Effect of Anthracene on the Interaction Between Platymonashelgolandica var. tsingtaoensis and Heterosigma akashiwoin Laboratory Cultures
BI Rong, WANG You, WANG Renjun, LI Wei, and TANG Xuexi
?
Effect of Anthracene on the Interaction Betweenvar.andin Laboratory Cultures
BI Rong, WANG You, WANG Renjun, LI Wei, and TANG Xuexi*
,,,266003,
Two species of marine phytoplankton,var.and, were cultivated in bi-algal cultures to investigate the effect of anthracene (ANT) on the interaction between them. Without ANT,out-competedat low initial biomass ratios (():()=1:4 and 1:1), but not at the highest (:=4:1). This observation was consistent with the description in Lotka-Volterra two species competition model. It was found thatwas excluded at low initial biomass ratios, while the unstable equilibrium between two species was predicted at the highest. For both species, carrying capacity and maximal specific growth rate decreased in bi-algal cultures compared to those in monocultures.exhibited a higher sensitivity to ANT than. This resulted markedly in a reduced cell density ofbut an increased cell density of. Carrying capacity ofwas consistently higher in bi-algal cultures with ANT than those without ANT, suggesting that ANT, through the elimination of, generated the dominance ofindependently of initial biomass ratios. This study showed a density-dependent effect of harmful alga () on dietary alga (), and indicated that ocean pollutant ANT could induce the succession of marine phytoplankton.
anthracene; interspecific competition; phytoplankton; population growth
1 Introduction
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous environmental pollutants, which are well known as their lipophilic characteristics, environmental persistence and genotoxicity (Cerniglia, 1992; Hong, 2008). PAHs are mainly produced by anthropogenic activities in aquatic environment (Eisler, 1987). On the continental shelf of China, the average concentration of anthropogenic PAHs is 82ngg?1in sediments, a low to moderate concentration in comparison with other shelf regions worldwide (Liu, 2012). PAHs can associate with particulate materials in aquatic environment as shown in many studies. They may accumulate in marine algae (Warshawsky, 1995; Kirso and Irha, 1998; Hong, 2008; Echeveste, 2011), copepods (Stringer, 2012), other crustaceans (Neff, 1976; Ober-d?rster, 2000), bivalve mollusks (Piccardo, 2001; Wang, 2011) and fish (Neff, 1976; Cheung, 2007; Essumang, 2012).
It is well known that there are toxic effects of PAHs on algae (Gala and Giesy, 1992; Gala and Giesy, 1994; Brack, 2003; Zbigniew and Wojciech, 2006; Pokora and Tukaj, 2010; Othman, 2012). Aksmann and Tukaj (2004) found that the green algaisolated from southern Baltic Sea was inhibited by anthracene (ANT) at concentrations exceeding 0.05mgL?1under laboratory conditions. A similar result was reported by Hong. (2008) in(Greville) Cleve andsp. These algae were capable of degrading PAHs, however their growth was inhibited by phenanthrene and fluoranthene. Hjorth. (2007) reported the effect of pyrene contamination on a natural plankton community. They found that pyrene induced the change in community composition and declined the abun- dance of phytoplankton. However, few attempts have been made to study the responses of marine phytoplankton competition and succession to PAHs stress.
Of all PAHs, ANT is one of the priority pollutants identified by United States Environmental Protection Agency (USEPA, 2005). It is found as a single compound in water bodies and the linear frame of this molecule is also found in the structures of many other toxic compounds such as benzo[]anthracene (Zbigniew and Wojciech, 2006). In Jiaozhou Bay, China, the concentration of ANT is higher than both the ERL (Effects range-Low) and the ISQV-Low (Interim sediment quality values-Low) values, suggesting that ANT can induce potential detrimental effect on marine organisms in this area (Long, 1995; Chapman, 1999; Yang, 2003).
In this study, we focused on the effect of ANT on population growth and interspecific competition between two species of marine phytoplankton,var.(Chlorophyceae)and(Raphidophyceae).is widely used as feed in aquaculture. Blooms ofare common to coastal regions around the world and have been implicated in fish-killing in aquaculture (Hallegraeff and Hara, 2003). The following questions were addressed in this study: (i) Which species has a higher competitive ability under the presence or absence of ANT? (ii) Are the interaction betweenanddensity-dependent? (iii) Does ANT affect the interaction between these two species?
2 Materials and Methods
2.1 Chemicals
ANT (high purity, Sigma-Aldrich (Shanghai) Trading Co., Ltd., USA) was dissolved in dimethylsulfoxide (DMSO) (AMRESCO, USA) to 1gL?1. The ANT-DMSO solution was stored at room temperature 24h prior to use and protected from light. Exposure solutions were prepared by diluting the stock solution with algal culture medium. Delivery of ANT to water with DMSO could achieve a much higher ANT concentration than dissolving it in water directly (Huang, 1997).
2.2 Organisms
Two species of marine phytoplankton,and, were used in this study. Both species were obtained from the Marine Microalgae Research Center, Ocean University of China.
2.3 Phytoplankton Monoculture
andwere precultured until the exponential growth phase. Algae from the exponential growth phase were used as an inoculum for the subsequent experimental batch culture. The initial pH was 8 and the salinity was adjusted to 30. Cultures were kept under a constant temperature of 20℃ with the light intensity of 76μmol photons m?2s?1at a light:dark cycle of 12h:12h in illuminating incubators. Initial cell densities were set as 0.125×104, 0.5×104, and 2×104cellsmL?1forand 4×104cellsmL?1for, each in triplicate. The algal cultures were kept in 100mL Erlenmeyer flasks containing 60mL culture medium. The culture medium was prepared based on the modified f/2 medium (Guillard, 1975). All flasks were shaken manually twice a day at a set time.
The experiment lasted 30 days. A 0.5mL culture was collected at two-day intervals to measure the algal growth. Algal cell density was determined using a haemocytometer and an optical microscope (OlympusCX31, Japan).
2.4 Bi-algal Culture Without ANT
Interaction between phytoplankton is expected to be size-dependent according to surface/volume considerations (Moloney and Field, 1991; Cavender-Bares, 2001; Armstrong, 2003). In bi-algal cultures of the present study, the initial cell density of each alga was adjusted based on the volume of individual cells,., 4449 μm3for(Sun, 2004) and 522μm3for(Kamiyama and Arima, 2001). Biomass of each species was estimated as cell volume (μm3) that was calculated as product of the cell density (cellsmL?1) and the volume of individual cells (μm3) (Sommer, 1994). The initial biomass ratios of(P)to(H) were set as 1:4, 1:1, and 4:1.was inoculated at a density of 4×104cellsmL?1into cultures ofat three different cell densities, 0.125×104(:=1:4), 0.5×104(:=1:1) and 2×104cells mL?1(:=4:1). Bi-algal cultures were kept in 100mL Erlenmeyer flasks under the same culture conditions and setup procedures as those in phytoplankton monoculture.
2.5 Algal EC50Calculation and Dose-Response Curve
Chemicals were delivered to the culture medium at final ANT concentrations in a range from no effect to maximal effect. Based on the results in our preliminary experiments and previous studies (Huang, 1997), the concentration gradient was set with logarithmic equal-interval of 0.476 for(0, 0.1, 0.3, 0.9, 2.7, 8.0mgL?1) and 0.386 for(0, 0.1, 0.2, 0.6, 1.4, 3.5mgL?1) to calculate EC50at 96h. To exclude the effect of initial cell density on the determination of EC50, the initial cell density for each species was set to obtain equal biomass,, 0.5×104cellsmL?1forand 4×104cellsmL?1for. The effect of DMSO (0.3%) on algal growth was tested in a separate control.
2.6 Bi-algal Culture with ANT
An effective concentration of ANT was determined as 2.8mgL?1in bi-algal cultures with ANT based on the preliminary experiments. At this concentration, ANT showed significant effects on the growth ofand. All other culture conditions were the same as those in bi-algal cultures without ANT.
2.7 Data Analysis and Statistics
The experiment data were log-transformed, and a logistic growth curve was fit to the following equation:
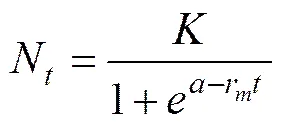
whereNis the cell density at time(104cellsmL?1);is the carrying capacity of the population (104cells mL?1), which is defined as maximum sustainable population density (biomass) in a given ecosystem (Lampert and Som- mer, 2007);is the sampling time (d);ris the maximal specific growth rate (d?1);is a constant determining the initial cell density of algae(N).
. (2)
Eq. (1) was applied to the growth phase before the maximal cell density was reached.
EC50values at 96h were estimated in terms of interpolated concentration that would inhibit algal population growth by 50% over a specific period of time (96h) (Walsh, 1987). The relative growth rate was defined by the following formula,
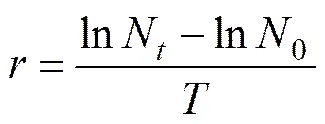
wheredenotes the relative growth rate (d?1); and0,Nare the population densities (104cellsmL?1) at times 0 and(d); andis the time interval (d). The EC50after 96h was derived by straight-line graphical interpolation (Green- berg, 1985).
The interaction betweenandat different initial biomass ratios (1:4, 1:1, or 4:1) were analyzed using the Lotka-Volterra two species competition model expressed by the following equations:
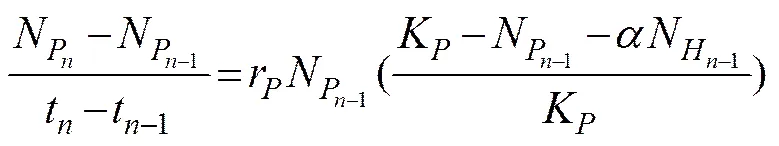
and
, (5)
K>K/,KK/α,excludes;
K >K/α,KK/,excludes;
KK/,KK/α,andhave a coexistence;
K>K/,K>K/α,andhave an unstable equilibrium.
Wilcoxon Signed Ranks Test was conducted to analyze the differences in algal growth between monocultures and bi-algal cultures without ANT, as well as in bi-algal cultures with and without ANT, with the significance level of<0.05. Estimated values of growth parameters were obtained by the nonlinear regression in SPSS version 16.0.
3 Results
3.1 Phytoplankton Growth in Bi-algal Culture Without ANT
Overall, cell densities ofandin bi-algal cultures were lower than those in monocultures (Fig.1). Especially for, cell density was dramatically decreased in bi-algal cultures independently of initial biomass ratios. At the stationary growth phase, cell density ofin monocultures was about 5 to 25 times higher than that in bi-algal cultures. But for, the growth suppression in bi-algal cultures was less pronounced as cell density was ca. one to two times higher in monocultures than that in bi-algal cultures. Significant suppression of population growth was observed for both algal species in bi-algal cultures but not in monocultures (Wilcoxon Signed Ranks Test,=?3.181,=0.001,=16 for;=?2.244,=0.025,=16 for.).
The population growth of two algal species was determined according to the logistic growth model (Eq. (1)) with estimated parameters plotted in Fig.2. Carrying capacity was consistently high in monocultures independently of initial biomass ratios (Fig.2a). Compared to that in monocultures, carrying capacity in bi-algal cultures was decreased by 97%, 90% and 69% for, and by 13%, 41% and 50% forat initial biomass ratios of:1:4, 1:1 and 4:1, respectively. Similarly, maximal specific growth rate for both algal species was lower in bi-algal cultures than that in monocultures (Fig.2b).
out-competedat:=1:4 and 1:1 (Figs.1a and b) but not at:=4:1 (Fig.1c). Furthermore, the Lotka-Volterra two species competition model showed a similar result with the experimental result above.was excluded at:=1:4 and 1:1 withK>K/andK <K/α(Table 1). AndK>K/andK >K/αwere observed at:=4:1, showing a potential coexistence, but this equilibrium was unstable.
3.2 Algal EC50Values and Dose-Response Curve
Relative growth rates ofwere enhanced at the lowest ANT concentration (Fig.3a). However, the enhancement of relative growth rates ofwas not statistically significant at the lowest ANT concentration (Fig.3b). For both algal species, growth inhibition markedly increased with increasing ANT concentrations. The 96h EC50value was 7.0mgL?1forand 3.1mgL?1for, respectively. DMSO had no detectable effect on the growth ofor.
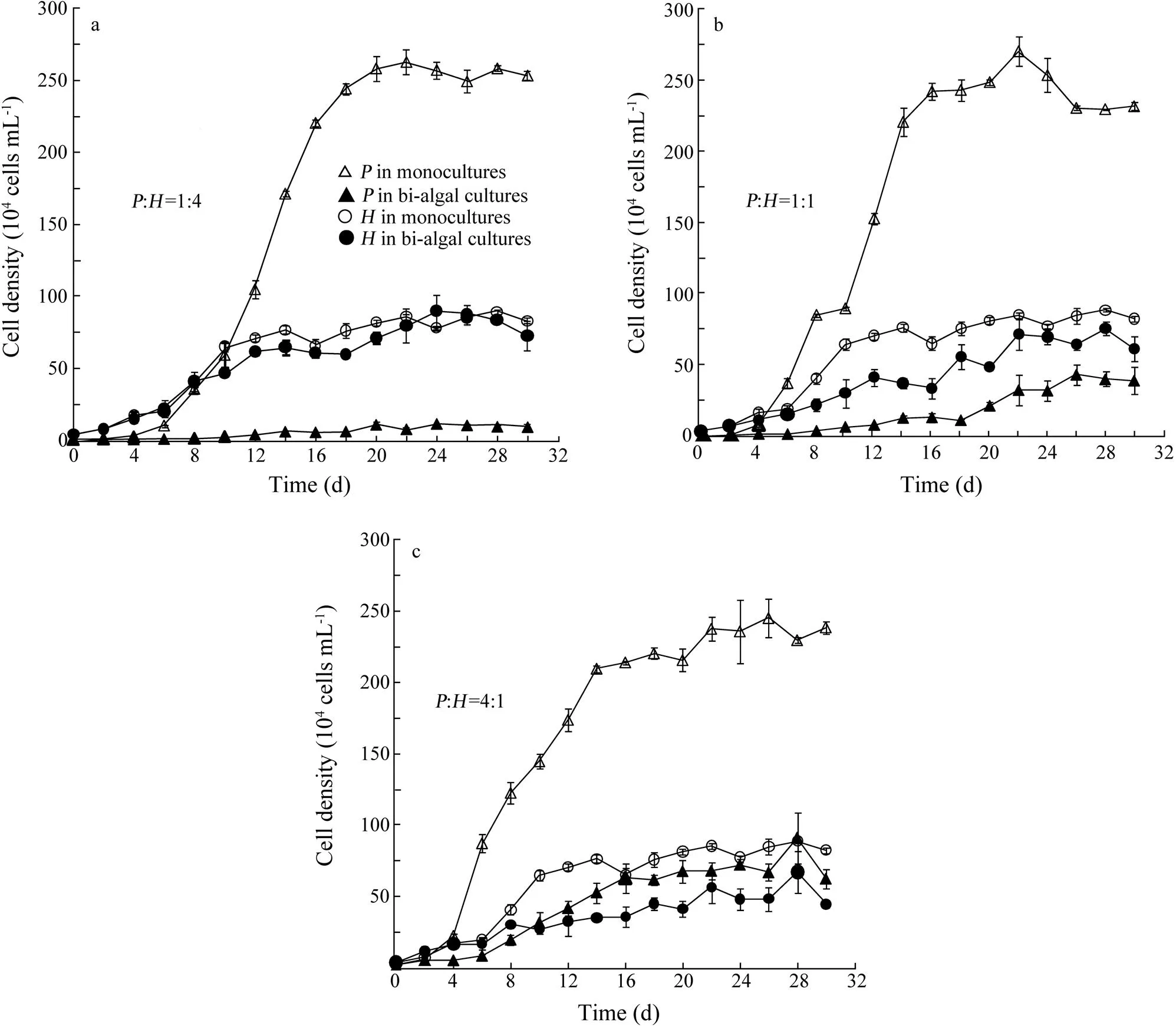
Fig.1 Population growth of Platymonas helgolandica and Heterosigma akashiwo in monocultures and bi-algal cultures at different biomass ratios. (a) the initial biomass ratio of P. helgolandica (P) to H. akashiwo (H)=1:4 with initial cell densities of 0.125×104cellsmL?1 for P. helgolandica and 4×104cellsmL?1 for H. akashiwo; (b) P:H=1:1 with initial cell densities of 0.5×104cellsmL?1 for P. helgolandica and 4×104cellsmL?1 for H. akashiwo; and (c) P:H=4:1 with initial cell densities of 2×104cellsmL?1 for P. helgolandica and 4×104cellsmL?1 for H. akashiwo. Values are shown as mean±SD.
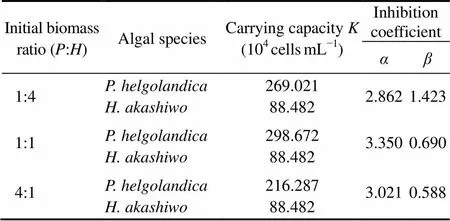
Table 1 Estimated parameters of Platymonas helgolandica and Heterosigma akashiwo obtained by nonlinearregression according to the Lotka-Volterra twospecies competition model
Note: ‘’ and ‘’ indicateand, respectively.
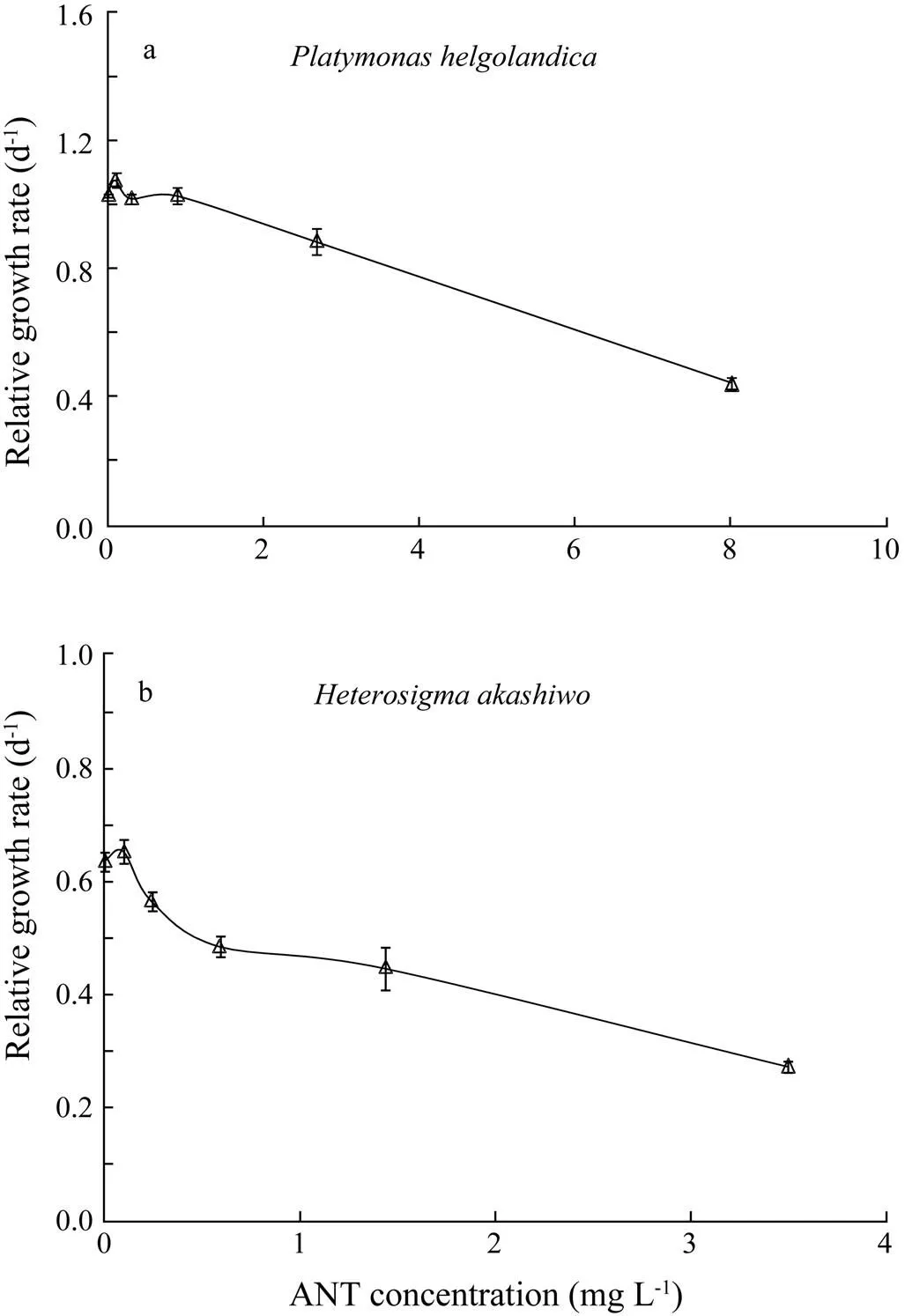
Fig.3 Relative growth rates of (a) Platymonas helgolandica and (b) Heterosigma akashiwo under anthracene (ANT) stress for 96h. Values are shown as mean±SD.
3.3 Phytoplankton Growth in Bi-algal Culturewith ANT
The interaction betweenandunder ANT stress (the ANT concentration=2.8mgL?1) is shown in Fig.4. Cell density ofwas significantly higher than that in bi-algal cultures without ANT at three biomass ratios (Wilcoxon Signed Ranks Test,=?3.180,=0.001,=16 at:=1:4;=?3.351,=0.001,=16 at:=1:1;=?3.351,=0.001,=16 at:=4:1). At:=1:4 (Fig.4a), however, cell density ofwas similar between two treatments (with and without ANT) from day 0 to day 6 (Wilcoxon Signed Ranks Test,=?1.604,=0.109,=4) and decreased to zero after day 8. Cell density ofmarkedly increased on day 8 exactly when the growth ofwas terminated. Similarly, at:=1:1 and 4:1 (Figs.4b and c), cell density ofwas similar between two treatments from day 0 to day 4 (Wilcoxon Signed Ranks Test,=0,=1.000,=3 at:=1:1;=?1.604,=0.109,=3 at:=4:1) but decreased to zero after day 6. On day 6, cell density ofbegan to increase. As shown in Fig.4, ANT, through the elimination of, promoted the growth of.
To investigate the effect of biomass ratio on carrying capacity, the growth ofwas fit to the logistic growth model (Eq. (1)), while the growth period ofwas too short to be fit to Eq. (1). The estimated carrying capacity forwas consistently higher with ANT than that without ANT (Fig.5a). The carrying capacity ofwith ANT was 18, nine or four times higher than that without ANT at the biomass ratio of 1:4, 1:1 or 4:1, respectively. However, there was no consistent pattern in the change of maximal specific growth rate (Fig.5b).
4 Discussion
It has been well known that algal species differ in tolerance to various contaminants (Blanck, 1984; Dijkman, 1997; Zbigniew and Wojciech, 2006; Hong, 2008; Echeveste, 2010). In this study, the tolerance ofto ANT was greater than that ofThe high variability in sensitivity of different algal species to the same chemical can be explained by the morphology, cytology, physiology and genetics of the organisms (Rojí?ková-Padrtová and Mar?álek, 1999).is cell wall free, whileis covered by a wall mostly consisting of cellulose (Guo, 1994; Wang, 2000). The algal cell wall has been considered as a physical barrier of resisting benzo[α]pyrene and its phototoxic products (Warshawsky, 1995). On the other hand, the sensitivity of phytoplankton to PAHs varies with the cell size (Tang, 1998; Del Vento and Dachs, 2002; Echeveste, 2010; Othman, 2012). Tang. (1998) suggested that freshwater algae with a high ratio of surface area to biovolume possessed a high potential of accumulating atrazine (one of the most widely used pesticides). A strong positive linear relationship was observed between LC50(the PAH concentration at which cell population will be decreased by a half) and cell volume of six marine phytoplankton species, for both phenanthrene and pyrene (Echeveste, 2010). As the cell volume ofis much lower than that of,it is therefore likely that differences in both cell surface structure and cell size betweenandmight account for their different response to ANT stress.
As shown in Fig.4, it is interesting that cell density ofincreased exactly on the day when the growth ofwas terminated. The sharp decrease in the growth ofindicated thatmay accumulate large amounts of ANT and remove ANT from the medium, and thus cell density ofbegan to decline due to the toxicity of ANT. Toxicant removal and/or competitor decline may have enabled the growth of.
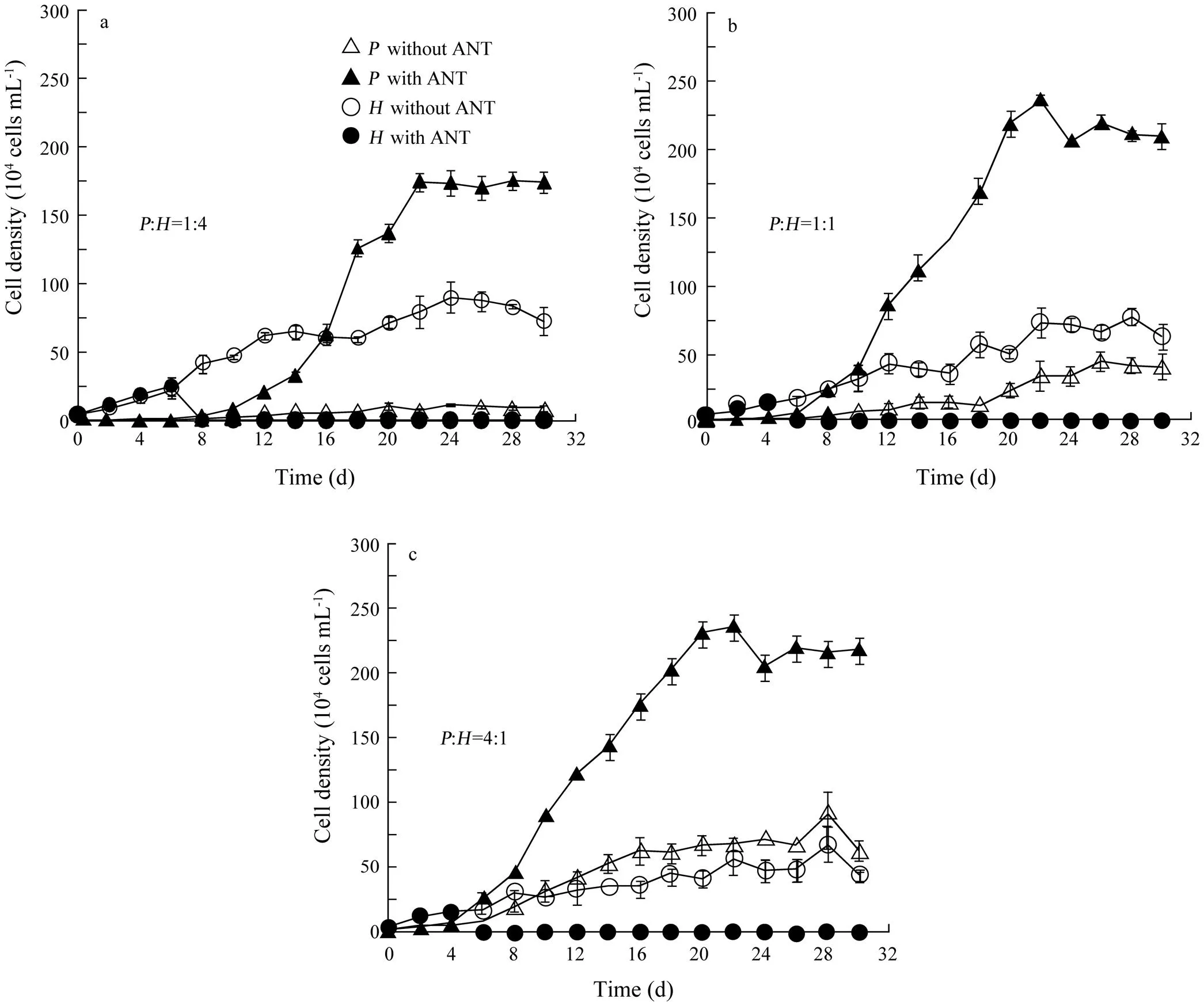
Fig.4 Population growth of Platymonas helgolandica and Heterosigma akashiwo in bi-algal cultures with and without anthracene (ANT) at initial biomass ratios of P. helgolandica (P) to H. akashiwo (H)=(a) 1:4, (b) 1:1, and (c) 4:1. The corresponding initial cell densities were the same as those in Fig.1. The concentration of ANT was 2.8mgL?1 in bi-algal cultures with ANT. Values are shown as mean±SD.
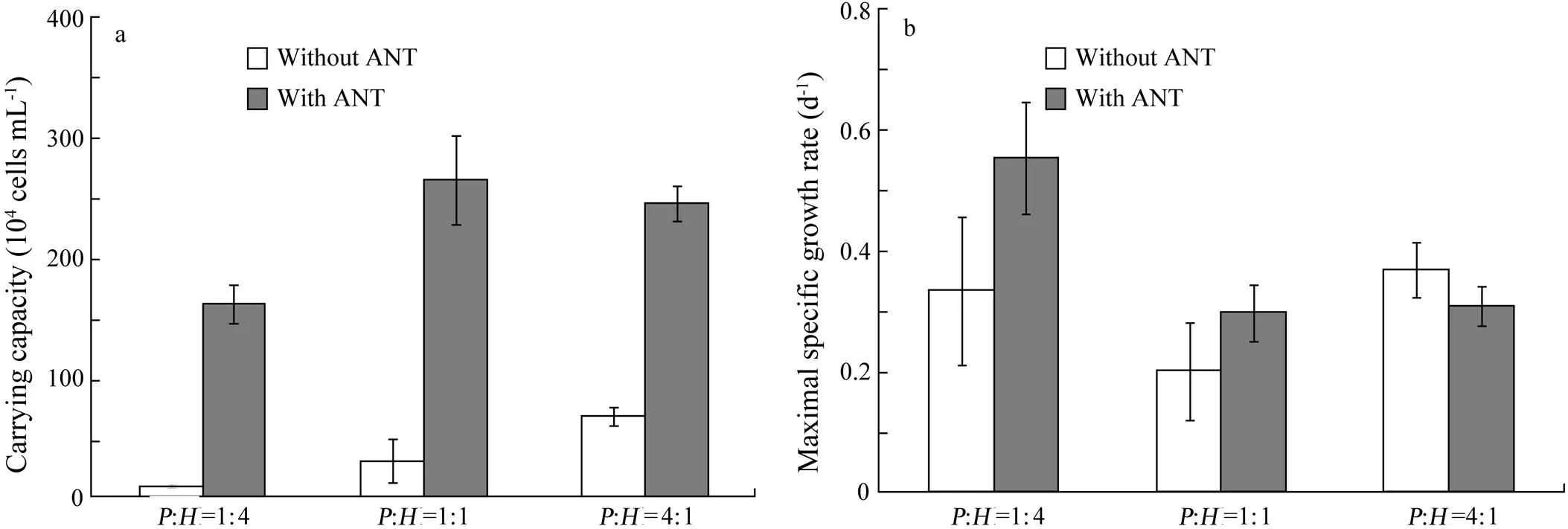
Fig.5 The carrying capacity (a) and maximal specific growth rate (b) of Platymonas helgolandica with and without anthracene (ANT) at biomass ratios of P. helgolandica (P) to H. akashiwo (H)=1:4, 1:1, and 4:1. Values are shown as mean±SD.
In the light of the above, it is not surprising that the difference in carrying capacity ofbetween treatments (with and without ANT) became small when initial biomass ratios oftoincreased (Fig.5). In the first case (:=1:4), a higher biomass ofcould accumulate a higher amount of ANT than in the second (:=1:1) and the third case (:=4:1). Thus,was probably exposed to a larger amount of ANT in the second or third case.
The results in the present study revealed that initial biomass ratio affected the interaction betweenandin bi-algal cultures without ANT.out-competedat lower biomass ratios ofto(:=1:4 and 1:1 respectively). However, there was an unstable equilibrium between these two species at higher biomass ratio (:=4:1). This result is consistent with those of previous studies, indicating the density-dependent effect of harmful algae to other algal species (Hao, 2008; Tillmann and Hansen, 2009; Wei, 2012). For example, Hattenrath-Lehmann and Gobler (2011) reported the density-dependent inhibition of the harmful algato natural phytoplankton assemblage in bi-algal laboratory experiments. Furthermore, our results showed thatcould excludeat a higher initial biomass ratio ofto, indicating the potential ofas a biological control ofblooms in aquaculture.
A high competitive capability of a species due to its high pollutant tolerance is believed to be responsible for the change or reverse of the interspecific competition (Niu, 2003). In the present study, the high sensitivity ofto ANT resulted in the elimination ofin bi-algal cultures with ANT. Consequently, the dominance ofwas generated. This result is in agreement with the suggestion of Hjorth. (2007) that PAHs might be an important stress for marine phytoplankton communities.
Hormesis is an adaptive response with distinguishing biphasic dose-response characteristics that are either directly induced or the result of compensatory biological processes following an initial disruption in homeostasis (Calabrese, 1999; Calabrese and Baldwin, 2002). In the present study, only one dose in the dose-response curve ofshowed a stimulatory effect, thus it could be a low or moderate evidence classification of hormesis (Calabrese and Baldwin, 1998). The stimulatory effect of ANT was also reported by Wang. (2002), low concentration of ANT could stimulate the growth ofand. However, the results in the present study did not reveal statistically significant low-dose stimulation on the dose-response curve of. This could be considered as no or low evidence of hormesis (Calabrese and Baldwin, 1998). There is evidence that hormesis is infrequently observed even though it is a general biological phenomenon due to a combination of facts such as study design and statistical power (Calabrese and Baldwin, 1998; Calabrese, 2008). No single mechanism accounts for the general occurrence of hormesis, although hormesis has been explained mechanistically in some cases (Calabrese, 2008). Further research would be required to decipher the mechanism of the stimulatory effect of ANT on algal growth.
5 Conclusions
The present study provides empirical evidence for the interaction between the harmful algaand the dietary algawith and without ANT. Density-dependent interaction was observed in bi-algal cultures without ANT.exhibited a higher sensitivity to ANT, resulting in the diminishing ofand the dominance of. Different cell surface structures and cell sizes might explain different responses of phytoplankton to ANT. Further studies are suggested to explore the physiological principles of algal species-specific responses to ANT, as well as the potential effects of ANT to higher trophic levels via bottom-up processes in marine ecosystems.
Acknowledgements
We thank Drs. Shungudzemwoyo Garaba, Changwei Bian and Ying Wang for their helpful suggestions on the manuscript. We sincerely appreciate the valuable advice provided by Dr. Ulrich Sommer on an earlier version of this manuscript and insightful comments from anonymous reviewers. This work was supported by the Ocean Public Welfare Scientific Research Projects, State Oceanic Administration People’s Republic of China (Grant Nos. 200905020, 2010225007, and 201305009), and the National Natural Science Foundation of China (Grant No. 31070458).
Aksmann, A., and Tukaj, Z., 2004. The effect of anthracene and phenanthrene on the growth, photosynthesis, and SOD activity of the green algadepends on the PAR irradiance and CO2level., 47: 177-184.
Armstrong, R. A., 2003. A hybrid spectral representation of phytoplankton growth and zooplankton response: The ‘control rod’ model of plankton interaction., 50: 2895-2916.
Blanck, H., Wallin, G., and W?ngberg, S.-?., 1984. Species-dependent variation in algal sensitivity to chemical com- pounds., 8: 339-351.
Brack, W., Altenburger, R., Küster, E., Meissner, B., Wenzel, K.-D., and Schüürmann, G., 2003. Identification of toxic pro- ducts of anthracene photomodification in simulated sunlight., 22: 2228-2237.
Calabrese, E. J., and Baldwin, L. A., 1998. Hormesis as a bio-logical hypothesis., 106: 357-362.
Calabrese, E. J., 1999. Evidence that hormesis represents an ‘overcompensation’ response to a disruption in homeostasis., 42: 135-137.
Calabrese, E. J., and Baldwin, L. A., 2002. Defining hormesis., 21: 91-97.
Calabrese, E. J., 2008. Hormesis: Why it is important to toxicology and toxicologists., 27: 1451-1474.
Cavender-Bares, K. K., Rinaldo, A., and Chisholm, S. W., 2001. Microbial size spectra from natural and nutrient enriched ecosystems., 46: 778-789.
Cerniglia, C. E., 1992. Biodegradation of polycyclic aromatic hydrocarbons., 3: 351-368.
Chapman, P. M., Allard, P. J., and Vigers, G. A., 1999. Development of sediment quality values for Hong Kong special administrative region: A possible model for other jurisdictions., 38: 161-169.
Cheung, K. C., Leung, H. M., Kong, K. Y., and Wong, M. H., 2007. Residual levels of DDTs and PAHs in freshwater and marine fish from Hong Kong markets and their health risk assessment., 66: 460-468.
Del Vento, S., and Dachs, J., 2002. Prediction of uptake dynamics of persistent organic pollutants by bacteria and phytoplankton., 21: 2099-2107.
Dijkman, N. A., van Vlaardingen, P. L. A., and Admiraal, W. A., 1997. Biological variation in sensitivity to N-heterocyclic PAHs; effects of acridine on seven species of micro-algae., 95: 121-126.
Echeveste, P., Agustí, S., and Dachs, J., 2010. Cell size depen-dent toxicity thresholds of polycyclic aromatic hydrocarbons to natural and cultured phytoplankton populations., 158: 299-307.
Echeveste, P., Agustí, S., and Dachs, J., 2011. Cell size dependence of additive versus synergetic effects of UV radiation and PAHs on oceanic phytoplankton., 159: 1307-1316.
Eisler, R., 1987. Polycyclic aromatic hydrocarbon hazards to fish, wildlife, and invertebrates: A synoptic review., 11: 93.
Essumang, D. K., Dodoo, D. K., and Adjei, J. K., 2012. Polycyclic aromatic hydrocarbon (PAH) contamination in smoke-cured fish products., 27: 128-138.
Gala, W. R., and Giesy, J. P., 1992. Photo-induced toxicity of anthracene to the green alga,., 23: 316-323.
Gala, W. R., and Giesy, J. P., 1994. Flow cytometric deter-mination of the photoinduced toxicity of anthracene to the green alga., 13: 831-840.
Greenberg, A. E., Trussell, R. R., Clesceri, L. S., and Franson, M. A. H., 1985.. 16 edition. American Public Health Association, Washington, D. C., 1268pp.
Guillard, R. R. L., 1975. Culture of phytoplankton for feeding marine invertebrates. In:. Smith, W., and Chanley, M., eds., Plenum Publishing Corporation, New York, 26-60.
Guo, Y., 1994. Studies on(Hada) Hada in the Dalian bight, Liaoning, China., 25: 211-215.
Hallegraeff, G. M., and Hara, Y., 2003. Taxonomy of harmful marine raphidophytes. In:.Hallegraeff, G. M., and Anderson, D. M., eds., UNESCO Publishing, Paris, 511-522.
Hao, W. J., Wang, Y., and Tang, X. X., 2008. Interactions between two marine microalgaes:Hulburt andvar.in under controlled laboratory co-culture., 47: 98-105.
Hattenrath-Lehmann, T. K., and Gobler, C. J., 2011. Alle-lopathic inhibition of competing phytoplankton by North American strains of the toxic dinoflagellate,: Evidence from field experiments, laboratory experiments, and bloom events., 11: 106-116.
Hjorth, M., Vester, J., Henriksen, P., Forbes, V., and Dahll?f, I., 2007. Functional and structural responses of marine plankton food web to pyrene contamination., 338: 21-31.
Hong, Y. W., Yuan, D. X., Lin, Q. M., and Yang, T. L., 2008. Accumulation and biodegradation of phenanthrene and fluo-ranthene by the algae enriched from a mangrove aquatic ecosystem., 56: 1400-1405.
Huang, X. D., McConkey, B. J., Babu, T. S., and Greenberg, B. M., 1997. Mechanisms of photoinduced toxicity of photo- modified anthracene to plants: Inhibition of photosynthesis in the aquatic higher plant(duckweed)., 16: 1707-1715.
Kamiyama, T., and Arima, S., 2001. Feeding characteristics of two tintinnid ciliate species on phytoplankton including harmful species: Effects of prey size on ingestion rates and selectivity., 257: 281-296.
Kirso, U., and Irha, N., 1998. Role of algae in fate of carcinogenic polycyclic aromatic hydrocarbons in the aquatic environment., 41: 83-89.
Lampert, W., and Sommer, U., 2007.. Oxford University Press, New York, 324pp.
Liu, L. Y., Wang, J. Z., Wei, G. L., Guan, Y. F., and Zeng, E. Y., 2012. Polycyclic aromatic hydrocarbons (PAHs) in continen- tal shelf sediment of China: Implications for anthropogenic influences on coastal marine environment., 167: 155-162.
Long, E. R., MacDonald, D. D., Smith, S. L., and Calder, F. D., 1995. Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments., 19: 81-97.
Moloney, C. L., and Field, J. G., 1991. The size-based dynamics of plankton food webs. I. A simulation model of carbon and nitrogen flows., 13: 1003-1038.
Neff, J. M., Cox, B. A., Dixit, D., and Anderson, J. W., 1976. Accumulation and release of petroleum-derived aromatic hydrocarbons by four species of marine animals., 38: 279-289.
Niu, M. G., 2003. Study on the resource competitions and succession laws among the algae in eutrophication water. Master thesis.Suzhou University, Suzhou.
Oberd?rster, E., Brouwer, M., Hoexum-Brouwer, T., Manning, S., and McLachlan, J. A., 2000. Long-term pyrene exposure of grass shrimp,, affects molting and reproduction of exposed males and offspring of exposed females., 108: 641-646.
Othman, H. B., Leboulanger, C., Le Floc’h, E., Hadj Mabrouk, H., and Sakka Hlaili, A., 2012. Toxicity of benz()anthracene and fluoranthene to marine phytoplankton in culture: Does cell size really matter?, 243: 204-211.
Piccardo, M. T., Coradeghini, R., and Valerio, F., 2001. Polycyclic aromatic hydrocarbon pollution in native and caged mussels., 42: 951-956.
Pokora, W., and Tukaj, Z., 2010. The combined effect of anthracene and cadmium on photosynthetic activity of three(Chlorophyta) species., 73: 1207-1213.
Rojí?ková-Padrtová, R., and Mar?álek, B., 1999. Selection and sensitivity comparisons of algal species for toxicity testing., 38: 3329-3338.
Sommer, U., 1994. Are marine diatoms favoured by high Si:N ratios?, 115: 309-315.
Stringer, T. J., Glover, C. N., Keesing, V., Northcott, G. L., and Tremblay, L. A., 2012. Development of a harpacticoid cope- pod bioassay: Selection of species and relative sensitivity to zinc, atrazine and phenanthrene., 80: 363-371.
Sun, J., Liu, D. Y., Chen, Z. T., and Wei, T. D., 2004. Growth of,andand their survival strate- gies under different N/P ratios., 15: 2122-2126.
Tang, J., Hoagland, K. D., and Siegfried, B. D., 1998. Uptake and bioconcentration of atrazine by selected freshwater algae., 17: 1085-1090.
Tillmann, U., and Hansen, P. J., 2009. Allelopathic effects ofon other algae: Evidence from mixed growth experiments., 57: 101-112.
USEPA, 2005.. Environmental Protection Agency, United State, 53pp.
Walsh, G. E., Deans, C. H., and McLaughlin, L. L., 1987. Comparison of the EC50s of algal toxicity tests calculated by four methods., 6: 767-770.
Wang, L., Pan, L., Liu, N., Liu, D., Xu, C., and Miao, J., 2011. Biomarkers and bioaccumulation of clamin response to combined cadmium and benzo[α]pyrene exposure., 49: 3407-3417.
Wang, Y., Tang, X. X., and Li, Y. Q., 2000. The joint toxic effect of anthracene and profenofos on marine microalga., 24: 5-6.
Wang, Y., Tang, X. X., Li, Y. Q., and Liu, Y., 2002. Stimulation effect of anthracene on marine microalgae growth., 13: 343-346.
Warshawsky, D., Cody, T., Radike, M., Reilman, R., Schumann, B., LaDow, K., and Schneider, J., 1995. Biotransformation of benzo[]pyrene and other polycyclic aromatic hydrocarbons and heterocyclic analogs by several green algae and other algal species under gold and white light., 97: 131-148.
Wei, J., Zhao, W., Yang, W. D., and Ge, Y., 2012. Effects of initial biomass ratio on the interspecific competition outcome between three marine microalgae species., 32: 1124-1132.
Yang, Y., Mai, B., Pan, J., Yin, X., and Li, F., 2003. Distribution and sources of polycyclic aromatic hydrocarbon in sediments of Jiaozhou Bay., 22: 38-43.
Zbigniew, T., and Wojciech, P., 2006. Individual and combined effect of anthracene, cadmium, and chloridazone on growth and activity of SOD izoformes in threespecies., 65: 323-331.
(Edited by Qiu Yantao)
DOI 10.1007/s11802-015-2345-2
ISSN 1672-5182, 2015 14 (1): 105-113
(March 28, 2013; revised July9, 2013; accepted October20, 2014)
? Ocean University of China, Science Press and Spring-Verlag Berlin Heidelberg 2015
. Tel: 0086-532-82032952 E-mail: tangxx@ouc.edu.cn
 Journal of Ocean University of China2015年1期
Journal of Ocean University of China2015年1期
- Journal of Ocean University of China的其它文章
- A Comparative Study of CART and PTM for Modelling Water Age
- Numerical Study of the Secondary Circulations in Rip Current Systems
- Application of Numerical Simulation for Analysis of Sinking Characteristics of Purse Seine
- The Structure and Formation Mechanism of a Sea Fog Event over the Yellow Sea
- Molecular Cloning, Expression Pattern, and 3D Structural Prediction of the Cold Inducible RNA - Binding Protein (CIRP) in Japanese Flounder (Paralichthys olivaceus)
- Effects of the Amplitude and Frequency of Salinity Fluctuation on the Body Composition and Energy Budget of Juvenile Tongue Sole (Cynoglossus semilaevis)
