation at the site of peripheral nerve lesion
- A self-made, low-cost infrared system for evaluating the sciatic functional index in mice
- Methylprednisolone exerts neuroprotective effects by regulating autophagy and apoptosis
