Efficient Removal Phenol Red over Ternary Heterostructured Ag-Bi2MoO6/BiPO4Composite Photocatalyst
Da-yu Jiang,Da Xu,JiaZheng,Yang Yang,Chang Liu,Yu-shuang Wang,Guang-bo Che, Xue Lin,Li-min ChangKey Laboratory of Preparation and Applications of Environmental Friendly Materials,Ministry of Education,Jilin Normal University,Changchun 130103,China
Efficient Removal Phenol Red over Ternary Heterostructured Ag-Bi2MoO6/BiPO4Composite Photocatalyst
Da-yu Jiang,Da Xu,Jia?Zheng,Yang Yang,Chang Liu,Yu-shuang Wang,Guang-bo Che, Xue Lin?,Li-min Chang
Key Laboratory of Preparation and Applications of Environmental Friendly Materials,Ministry of Education,Jilin Normal University,Changchun 130103,China
The fabrication of multicomponent composite systems may provide benefits in terms of charge separation and the retardation of charge pair recombination.In this work,a ternary heterostructured Ag-Bi2MoO6/BiPO4composite was fabricated through a low-temperature solution-phase route for the first time.The XRD,SEM,EDX and XPS results indicated the prepared sample is a three-phase composite of BiPO4,Bi2MoO6,and Ag.Ag nanoparticles were photodeposited on the surface of Bi2MoO6/BiPO4nanosheets,which not only increase visible-light absorption via the surface plasmon resonance,but also serve as good electron acceptor for facilitating quick photoexcited electron transfer.The interface between Bi2MoO6and BiPO4facilitates the migration of photoinduced electrons from Bi2MoO6to BiPO4,which is also conductive to reduce the recombination of electron-holes.Thus,the ternary heterostructured Ag-Bi2MoO6/BiPO4composite showed significant photocatalytic activity,higher than pure Bi2MoO6,BiPO4,and Bi2MoO6/BiPO4.Moreover,the possible photocatalytic mechanism of the Ag-Bi2MoO6/BiPO4heterostructure related to the band positions of the semiconductors was also discussed.In addition,the quenching effects of different scavengers revealed that the reactive·OH and O2·?play a major role in the phenol red decolorization.
Heterostructure,Bi2MoO6,BiPO4,Ag,Photocatalysis,Visible light
I.INTRODUCTION
In recent decades,TiO2has been applied and investigated broadly for the photodegradation of organic pollutants in water owing to its low cost,strong oxidizing power,and nontoxic nature[1].However,the main drawbacks of limited visible light utilization and low quantum yields limit its practical applications[2-4].Therefore,many efforts have been made to fabricate excellent visible-light-driven photocatalysts such as Bi2WO6[5],Bi2MoO6[6],BiVO4[7],InVO4[8], etc.Among these photocatalysts,Bi2MoO6has been found to show excellent visible-light-driven photocatalytic activity for water splitting and for the degradation of organic contaminants[9-12].However,further research on the enhancement of the Bi2MoO6photocatalytic performance is still indispensable because of its poor quantum yield.
Among a variety of methods,the construction of composite photocatalysts has been proven to be an effective method for improving photocatalytic activity for the degradation of organic contaminants[13,14].In the composite photocatalysts,the interface between coupled semiconductors and/or metals can lead to more efficient interfacial charge transfer and enhance the photoinduced charge separation.So far,a variety of Bi2MoO6-based photocatalysts have been prepared,such as Bi2MoO6/TiO2[15],Bi2MoO6/C[16], Bi2MoO6/Bi2O3[17],and Ag/Bi2MoO6[18].In our previous work,we presented the hydrothermal synthesis of Bi2MoO6/BiPO4[19],Bi2MoO6/SiO2[20],and Bi2MoO6/BiVO4[21].The as-synthesized composites showed superior photocatalytic activities than that of pure Bi2MoO6.Motivated by the above efforts,we further research the synthesis of ternary heterostructured Ag-Bi2MoO6/BiPO4composite.In addition,to the best of our knowledge,there are no reports on the synthesis and photocatalytic activity of this material.
Recent years,BiPO4has been paid much attention,which has been proven to show much higher photocatalytic activity than TiO2(P25)for the degradation of organic contaminants under UV light[22]. Many studies revealed that BiPO4based photocatalysts displayed excellent photocatalytic performances under visible light irradiation,such as Ag3PO4/BiPO4, Ag/Ag3PO4/BiPO4etc.[23,24].
In this work,the composite photocatalysts of ternaryheterostructured Ag-Bi2MoO6/BiPO4were successfully synthesized for the first time. The phenol red was used as a mode compound to investigate the photocatalytic performances of the ternary heterostructured composites under visible-light irradiation(λ>420 nm). The results demonstrated that compared with pure Bi2MoO6,BiPO4,and Bi2MoO6/BiPO4composite,the ternary heterostructured Ag-Bi2MoO6/BiPO4composite photocatalysts had a remarkably enhanced phenol red photodegradation activity under visible-light irradiation.The 1.27%Ag-Bi2MoO6/BiPO4catalyst performed the best in the degradation of phenol red.Moreover,the possible photocatalytic mechanism of the Ag-Bi2MoO6/BiPO4heterostructure related to the band positions of the semiconductors was also discussed in detail.
?Authors to whom correspondence should be addressed.E-mail: jlsdlinxue@126.com,Tel.: +86-15694349717,FAX:+86-434-3291890
II.EXPERIMENTS
A.Preparation of photocatalysts
1.Preparation of Bi2MoO6/BiPO4photocatalyst
All chemicals were analytic grade and used without further purification.More details about the preparation of Bi2MoO6/BiPO4,can be found in our previous work [19].In a typical procedure,Bi(NO3)3·5H2O(2 mmol) was firstly dissolved with Na2MoO4·2H2O(1 mmol)or Na3PO4·12H2O(2 mmol)in 15 mL of distilled water.
For synthesis of Bi2MoO6,the pH value of solution was adjusted to 6 by adding concentrated ammonia. The mixture was then transferred into a 20 mL Teflonlined stainless steel autoclave,and heated to 160°C for 24 h.After reaction,the obtained solid was washed with ethanol and distilled water several times,and dried at 80°C for 10 h.For synthesis of BiPO4,the pH value of solution was adjusted to 1 by adding 1 mol/L HNO3. The mixture was then transferred into a 20 mL Teflonlined stainless steel autoclave.The autoclave was kept at 160°C for 24 h,and work-up of the products was described above.For synthesis of Bi2MoO6/BiPO4composite,Bi(NO3)3·5H2O(3 mmol)and total 2 mmol Na2MoO4+NaPO4(molar ratio of Mo:P was 1:1)were dissolved in 15 mL of distilled water.The pH value of the mixture was adjusted to 1 by adding 1 mol/L HNO3. The mixture was then transferred into a 20 mL Teflonlined stainless steel autoclave.The autoclave was kept at 160°C for 24 h,and work-up of the products was described above.The obtained products were denoted as Bi2MoO6,BiPO4,Bi2MoO6/BiPO4,respectively.
2.Preparation of Ag-Bi2MoO6/BiPO4photocatalyst
The synthesized Bi2MoO6/BiPO4composite(0.1 g) were mixed with 200 mL of deionized water followed by ultrasonication for 30 min.Then,1.0 mL of 5% polyethylene glycol(PEG)2000 solution was added and the dispersion was stirred for another 30 min.For deposition of silver on the surface of the composite,a photodeposition method was used as follows:A certain amount of AgNO3solution(2.7 mg/mL)was added to the dispersion.Then the suspension was transferred to a water-cooled reactor(250 mL)and irradiated under a Xe lamp with 300 mW/cm2illumination intensity for 60 min.The precipitates were washed with deionized water and ethanol twice,respectively.The final products were dried at 80°C for 6 h in a vacuum box, denoted as 0.65%Ag-Bi2MoO6/BiPO4,and 1.27%Ag-Bi2MoO6/BiPO4.
B.Characterization of photocatalysts
The crystal structures of the samples were characterized by X-ray diffraction(XRD)on a Rigaku (Japan)D/max 2500 X-ray diffractometer(Cu Kα radiation,λ=0.15418 nm).The morphologies and structure details of the as-synthesized samples were detected by using field emission scanning microscopy (FESEM,JSM-6700F)and transmission electron microscopy(TEM,JEM-2100F).The chemical compositions of the as-fabricated compounds were determined by scanning electron microscope-X-ray energy dispersion spectra(SEM-EDX,JSM-6700F).X-ray photoelectron spectroscopy(XPS)analysis was performed with an ESCALa-b220i-XL electron spectrometer(VGScientific,England)using 300 W Al Kα radiation.The photoluminescence(PL)spectra of the photocatalysts were obtained by a F4500(Hitachi,Japan)photoluminescence detector with an excitation wavelength of 325 nm.The UV-Vis diffuse reflectance spectra(DRS) were recorded using a scan UV-Vis spectrophotometer (UV-2550).
C.Photocatalytic activities studies
The photocatalytic properties of the as-prepared samples were evaluated using phenol red as a model compound.The phenol red is a very stable compound, which has been used widely as a representative reaction for examining the performance of numerous visible light active catalysts.In experiments,the phenol red solution (0.01 mmol/L,100 mL)containing 0.05 g of photocatalyst were mixed in a pyrex reaction glass.A 300 W Xe lamp(with illumination intensity of 100 mW/cm2)was employed to provide visible light irradiation.The distance between the lamp and the sample was 10 cm.A 420 nm cut-off filter was inserted between the lamp and the sample to filter out UV light(λ<420 nm).Prior to visible light illumination,the suspension was strongly stirred in the dark for 60 min to ensure the establishment of adsorption-desorption equilibrium.Then the solution was exposed to visible light irradiation under magnetic stirring.At given time intervals,4 mL of the suspension was periodically collected and analyzed after centrifugation.The phenol red concentration wasanalyzed by a UV-2550 spectrometer to record intensity of the maximum band at 432 nm in the UV-Vis absorption spectra.
D.Active species trapping experiments
For detecting the active species during photocatalytic reactivity,some sacrificial agents,such as 2-propanol(IPA),ammonium oxalate(AO),and 1,4-benzoquinone(BQ)were used as the hydroxyl radical (·OH)scavenger,hole(h+)scavenger and superoxide radical(O2·?)scavenger,respectively.The method was similar to the former photocatalytic activity test with the addition of 1 mmol of quencher in the presence of phenol red.
III.RESULTS AND DISCUSSION
Figure1showstheXRD patternsoftheassynthesized Bi2MoO6,BiPO4,Bi2MoO6/BiPO4,and Ag-Bi2MoO6/BiPO4composites.The diffraction peaks of Bi2MoO6and BiPO4can be exactly indexed as JCPDS No.15-0767 and No.21-0121.When coupling the two semiconductors,the main characteristic diffraction peaks of Bi2MoO6and BiPO4did not change obviously.The Ag-Bi2MoO6/BiPO4composites showed a coexistence of Bi2MoO6phase(JCPDS No.15-0767) and BiPO4phase(JCPDS No.21-0121),showing that the mixture of Bi2MoO6and BiPO4is the main existing form of the composite samples. In addition, there is no any diffraction peaks of silver species(38.1°, 44.2°,64.4°,and 77.4°for Ag)can be observed for the Ag-Bi2MoO6/BiPO4samples,suggesting that all the as-synthesized composites possess the same crystal structure.This may be due to the low concentration (0.65wt%?1.27wt%)or small crystal size of Ag.Furthermore,the changes of all diffractions and lattice parameters were not detectable,which indicates that Ag related species resided in the lattice sites and have no separate phase.

FIG.1 XRD patterns of the as-synthesized samples.

FIG.2 SEM images of(a)as-synthesized Bi2MoO6, (b) BiPO4, (c) Bi2MoO6/BiPO4, (d) 1.27%Ag-Bi2MoO6/BiPO4,(e)TEM,and(f)HRTEMimages of 1.27%Ag-Bi2MoO6/BiPO4.
The Ag-Bi2MoO6/BiPO4samples were synthesized through two main processes. The first step was taken to prepare Bi2MoO6/BiPO4composite. On this basis,the second step was taken to load Ag on the surface of Bi2MoO6/BiPO4sample. Figure 2(a)?(d)shows the SEM images of the as-prepared Bi2MoO6,BiPO4,Bi2MoO6/BiPO4,and 1.27%Ag-Bi2MoO6/BiPO4samples.For the pure Bi2MoO6sample(Fig.2(a)),the morphology is nanosheet.And the pure BiPO4product is irregularly shaped flaky crystals with sizes between 200 and 800 nm(Fig.2(b)).As for the Bi2MoO6/BiPO4composite(Fig.2(c)),it can be observed that there are sheet-like crystals with average size of around 500 nm.It can be observed that the Ag-Bi2MoO6/BiPO4sample display a sheet-like morphology(Fig.2(d)),indicating that low amount Ag loading didn't have significant influence on the morphology of Bi2MoO6/BiPO4crystals.It also can be seen that the as-fabricated Ag-Bi2MoO6/BiPO4composite include Ag nanoparticles assembling uniformly on the surface of Bi2MoO6/BiPO4nanosheets(Fig.2(e)). HRTEM image further confirm the formation of a novel ternary heterostructure(Fig.2(f)).By measuring the lattice fringes,the resolved interplanar distances are about 0.204,0.275,and 0.171 nm,which correspondsto the(200)plane of Ag,the(200)plane of Bi2MoO6, and the(302)plane of BiPO4,respectively.EDX elemental microanalysis confirms Bi,Mo,P,Ag,and O as major elements in the ternary heterostructured Bi2MoO6/BiPO4composite(Fig.3(a)). In addition, the EDS analysis indicates that the loading percentage of Ag was 0.65%and 1.27%,respectively(as shown in Table I).The formation of the Ag-Bi2MoO6/BiPO4heterostructure was also confirmed by the elemental mapping of the as-prepared 1.27%Ag-Bi2MoO6/BiPO4sample(Fig.3(b)?(g)).Maps of Bi M,Mo L,P K,O K,and Ag L have the same shape and location,demonstrating the existence of Bi2MoO6,BiPO4,and Ag in the Ag-Bi2MoO6/BiPO4composite.This gives solid evidence of the formation of Ag-Bi2MoO6/BiPO4heterostructure.

FIG.3 EDX spectrum (a)and the corresponding EDSelementalmappingimages(b-g)of1.27%Ag-Bi2MoO6/BiPO4sample.The bars in figures are 5μm.
XPS spectra for 1.27%Ag-Bi2MoO6/BiPO4composite are presented to determine the oxidation state and elemental composition for each member of the heterostructure,as shown in Fig.4.The Bi 4f fine XPS spectrum of the sample is shown in Fig.4(a).XPS signals of Bi 4f are observed at binding energies at about 163.63 eV(Bi 4f7/2)and 158.32 eV(Bi 4f5/2),ascribed to Bi3+[25].The Mo 3d peaks are detected at 261.59 and 234.75 eV(Fig.4(b)),indicating a six-valent oxidation state for Mo6+[26].P 2p fine XPS spectrum of the sample is shown in Fig.4(c).XPS signals of P 2p were detected at binding energies around 132.37 eV(P 2p), attributed to P of PO43?[25].The wide and asymmetric peak of the O 1s spectrum indicated that there might be more than one chemical state according to the binding energy(Fig.4(d)).The peaks at 530.25 and 529.18 eV related to P?O(lattice O)[25]and Mo?O (lattice O)[26],respectively.As illustrated in Fig.4(e), typical peaks of Ag 3d can be observed,in which the peaks at 367.08 and 373.21 eV are ascribed to Ag 3d3/2(Ag0)and Ag 3d5/2(Ag0)[27,28].

TABLE I The EDS ofthe as-prepared x%Ag-Bi2MoO6/BiPO4sample.
Figure 5(a)shows the UV-Vis diffuse reflectance spectra(DRS)of as-fabricated Bi2MoO6,BiPO4, Bi2MoO6/BiPO4and Ag-Bi2MoO6/BiPO4samples. All the absorbance edges of Ag-Bi2MoO6/BiPO4composites showed marked red shifts,which can be attributed to the surface plasmon resonance(SPR)of the loading Ag,further confirming the existence of Ag particles.The band gap energies of the pure Bi2MoO6and BiPO4can be calculated by the following formula:

where α,ν,Egand A are absorption coefficient,light frequency,the band-gap energy,and a constant,respectively.n is determined by the type of optical transition of a semiconductor(n=1 for a direct transition and n=4 for an indirect transition).For BiPO4and Bi2MoO6, the values of n are 4 and 1 for the indirect transition and direct transition[22,23],respectively.According to Eq.(1),the band-gap energy(Eg)of Bi2MoO6can be estimated from a plot of(αhν)2versus energy(hν), and the Egof BiPO4can be estimated from a plot of(αhν)1/2versus energy(hν).Thus,the band gaps of the as-prepared BiPO4and Bi2MoO6are estimated to be 3.40 and 2.56 eV,respectively(as illustrated in Fig.5(b)).
In order to clearly understand the formation of Ag/Bi2MoO6/BiPO4heterojunction,the initial energy band structures of Bi2MoO6and BiPO4were provided. The band positions of Bi2MoO6and BiPO4were obtained by the following empirical formulas:


FIG.4 XPS spectra of 1.27%Ag-Bi2MoO6/BiPO4sample.(a)Bi 4f spectrum,(b)Mo 3d spectrum,(c)P 2p spectrum,(d) O 1s spectrum,(e)Ag 3d spectrum.

FIG.5(a)UV-Vis DRS of the as-prepared samples.(b)The plots of(αhν)n/2versus photon energy(hν)for the band-gap energies of Bi2MoO6and BiPO4.
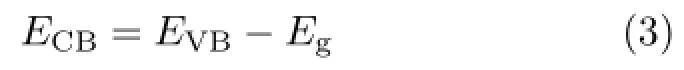
where EVBis the valence band edge potential,ECBis the conduction band edge potential,X is the electronegativity of the semiconductor,which is the geometric mean of the electronegativity of the constituent atoms,and the value of X for Bi2MoO6and BiPO4is ca.5.50 and 6.49 eV,respectively.Eeis the energy of free electrons on the hydrogen scale(about 4.5 eV),Egis the band gap energy of the semiconductor.Based on the band gap positions,the CB and VB edge potentials of Bi2MoO6are determined to be?0.28 and 2.28 eV, respectively.The CB and VB edge potentials of BiPO4are determined to be 0.30 and 3.70 eV,respectively. The energy band structure diagram of Bi2MoO6and BiPO4is thus schematically illustrated,as displayed in Fig.6.Since the CB potential of Bi2MoO6is more negative than that of BiPO4(Fig.6),the electrons will diffusion from Bi2MoO6to BiPO4,resulting in accumulation of negative charges in BiPO4close to the junction.In addition,Ag nanoparticles on the surface of the composites capture electrons effectively,which is also beneficial to electrons transmission from BiPO4or Bi2MoO6to Ag nanoparticles.These charge transfer would reduce the electron-hole pair recombination and prolong the life-time of charges,thus improving the photocatalytic efficiency.

FIG.6 Schematic diagram of the separation and transfer of photogenerated charges in the heterostructured composite under visible light irradiation.

FIG.7(a)Photodegradation efficiencies of phenol red as a function of irradiation time for different photocatalysts.(b) UV-visible spectral changes of phenol red in an aqueous 1.27%Ag-Bi2MoO6/BiPO4dispersion as a function of irradiation time under visible light illumination.
The photocatalytic performances of as-synthesized samples were studied by comparing degradation rates of phenol red under visible light irradiation(Fig.7(a)). The blank test demonstrates that the degradation of phenol red was extremely slow without any photocatalyst under visible light illumination.From the catalytic experiments,Ag-Bi2MoO6/BiPO4samples were detected to be more photoactive towards phenol red solution than pure Bi2MoO6,BiPO4,and Bi2MoO6/BiPO4composite.Additionally,it can be seen that the photocatalytic efficiency are significantly affected by the content of Ag loading.With the Ag content increasing,the phenol red degradation rate increase.Furthermore,the highest degradation rate was obtained from 1.27%Ag-Bi2MoO6/BiPO4sample with almost 100%of phenol red removal.This increase may be attributed to the capturing of electrons by the deposited Ag to hinder the recombination of hole-electron pairs[25,26].Visible light irradiation of an aqueous phenol red by 1.27%Ag-Bi2MoO6/BiPO4sample led to an apparent decrease in absorption(Fig.7(b)).The comparison of PL spectra of the as-prepared photocatalysts under the excitation wavelength of 325 nm is shown in Fig.8.Compared with pure Bi2MoO6,and Bi2MoO6/BiPO4sample,the PL peak intensity of Ag-Bi2MoO6/BiPO4sample decreased obviously.These results reveal that the heterojunction effect contributes to the effective electron-hole pair separation,which could be a reason for the heterostructured Ag-Bi2MoO6/BiPO4composites showing superior photocatalytic performances under visible light illumination.
For detecting the main oxidative species in the photocatalytic process,the trapping experiments of radicals and holes in the presence of various scavengers were operated(Fig.S1 in supplementary materials).Under the visible-light irradiation of the as-prepared 1.27%Ag-Bi2MoO6/BiPO4composite,the photodegradation rate of phenol red slightly decreased after the addition of hole scavenger AO,which shows that holes are not the main active species that are responsible for the degradation of phenol red in current photocatalytic systems. However,the photodegradation rate of phenol red was decelerated significantly after the addition of superoxide radical scavenger BQ as well as IPA(hydroxyl radical scavenger).It shows that the active species including O2·?and·OH played the major role in the degrada-tion of phenol red over the 1.27%Ag-Bi2MoO6/BiPO4composite under visible light illumination.

FIG.8 Room temperature PL spectra of the as-synthesized photocatalysts.
IV.CONCLUSION
Ternary heterostructured Ag-Bi2MoO6/BiPO4composite photocatalyst was successfully synthesized,and the composite sample showed excellent visible-light induced photocatalytic activity. And the as-prepared 1.27%Ag-Bi2MoO6/BiPO4composite had very obviously enhanced visible light photocatalytic activity for the degradation of phenol red in solution.The photocatalytic activity enhancement of the ternary heterostructured composite could be attributed to its strong absorption in the visible region due to the surface plasmon resonance resulting from Ag nanoparticles loading and low recombination rate of the electron-hole pairs because of formation of the ternary heterostructure.This work indicated that the composite effect created among semiconductors is of great importance in determining the photocatalytic performances.
Supplementary materials:Figure S1 shows trapping experiments of active species during the photocatalytic degradation of phenol red over 1.27%Ag-Bi2MoO6/BiPO4sample under visible light irradiation.
V.ACKNOWLEDGMENTS
This work is supported by the National Natural Science Fundation of China(No.21407059,No.21576112), and the Science and Technology Research Project of the Department of Education of Jilin Province (No.2015220).
[1]S.Fukuzumi,T.Kobayashi,and T.Suenobu,Angew. Chem.Int.Edit.50,728(2011).
[2]Y.F.Hu,Y.X.Li,S.Q.Peng,G.X.Lv,and S.B.Li, Acta Phys.-Chim.Sin.24,2071(2008).
[3]D.Xu,A.M.Gao,and W.L.Deng,Acta Phys.-Chim. Sin.24,1219(2008).
[4]B.X.Li,Y.F.Wang,and T.X.Liu,Acta Phys.-Chim. Sin.27,2946(2011).
[5]J.Y.He,W.M.Wang,F.Long,Z.G.Zou,Z.Y.Fu, and Z.Xu,Mater.Sci.Eng.B 177,967(2012).
[6]G.H.Tian,Y.J.Chen,X.Y.Meng,J.Zhou,W. Zhou,K.Pan,C.G.Tian,Z.Y.Ren,and H.G.Fu, ChemPlusChem 78,117(2013).
[7]Y.Yan,S.F.Sun,Y.Song,X.Yan,W.S.Guan,X.L. Liu,and W.D.Shi,J.Hazard.Mater.250/251,106 (2013).
[8]Y.Wang,H.X.Dai,J.G.Deng,Y.X.Liu,Z.X.Zhao, X.W.Li,and H.Arandiyan,Chem.Eng.J.226,87 (2013).
[9]W.Z.Yin,W.Z.Wang,and S.M.Sun,Catal.Commun.11,647(2010).
[10]H.G.Yu,Z.F.Zhu,J.H.Zhou,J.Wang,J.Q.Li,and Y.L.Zhang,Appl.Surf.Sci.265,424(2013).
[11]M.Y.Zhang,C.L.Shao,P.Zhang,C.Y.Su,X.Zhang, P.P.Liang,Y.Y.Sun,and Y.C.Liu,J.Hazard.Mater. 225/226,155(2012).
[12]L.W.Zhang,T.G.Xu,X.Zhao,and Y.F.Zhu,Appl. Catal.B 98,138(2010).
[13]F.J.Zhang,S.F.Zhu,F.Z.Xie,J.Zhang,and Z.D. Meng,Sep.Purif.Technol.113,1(2013).
[14]D.L.Jiang,L.L.Chen,and J.J.Zhu,M.Chen,W. D.Shi,and J.M.Xie,Dalton Trans.42,15726(2013).
[15]M.Y.Zhang,C.L.Shao,J.B.Mu,Z.Y.Zhang,Z.C. Guo,P.Zhang,and Y.C.Liu,CrystEngComm 14,605 (2012).
[16]M.Y.Zhang,C.L.Shao,J.B.Mu,X.M.Huang,Z.Y. Zhang,Z.C.Guo,P.Zhang,and Y.C.Liu,J.Mater. Chem.22,577(2012).
[17]Y.S.Xu,Z.J.Zhang,and W.D.Zhang,Mater.Res. Bull.48,1420(2013).
[18]B.Yuan,C.H.Wang,Y.Qi,X.L.Song,K.Mu,P. Guo,L.T.Meng,and H.M.Xi,Colloids Surf.A 425, 99(2013).
[19]X.Lin,D.Liu,X.Y.Guo,N.Sun,S.Zhao,L.M. Chang,H.J.Zhai,and Q.W.Wang,J.Phys.Chem. Solids 76,170(2015).
[20]X.Lin,X.Y.Guo,D.Liu,Q.W.Wang,H.J.Zhai, and L.M.Chang,Mater.Res.Bull.63,72(2015).
[21]X.Lin,X.Y.Guo,Q.W.Wang,L.M.Chang,and H. J.Zhai,Acta Phys.-Chim.Sin.30,2113(2014).
[22]C.S.Pan and Y.F.Zhu,Environ.Sci.Technol.44, 5570(2010).
[23]H.L.Lin,H.F.Ye,B.Y.Xu,J.Cao,and S.F.Chen, Catal.Comm.37,55(2013).
[24]T.Y.Huang,Y.J.Chen,C.Y.Lai,and Y.W.Lin, RSC Adv.5,43854(2015).
[25]S.Y.Wu,H.Zheng,Y.W.Lian,and Y.Y.Wu,Mater. Res.Bull.48,2901(2013).
[26]P.Zhang,C.L.Shao,M.Y.Zhang,Z.C.Guo,J.B. Mu,Z.Y.Zhang,X.Zhang,and Y.C.Liu,J.Hazard. Mater.217/218,422(2012).
[27]Y.F.Chen,W.X.Huang,D.L.He,Y.Situ,and H. Huang,ACS Appl.Mater.Inter.6,14405(2014).
[28]B.Yuan,C.H.Wang,Y.Qi,X.L.Song,K.Mu,P. Guo,L.T.Meng,and H.M.Xi,Colloid.Surface.A 425,99(2013).
(Dated:Received on February 29,2016;Accepted on May 26,2016)
 CHINESE JOURNAL OF CHEMICAL PHYSICS2016年5期
CHINESE JOURNAL OF CHEMICAL PHYSICS2016年5期
- CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- Preparation of Bio-hydrogen and Bio-fuels from Lignocellulosic Biomass Pyrolysis-Oil
- Combination Computing of Support Vector Machine,Support Vector Regression and Molecular Docking for Potential Cytochrome P450 1A2 Inhibitors
- Working Condition Real-Time Monitoring Model of Lithium Ion Batteries Based on Distributed Parameter System and Single Particle Model
- Hydrodeoxygenation of Anisole over Ni/α-Al2O3Catalyst
- Highly Efficient and Selective Removal of Pb(II)ions by Sulfur-Containing Calcium Phosphate Nanoparticles
- Photo-depositing Ru and RuO2on Anatase TiO2Nanosheets as Co-catalysts for Photocatalytic O2Evolution from Water Oxidation
