Protective effect of rhizome extracts of the herb,vacha(Acorus calamus)against oxidative damage:An in vivo and in vitro study
M.DevkiR.NirupmM.NirupmH.N.Yjurvedi
a Department of Zoology,University of Mysore,Manasagangotri,Mysore 570 006,India
b Department of Health Technology and Informatics,The Hong Kong Polytechnic University,Hong Kong
Abstract The rhizome of Acorus calamus,an herb widely used in Indian system of medicine for many ailments including epilepsy,mental illness and rheumatism,was subjected to soxhlet extraction to elucidate antioxidant property of different solvent extracts using in vitro assays.The benzene extract was most potent in scavenging hydroxyl and superoxide radicals and in reducing 1,1-diphenyl-2-picryl hydrazyl and ferric reducing antioxidant power.In addition the benzene extract prevented oxidative damage to DNA and mitochondria.It was also effective in preventing stress-induced decrease in total plasma anti-oxidant activity as determined in vivo using rat model wherein stress was induced by exposing to restraint and forced swimming.The minimum effective dose of the benzene extract was 5 mg/kg body weight(oral),and at this dose,its effect was similar to the same dose of a standard anti-oxidant, ascorbic acid. The study for the firs time, clearly demonstrates a potent anti-oxidant activity of A.calamus combining in vitro and in vivo results.Hence,the therapeutic value of this herb maybe due to its anti-oxidant property.
Keywords: Acorus calamus;Antioxidant;Forced swimming;Radical scavenging;DNA protection assay
1. Introduction
The reactive oxygen species (ROS) and reactive nitrogen species (RNS) are known to cause damage to lipids, proteins,enzymes,and nucleic acids leading to cell or tissue injury and are implicated in the processes of aging as well as in wide range of degenerative diseasesviz.cancer,atherosclerosis,diabetes, liver injury, Alzheimer, Parkinson, and coronary heart pathologies,etc.[1,2]. Antioxidant based drugs and formulations have appeared during last three decades [3]. Currently available synthetic antioxidants like butylated hydroxyanisole(BHA), butylated hydroxytoluene (BHT), tertiary butylated hydroquinon and gallic acid esters have been suspected to cause or prompt negative health effects. Hence, strong restrictions have been imposed on their application and there is a trend to substitute them with naturally occurring antioxidants.Moreover, these synthetic antioxidants also show low solubility and moderate antioxidant activity [4]. The search for natural antioxidants as alternatives is therefore of great interest.
Recent studies have shown that a number of plant products including polyphenols,terpenes and various plant extracts exert an antioxidant action[5,6].Our present study focuses on one of the most potent Indian medicinal herbAcorus calamus(vacha or sweet fla or buch plant)for its antioxidant property.It belongs to Acoraceae family and has been used in the Indian and Chinese system of medicine for several decades to treat diseases,especially the central nervous system(CNS)abnormalities[7,8].The rhizome of this plant is widely used in the treatment of several ailments like epilepsy,mental ailments,chronic diarrhea,dysentery,bronchial catarrh,intermittent fevers glandular,abdominal tumors, kidney and liver troubles, rheumatism, sinusitis and eczema [9]. However, there is scanty information on antioxidant properties ofA. calamusand it is not known whether it has DNA protective activity.Hence,the aim of this study was to determine the antioxidant potential ofA.calamususing different solvent extracts of rhizome usingin vitroassays including DNA protection.
Stress leads to hyper secretion of glucocorticoids[10]and an increased secretion of glucocorticoids(GCs)results in overproduction of reactive oxygen species [11] and leads to oxidative damage [12]. Hence there is a need to prevent stress induced oxidative damage. Several studies demonstrate suppression of long term stress-induced oxidative damage following administration of herbal extracts[13,14].Thus far there is no information as to whether the altered antioxidant status due to short term stress exposure is prevented by herbal extracts.Earlier we have reported that acute stressi.e.1 h exposure of adult male rats to restraint followed by forced swimming for 15 min after a gap of 4 h alters the antioxidant status [15]. Hence this is a good model for speedy assessment of total antioxidant activityin vivo.The present study using this model investigates whether benzene extractofrhizomepreventsstressinducedchangesinantioxidant status using total antioxidant assay.
2. Materials and methods
2.1. Chemicals
L-ascorbic acid, gallic acid, 1,1-diphenyl-2-picryl hydrazyl(DPPH), ethylene diamine tetra acetic acid (EDTA), nitro blue tetrazolium (NBT), β-nicotinamide adenine dinucleotide (β-NADH), ferric chloride (FeCl3), ferrozine,trichloroacetic acid (TCA), ferric chloride, guanidine hydrochloride, 5-methylphenazinium methosulphate (PMS),2,4-dinitrophenylhydrazine (2,4-DNPH), hydrogen peroxide(H2O2), dichlorofluorescei (DCF), 2',7'dichlorofluorescei diacetate (DCF-DA) and 2,4,6 tripyridyl-s-triazine (TPTZ)were purchased from E.Merck India.
2.2. Experimental animals
Adult male Wistar rats (25) weighing 180–200 g were obtained from the inbred colony of the central animal facility of the University of Mysore and were maintained(2 or 3 rats/cage)under 12 h:12 h light and dark cycle.The animals were provided standard rat chow and waterad libitum.The experimental protocols were approved by the Institutional animal ethics committee.
2.3. Plant material and preparation of the extracts
The rhizome of theA.calamuswas shade dried and a coarse powder was prepared (particle size ~0.25 mm) and was subjected to successive extraction using solvents with increasing polarityviz.petroleum ether,benzene,chloroform,ethanol,cold water,hot water and 0.2 N sodium hydroxide(NaOH)by continuous percolation process in a soxhlet apparatus.The aqueous extract was prepared by maceration with water. Each extract was concentrated by distilling off the solvent and evaporating to dryness followed by dissolution in rectifie spirit.
2.4. Determination of percentage of inhibition
Effica y ofA. calamusextracts at different concentrations(0.02, 0.04, 0.06, 0.08 and 0.1 mg/mL) was measured as percentage of inhibition of free radicals generationin vitroassays involving superoxide,hydroxyl,and DPPH radicals and ferrous ion chelating activity. The inhibition percentage (I%)was calculated using the formula(Eq.(1)),

where Ac is the absorbance of the control;As is the absorbance of the sample containing plant extract.
2.5. In vitro anti-oxidant assays:1,1-diphenyl-2-picryl hydrazyl(DPPH)radical scavenging activity
Reduction of DPPH radical by an antioxidant results in the loss of absorption at 517 nm[16].Each extract(0.3 mg/mL)ofA.calamuswas mixed with 5 mL of 0.1 mmol/L methanolic solution of DPPH and incubated at 20?C for 20 min in darkness.The control was prepared without plant extract and methanol was used for the base line correction.Then the absorbance of the sample was measured at 517 nm.The difference in absorbance between control and plant extract mixed samples indicated reducing activity of the extracts.
2.6. Superoxide anion radical scavenging activity
The superoxide anion scavenging activity of the extracts was determined by using Liu[17]method.Methylphenazinium methosulphate and β-nicotinamide adenine dinucleotide were allowed to react to generate superoxide anions which were reduced by NBT. Reaction mixture contained 3 mL Tris HCl buffer (100 mmol/L, pH 7.4), 0.75 mL of NBT (300 μmol/L)solution, 0.75 mL of NADH (936 μmol/L) and 0.3 mL of different concentrations(0.02,0.04,0.06,0.08 and 0.1 mg/mL)of eachA.calamusextract.Reaction was started by adding 0.75 mL of PMS (120 μmol/L). The mixture was allowed to stand for 5 min at room temperature.The absorbance was read at 560 nm.The difference in absorbance between control and plant extract mixed samples indicated scavenging activity of the extracts.
2.7. Hydroxyl radical scavenging activity
Inhibition of hydroxyl radical(OH?)mediated peroxidation was carried out by deoxyribose assay[18].The reaction mixture contained different concentrations (0.02, 0.04, 0.06, 0.08 and 0.1 mg/mL)of each extract,50 μl of deoxyribose(2.8 mmol/L)in KH2PO4NaOH buffer(50 mmol/L pH 7.5),200 μl of FeCl3(100 mmol/L),104 mmol/L EDTA,ascorbic acid(100 mmol/L)and50 μlH2O2(4 mmol/L).Thefina reactionvolumewasmade up to 1 mL with distilled water and incubated at 37?C for 1 h.After incubation 1 mL each of TCA and TBA was added and again incubated at 100?C for 20 min and control was prepared without plant extract.The mixture was allowed to cool and the absorbance was read at 532 nm. The difference in absorbance between control and extract mixed samples indicated reducing activity of the extracts.
2.8. Assay for ferric reducing antioxidant power(FRAP)
The FRAP assay was performed according to the method of Benzie and Strain [19]. The FRAP reagent contained 2.5 mL of a 10 mmol/L TPTZ solution in 40 mmol/L HCl, 2.5 mL of 20 mmol/L FeCl3·6H2O and 25 mL of 300 mmol/L acetate buffer (pH 3.6). It was freshly prepared and warmed at 37?C.The reaction mixture contained 900 μl FRAP reagent, 90 μl water and 30 μl of one of the different concentrations(0.1,0.2 and 0.3 mg/mL)ofA.calamusextract and control was prepared without plant extract. The reaction mixture was incubated at 37?C for 30 min and the absorbance was measured at 593 nm.The standard curve was prepared by using different concentrations of FeSO4(10–100 μmol/L) and concentrations of the different samples were estimated using standard curve.
2.9. Ferrous ion-chelating assay
The ferrous ion chelating activity can be used to assay the antioxidants and it is measured by the decrease in the absorbance at 562 nm of the iron (II) and ferrozine complex [20]. Each extract(2 mL)was mixed with 3.7 mL of methanol and 0.1 mL of 2 mmol/L FeCl2. The reaction was initiated by addition of 0.2 mL of 5 mmol/L ferrozine and control was prepared without plant extract.After 10 min incubation at room temperature,the absorbance was measured at 562 nm. Methanol was used instead of plant extract in control system. Methanol (0.2 mL)insteadofferrozinesolutionwasusedasasampleblank,forerror correction because of unequal color of the sample solutions.
2.10. Mitochondrial preparation
The liver was washed with saline, weighed and put into ice-cold isolation buffer containing 0.25 mmol/L sucrose,10 mmol/L Tris, 0.5 mmol/L ethylenediaminetetraacetic acid(EDTA), pH 7.4. The tissue was carefully minced and rinsed to remove residual blood,and then homogenized in 2.5 volumes of isolation buffer.The homogenate was adjusted to 8 volumes with isolation buffer and centrifuged at 1000×gfor 10 min.The resultant supernatant fraction was decanted and saved and the pellet was washed once with 2 volumes of isolation buffer and the supernatant was centrifuged at 10,000×gfor 10 min.The mitochondria in the pellet obtained by the 10,000×gcentrifugation were resuspended with isolation buffer followed by washing twice with the buffer.All of the above operations were carried out at 4?C.The mitochondrial protein content was determined by using bovine serum albumin (BSA) as the standard.Fresh mitochondria were used immediately for reactive oxygen species(ROS).
2.11. ROS assay with DCF-DA in isolated rat liver mitochondria
Isolated liver mitochondria (0.25 mg) were added to 100 μl of isolation buffer in a 96 well plate and allowed to warm at room temperature for 5 min.Then 10 μl of FeSO4(15 mmol/L)and 10 μl of ascorbic acid(6 mmol/L)and 10 μl of DCFDA were added and then incubated with or without extract(concentrations at 0.02,0.04,0.06,0.08 and 0.1 mg/mL plant extract)for 30 min at 37?C. ROS generation was recorded fluorimetricall for 30 min with an excitation wavelength of 488 nm and an emission wavelength of 525 nm by micro plate fluoromete. ROS levels were quantifie from a dichlorofluorescei (DCF)standard curve and expressed as pmol DCF formed/min/mg protein[21].
2.12. DNA protection assay
DNA protection assay was performed using supercoiled pBR322 plasmid DNA as per the method described by Lee et al.[22].A mixture of 10 μl of plant extract and plasmid DNA(0.5 μg) were incubated for 10 min at room temperature followed by the addition of 10 μl of Fenton’s reagent(30 mmol/L H2O2, 50 μmol/L ascorbic acid and 80 μM FeCl3). The fina volume of the mixture was made up to 20 μl and incubated for 30 min at 37?C. The DNA was analyzed on 1% agarose gel using ethidium bromide staining.
2.13. Total phenolic content
Total phenol content was determined by Folin Ciocalteu reagent[23].Each plant extract(0.1 mL)or gallic acid(standard)was mixed with Folin Ciocalteu reagent(5 mL,1:10 diluted with distilled water)and aqueous Na2CO3(4 mL,1 mol/L).The mixture was allowed to stand for 10 min and the absorbance was measured at 765 nm.Total phenol content was estimated comparing the absorbance of samples(plant extracts)with gallic acid(standard)and expressed as gallic acid equivalent(mg)/g of dry mass of extract.
2.14. In vivo antioxidant action of benzene extract
This experiment was conducted to fin out the effect of benzene extract ofA.calamuson stress induced alterations in total antioxidant activity using FRAP assay. Adult male rats were randomLy segregated into 6 groups(n=5 rats/group).The rats in firs group served as controls and those in second group were exposed to stressors [24], restraint for 1 h and after 4 h interval to forced swimming for 15 min.Third,4th and 5th group of rats were administered with 5,10 and 20 mg/kg body weight of benzene extract ofA.calamusin 0.1 mL of carboxy methyl cellulose(CMC),respectively,prior to exposure to stressors similar to group 2.The 6th group rats received vitamin C(5 mg/kg body weight) prior to exposure to stress regime. The blood samples were collected at 0 h,2 h after restraint and 4 h after forced swimming exercise in all rats by tail snipping and used for the FRAP assay.The FRAP assay was conducted as described forin vitroassay(2.5.4),replacing the plant extracts with same volume of the plasma of rats from different experimental groups.
2.15. Statistical analysis
Mean value of each parameter was computed considering values of 3 assays/in vitromethod and 5 rats/groupin vivoassay and expressed as mean±SEM. The mean values of different groups of each parameter were compared by one way analysis of variance (ANOVA) followed by Duncan’s multiple test and judged significanp<0.05.

Fig.1. Effect of different extracts of A.calamus on reduction of DPPH.Values are mean±SD of three assays.
3. Results
3.1. DPPH radical scavenging activity
The petroleum ether and benzene extracts at 0.3 mg/mL showed strong radical scavenging activity with percentage decrease of 51.0%and 71%respectively,whereas,chloroform,alcohol,cold water,hot water and NaOH extracts showed relatively poor free radical scavenging activity of 28.23%,48.02%,23.5%,21.4%,22.4%and 23.9%,respectively(Fig.1).
3.2. Superoxide scavenging activity
Extracts ofA. calamusscavenged the superoxide radicals generated by photoreduction of ribofl vin and there was an increase in percentage of inhibition with increase in concentration of the extract.None of the extracts caused 50%or more inhibition of superoxide generations at 0.02 and 0.04 mg/mL concentrations whereas benzene and alcoholic extracts caused>50%inhibition at 0.06 mg/mL and above.Cold and hot water and NaOH extracts caused>50%inhibition at 0.08 mg/mL and 1 mg/mL concentrations (Table 1). Extract of benzene showed the highest scavenging activity with IC50value of 0.042 mg/mL compared with chloroform, alcohol, cold water, hot water and NaOH wherein IC50values were found to be 0.088,0.05,0.076,0.073 and 0.093 mg/mL,respectively.
3.3. Hydroxyl radical scavenging activity
Thedegradationofdeoxyribosemediatedbyhydroxylradical generated by Fe3+/ascorbate/EDTA/H2O2system was inhibited byA.calamusextracts.None of the extracts had 50%or more hydroxyl ion generation inhibition activity at 0.02, 0.04 and 0.06 mg/mL concentrations. Chloroform and benzene extracts caused>50%inhibition at 0.8 mg/mL and it was still higher at 0.1 mg concentration,whereas alcoholic,cold water and crude aqueousextractscaused<50%inhibitionat0.1 mg/mL(Table2).The IC50values were found to be 0.095, 0.066, 0.078, 0.1,0.1 and 0.088 mg/mL for petroleum ether,benzene,chloroform,alcohol,cold water and aqueous crude extracts respectively.
3.4. Ferric reducing antioxidant power(FRAP)
The antioxidant potentials of the different extracts ofA.calamuswere estimated from their ability to reduce TPRZ-Fe(III)complex to TPTZ-Fe (II). The benzene, chloroform and crude aqueous extracts showed an increase in antioxidant potential(total reducing ability of antioxidants)with increase in concentration of extracts(Table 3).Other extracts did not show reducing ability in this assay.
3.5. Ferrous ion-chelating assay
Fe2+ion chelating ability of different extracts ofA.calamusare shown in Fig.2.The cold and NaOH extracts showed highest Fe2+ion chelating ability 66.6% and 58.6% at 0.3 mg/mL whereas, in remaining extracts Fe2+ion chelating ability was<50%inhibition.

Table 1 Inhibitory effect of different solvent extracts of A.calamus on superoxide anion radical generation.

Table 2 Inhibitory effect of different solvent extracts of A.calamus on generation of hydroxyl radical.
3.6. Effects on ROS generation in isolated liver mitochondria
The amount of DCF formed was reduced with increasing concentrations of differentA.calamusextracts.Lowest amount of ROS was formed with benzene extract at all concentrations compared to all other extracts which was followed by petroleum ether and chloroform extractsi.e.the antioxidant potency of these three extracts was benzene>petroleum ether>chloroform extract (Table 4). Other extracts although showed antioxidant power in majority of cases the inhibition was less compared to control.
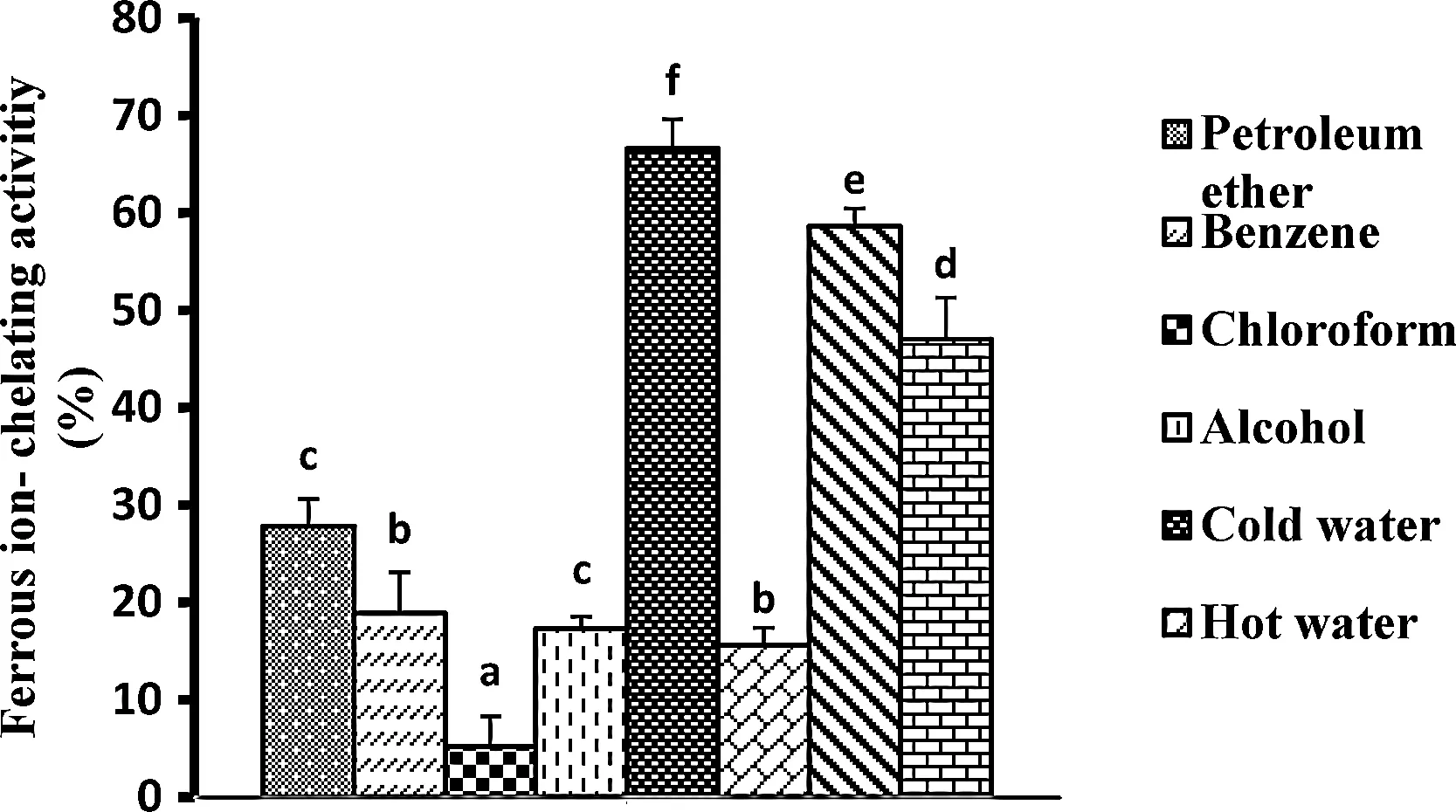
Fig.2. Ferrous ion chelating activity of different extracts of A.calamus.Values are mean±SD of three assays.
3.7. Total phenolic content
Fig. 3 shows total phenolic content of differentA. calamusextracts with respect to standard Gallic acid. The petroleum ether, benzene and chloroform extracts ofA. calamusshowed highest total phenolic content and it was 35 mg/g,40 mg/g and

Fig. 3. Total phenol content in different extracts of A. calamus. Values are mean±SD of three assays.
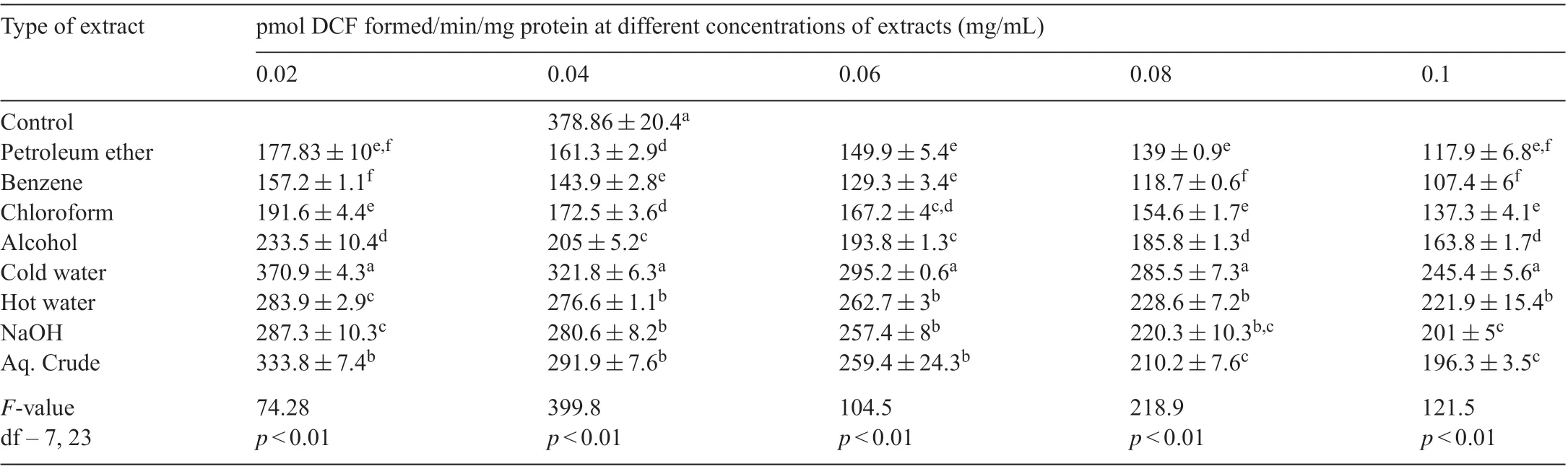
Table 4 Effect of different solvent extracts of A.calamus on free radical scavenging activity in isolated hepatic mitochondria.
35 mg/g respectively,calculated as Gallic acid equivalent of phenol. The total phenolic content of other extracts were 15 mg/g(alcohol), 10 mg/g (cold water), 18 mg/g (hot water), 16 mg/g(NaOH)and 18 mg/g(aqueous crude)respectively.
3.8. DNA protecting activity
Fig. 4 shows protective effects of different solvent extracts ofA.calamusagainst oxidative damage(nicks)of DNA which was studied on plasmid DNA pBR322.The lane-1 shows normal electrophoretic pattern of plasmid DNA.The lane 2 shows DNA nicks induced by Fenton reagent.The lanes 3–10 show marked reduction in the nicks in DNA induced by Fenton reagent and increase in native form of DNA.The petroleum ether,benzene,chloroform,alcohol and NaOH extracts were more effective in reducing Fenton reagent induced formation of nicks in DNA and in increasing native form of DNA compared with other extracts. Cold water extract was least effective in preventing DNA damage.

Fig.4. Protective effects of different extracts of rhizome of A.calamus on DNA damage caused by Fenton reagent.Lane 1,native pBR322 DNA;lane 2,Fenton’s reagent+DNA;lanes 3–10 Fenton’s reagent+DNA+one of the extracts of A.calamus,i.e.lane 3,petroleum ether;lane 4,benzene;lane 5,chloroform;lane 6,alcohol;lane 7,cold water;lane 8,hot water;lane 9,NaOH and lane 10,crude aqueous extract.
3.9. Total anti-oxidant potential in stressed rat(in vivo assay)
There was a significan decrease in the total antioxidant activity in stressed rats after 2 h of restraint which was further decreased 4 h after forced swimming compared to controls.Benzene extracts(5,10 and 20 mg/kg body weight) ofA.calamus as well as 5 mg kg/body weight of vitamin C treated stressed rats showed significantl higher plasma total antioxidant activity compared with stressed rats either 2 h after RS or 4 h after FS and it did not significantl differ from controls(Table 5).
4. Discussion
The present study clearly demonstrates anti-oxidant potential ofA.calamusextracts as shown by inhibition of generation of free radicals, protection of DNA and mitochondria from oxidative damage as revealed by differentin vitroassays and prevention of stress induced loss of anti-oxidant capacity throughin vivoassay.
Reactive oxygen species (ROS) including superoxide radicals, hydroxyl radicals, hydrogen peroxide and singlet oxygen are often generated as products of biological reactions or derived from exogenous factors. The ROS play an important role in cell metabolism including energy production,phagocytosis and intercellular signaling [25] and damage cells when present in excess. However, antioxidants scavenge the ROS to prevent theirdeleteriouseffects.Thereareenzymaticandnon-enzymatic mechanisms that maintain a balance in the oxidant and antioxidant activities. However, imbalance between oxidant and antioxidant systems leads to oxidative stress which results in the varietyofpathologicaleffectssuchasDNAdamage,carcinogenesis and various degenerative disorders such as cardiovascular diseases,aging and neuro-degenerative diseases[1,2].

Table 5 Effect of different concentrations of benzene extract of the rhizome of A.calamus on plasma total antioxidant activity in vivo.
Many plants were used for antioxidant activity for instance,
Acacia auriculiformis, Aegle marmelos, Campanula alliariifolia, Dimocarpus Longan, Gracilaria changii, Hyphaene thebaica, Kappaphycus alvarezii, Mangifera indica, Parmelia saxatilis, Rosmarius officinalis, etc.reported to have antioxidant property[26].The Indian medicinal herb,A.calamus,is a time tested and easily available herb in Asian region.Methanol[27,28], ethanol and hydro-alcoholic [29] extracts ofA. calamusare known to possess antioxidant activity as determined by DPPH method. In these studies only total antioxidant activity(DPPH method) was demonstrated whereas other scavenging activities and DNA and mitochondria protective effects,if any were not considered.The present study demonstrates that antioxidant property ofA. calamusis due to combined effects of different anti-oxidant mechanisms. Due to complex nature of phytochemicals a single method can not evaluate antioxidant activity of herbs.There are several ways antioxidants act i.e.by donating hydrogen to radicals,metal chelating ability,free radical scavenging activity,reducing power,and quenching singlet oxygen.But unfortunately,most of the assay methods measure any one of these activities.Hence,multiple antioxidant methods are required to reach the conclusion.
DPPH method is a stable free radical system and a very sensitive way to determine thein vitroantioxidant activity of plant extracts[30].The efficacie of anti-oxidants are often associated with their ability to scavenge stable free radicals.The petroleum ether and benzene extracts ofA.calamusshowed a higher free radical scavenging activity compared to other extracts in the present study in DPPH assay,indicating their potent anti-oxidant property. The DPPH assay also suggested that theA. calamuscontained compounds that are capable of donating hydrogen to a free radical in order to remove odd electron,which is responsible for the radical’s reactivity.
Superoxide anion is an oxygen-centered radical with selective reactivity and it is produced by a number of enzyme systems in auto-oxidation reactions and by non-enzymatic electron transfers that univalently reduce molecular oxygen. Here,benzene extract showed higher superoxide radical scavenging activity compared to other extracts. The probable mechanism of scavenging the superoxide ions is inhibition of generation of superoxide radicals in thein vitroreaction mixture.
The hydroxyl radical is highly reactive oxygen radical formed from the reactions of various hydroperoxides with transition metal ions. It attacks proteins, DNA, polyunsaturated fatty acids in membranes [31], abstracts hydrogen atoms from membrane lipids [32] and brings about peroxidic reaction of lipids. Hydroxyl radicals may cause fragmentation of sugar in DNA and DNA strand breaks [33]. The benzene extract ofA. calamuscaused inhibition of generation of hydroxyl radicals and the process was enhanced with increasing concentration of extracts and it caused maximum inhibition with low concentration compared to other extracts in the present experiment.
FRAP assay measures the reducing ability of antioxidants against oxidative effects of reactive oxygen species.The antioxidant potential is estimated by ability of anti-oxidants to reduce TPRZ-Fe(III)complex to TPTZ-Fe(II).Total antioxidant power may be considered analogous to total reducing power. In the current study chloroform and benzene extracts ofA. calamusexhibited greater total antioxidant power in FRAP assay compared to crude aqueous extract whereas other extracts did not show anti-oxidant activity.
Metal chelating capacity is claimed as one of the antioxidant mechanisms since it reduces the concentration of the catalytic transition metal in lipid peroxidation.The iron chelating capacity test measures the ability of antioxidants to compete with ferrizone in chelating ferrous ions[34].In the presence of samples possessing chelating activity,the formation of complexes is decreased.Among the eight extracts ofA.calamus,cold water,NaOH and crude aqueous extracts showed a higher chelating activity and the lowest activity was found in chloroform extract.The cold water extract showed maximum chelating activity when compared with other extracts.
Phenolic compounds may contribute directly to antioxidant action. It is reported that phenolic compounds have inhibitory effects on mutagenesis and carcinogenesis in humans [35].Polyphenols are potential protecting agents against lethal effects of oxidative stress and offer protection of DNA by chelating redox-active transition metal ions[36].Here,the benzene extract ofA.calamushad the highest phenolic content compared with other extracts.Similarly,the benzene extract exhibits high free radical scavenging action.Hence high anti-oxidant potential of benzene extract might be due to high free radical scavenging action coupled with high phenol content.
Hydroxyl radicals generated by the Fenton reaction cause oxidation induced breaks in DNA strands to yield its open circular or relaxed forms. Exposure of plasmid DNA to Fenton’s reagent ultimately results in DNA strand breaks,mainly due to the generation of hydroxyl radical and subsequent free radicalinduced reaction in plasmid DNA.Hydroxyl radicals react with nitrogenous bases of DNA producing base radicals and sugar radicals. The base radicals in turn react with the sugar moiety causing breakage of sugar phosphate backbone of nucleic acids resulting in strand breaks.Different extracts ofA.calamusexcept the cold water extract showed DNA damage protecting activity in our present study.A higher DNA protection activity was found in the petroleum ether,benzene,chloroform,alcohol and NaOH extracts compared with other extracts.The DNA protectiveactivityofA.calamusmaybeduetohighhydroxylradical scavenging action of the extracts,as hydroxyl radicals are major DNA damaging radicals. In addition high phenolic content of the extracts might also contribute for DNA protection.
It is well documented that mitochondria are the major source of intracellular ROS.The major single-organ oxygen consumers are the liver and brain, consuming 20.4% and 18.4%, respectively.Therefore,the liver mitochondria serve as an optimal sub cellular system to evaluate the effica y of the antioxidants. In the current study,the impact of antioxidant activity ofA.calamuson the biological system was investigated in isolated rat liver mitochondria by creating oxidative stress using FeSO4and ascorbic acid.We observed a decrease in the mitochondrial ROS production in the presence of benzene extract ofA. calamuscompared to other extracts.
It is to be noted that the benzene extract ofA. calamushas most potent antioxidant property as it was effective in allin vitroassays excepting ferrous iron chelating action, whereas other extracts did not exhibit anti-oxidant action on par with this extract. Howeverin vitrostudies have to be supported byin vivoobservations to determine whether extract has ability for antioxidant action in biological system.Hence,the antioxidant potential has to be testedin vivoby creating oxidative stress.Since benzene extract was more potent, it was tested for antioxidant actionin vivo.
There are many studies on antioxidant activity ofA.calamusfor instance,the ethanolic extract prevented acrylamide-induced hind limb paralysis, decreased GSH and GST, increased dopamine receptors in the corpus striatum [37]. Ethyl acetate and methanolic extracts(50 mg/kg bw)ofA.calamuswas effective in preventing noise stress induced oxidative damage[38,39].Similarly,methanolic extract prevents oxidative damage caused by nickel chloride[40]or ethanol induced oxidative stress[41].Above studies proved long term effect, the days of treatment periods are long and dose of the infusion is quite high. Our present study demonstrated the acute effect of theA. calamusextract on stress induced alterations with a lesser dose and also we proved thatA. calamusis effective in preventing the DNA damage induced by oxidative stress.
Stress is an inescapable fact of life and exposure to stressful situations is among the most common human experiences.It is reported that exposure to stress can stimulate many metabolic pathways leading to increased production of the oxygen free radicals[42,43].Various stressorsviz.immobilization[44,45],restraint [46] and cold stress [47] are known to induce oxidative stress.However an animal model which can be used to get results within a short duration(1 day)was used in our present study,wherein 2 h after restraint there was a significan decrease in plasma total antioxidant activity compared to initial level(0 h)and it was further significantl decreased 4 h after forced swimming exercise.Thus significan drop in plasma total antioxidant activity was achieved within 9 h of experimental duration in this rat model. This model mimics the human life situations,as a person is subjected to acute stressors several times in a day due to life events. Oral administration of benzene extract,prior to stress treatment, prevented stress induced decrease in plasma total antioxidant activity following RS or FS.A dose of 5 mg/kg body weight was minimum effective dose as its preventive effects were similar to antioxidant activity of a standard antioxidant vitamin C.
Thus present study clearly demonstrates that benzene extract ofA.calamusis a source of most potent antioxidant compound as it exerts its action by inhibiting different mechanisms of free radical generation or scavenging as shown byin vitroassays as well as its effica yin vivo.However,the components responsible for the above mentioned activity are currently unclear. Therefore,our studies are underway to isolate and identify the potent antioxidant molecules in the rhizome ofA.calamusand to study their health benefits
Funding
The work was supported by a grant from Ministry of Human Resource Development,Government of India,through the University Grants Commission to University of Mysore,under the Institution of Excellence scheme.
Conflict of interest
The authors declare that there are no conflict of interest.
 食品科學(xué)與人類(lèi)健康(英文)2016年2期
食品科學(xué)與人類(lèi)健康(英文)2016年2期
- 食品科學(xué)與人類(lèi)健康(英文)的其它文章
- The protective effect of dietary fl vonoid fraction from Acanthophora spicifera on streptozotocin induced oxidative stress in diabetic rats
- In vitro neuroprotective potentials of aqueous and methanol extracts from Heinsia crinita leaves
- Bioactive constituents from the leaves of Quercus phillyraeoides A.Gray for α-glucosidase inhibitor activity with concurrent antioxidant activity
- Antioxidant,antimicrobial and cytotoxic potential of condensed tannins from Leucaena leucocephala hybrid-Rendang
- Moringa oleifera:A review on nutritive importance and its medicinal application
