Phytochemical and biological studies of Butia capitata Becc. leaves cultivated in Egypt
Nagwa Mohamed Ammar, Mohammed Said Hefnawy, Sahar Youssef Al-Okbi, Doha Abdou Mohamed, Nabil Khamis El-Sayed, Amira Ahmed El -Anssary, Tom Mabry
1Pharmacognosy Department, National Research Centre, Dokki, El-Buhouth Street, 12622, Cairo, Egypt
2Pharmacognosy Department, Faculty of Pharmacy, Cairo University, Egypt
3Food Sciences and Nutrition Department, National Research Centre, Dokki, Cairo, Egypt
4Tanning Materials and Protein Chemistry, National Research Centre, Dokki, Cairo, Egypt
5Molecular Cell and Developmental Biology, The School of Biological Science, Botany Department, Taxas State University, Austin Texas 78713, USA
Phytochemical and biological studies of Butia capitata Becc. leaves cultivated in Egypt
Nagwa Mohamed Ammar1, Mohammed Said Hefnawy2, Sahar Youssef Al-Okbi3*, Doha Abdou Mohamed3, Nabil Khamis El-Sayed4, Amira Ahmed El -Anssary1, Tom Mabry5
1Pharmacognosy Department, National Research Centre, Dokki, El-Buhouth Street, 12622, Cairo, Egypt
2Pharmacognosy Department, Faculty of Pharmacy, Cairo University, Egypt
3Food Sciences and Nutrition Department, National Research Centre, Dokki, Cairo, Egypt
4Tanning Materials and Protein Chemistry, National Research Centre, Dokki, Cairo, Egypt
5Molecular Cell and Developmental Biology, The School of Biological Science, Botany Department, Taxas State University, Austin Texas 78713, USA
PEER REVIEW
Peer reviewer
Prof. Dr. Aisha Hussein Abou Zeid, Professor of Pharmacognosy, Pharmacognosy Department, National Research Centre, Cairo, Egypt.
E-mail: abouzeida@yahoo.co.uk
Tel: 0201111219882
Comments
This work has an added value since the authors evaluated for the first time the biological activity of the successive extracts of B. capitata leaves palm cultivated in Egypt. Also the research dealt with isolation and identification of the bioactive constituents of the extracts.
Details on Page 461
Objective:To study the antioxidant and anti-inflammatory activity of Butia capitata (B. capitata) leaf extracts along with phytochemical analysis of the proposed bioactive constituents.
Butia capitata leaves, antioxidant effect, anti-inflammatory activity, bioactive constituents, rats.
1. Introduction
Arecaceae, alternately known as Palmae is an ancient family and among the world’s larger plant families both in terms of number of species and abundance; it contains about 2 800 species. Until very recently, palms were classified into fifteen major groups which rely mostly on gross morphological
characteristics, such as induplicate or reduplicate and fan or feather leaves. These groups have been slightly reorganized into six subfamilies within the Arecaceae: Coryphoideae, Calamoideae, Nyphoideae, Ceroxyloideae, Arecoideae, and Phytelephantoideae[1,2].
Plants belonging to family Palmae possess many economic uses and biological activities such as tonic, diuretic and treatment of leprosy, asthma, bronchitis, fatigue, tuberculosis, abdominal complains, fever and vomiting.
The fruit ofButia capitata(B. capitata) from the genusButiais edible and used to make jams or jellies. Very few works were traced in the genusButia. Some authors reported the presence of cylindrin and lupeol methyl ether in the epicuticular waxes of the leaf ofB. capitataas well as tricin C-glycosyl flavones, luteolin, quercetin glycosides and kaempferol[3,4], while tricin 7-glucosides, isoorientin and caffeylshikimic acid were reported in the flowers[5].
The aim of this work is to study the anti-inflammatory and antioxidant activity of the different successive extracts ofB. capitataleaves, not previously studied, as well as the isolation and identification of the biologically active constituents.
2. Materials and methods
2.1. Phytochemical study
2.1.1. Materials and instruments
The volatile constituents fromB. capitatawere analyzed using a Finngan SSQ 7000 gas chromatograph coupled with a mass spectrometer.
All proton nuclear magnetic resonance spectra were run on a Bruker AMX-500, Varian Inova-500. The chemical shifts were reported in δ values (ppm) with tetramethylsilane as internal standard. hydrogen and carbon nuclear magnetic resonance (1H- and13C-NMR) spectra were recorded in dimethylsulfoxide. UV was recorded on UV-visible spectrophotometer: Beckman DU7 and Shimadzu UV 240 (PIN 204-5800) were used for recording UV spectra and measuring the absorbance in UV and visible range (UVPC). UV-VIS spectrophotometer in the region of 200-500 nm was used.
Electron ionization-mass spectrometry (EI-MS), heteronuclear multiple bond correlation (HMBC) and heteronuclear multiple quantum correlation (HMQC) were used.
High pressure liquid chromatography (HPLC) system: Agilent 1100 series (Agilent Technologies, Wald Brown, Germany). Quaternary pump: G/311A, degaser : G/1322A with variable wave length detector G 1314A. Autosampler: G1329A for investigation of alpha-tocopherol.
Thin layer chromatography (TLC) was preformed on Merck precoated silica gel 60 F254 plates while column chromatography was run using Merck silica gel (70-90) mesh as adsorbent.
Sephadex LH-20 (Pharmacia, Uppsala, Sweden), sheets of Whatman filter paper No. 1 were used for paper chromatography (PC). Sheets of Whatman filter paper (3 mm) were used for preparative paper chromatography. Cellulose plates, (E. Merck) and microcrystalline cellulose (E. Merck) were used for column chromatography.
Solvent system for TLC were benzene: ethyl acetate as 86:14 (v/v),n-butanol: acetic acid: water as 4:1:5 (v/v/v), acetic acid: water as 15:85 (v/v), methanol: chloroform as 9:1 (v/v).
The paper and plates were sprayed with 1% aluminium chloride reagent for detection of flavonoids[6], while vanillinsulphruic acid reagent was used for sterols[7].
Reagents for UV spectroscopic analysis of flavonoids were prepared according to Mabry,et al[8].
2.1.2. Plant material
Samples of the leaves ofB. capitataBecc. family Palmae were collected from the Orman Garden, Giza, Egypt, authenticated by Dr. Tereez Labib, Consultant of Plant Taxonomy, Ministry of Agriculture, Giza, Egypt. A voucher specimen was deposited in the National Research Centre Herbarium, Cairo, Egypt. The collected leaves were air dried, reduced to No. 36 powder and kept in tightly closed containers.
2.1.3. Investigation of the volatile constituents
FreshB. capitataleaves (500 g) were subjected to steam distillation in modified Lickens and Nikerson apparatus[9]. This method allowed the simultaneous extraction of the volatile components in an organic solvent (n-pentane). The solvent was evaporated carefully after dehydration over anhydrous sodium sulphate. The yielded volatiles were analyzed using gas chromatography-mass spectrometry (GC/MS) adopting the following conditions[10]: Capillary column: DB-5 fused silica (5% phenyl methyl polysiloxane), 30 m length, 0.25 mm inner diameter and 0.25 μm thickness. Carrier gas was helium at 13 psi. Oven temperature was programmed at 60 °C isothermal for 3 min, then heating to 260 °C at a rate of 4 °C/min, then isothermal at 260°C for 5 min. Injector temperature was 220 °C. Ionization energy was 70 eV and volume injected was 1 μL.
2.1.4. Preparation of different extracts of B. capitata leaves
Successive extracts ofB. capitataleaves (petroleum ether 40-60, ether, methanol and 50% aqueous methanol) were prepared by Soxhlet.
2. 1.5. Investigation of lipoidal matter
Petroleum ether (40-60 °C) extract ofB. capitataleaves was saponified using 10% alcoholic KOH solution to prepare the unsaponifiable matter and fatty acids[11,12]. Analysis was performed through TLC of aliquots of the unsaponifiable matter (USM) of the leaves where it was dissolved in chloroform, spotted on silica gel “G” plates alongside with different solution of authentics and developed with benzene: ethyl acetate (86:14, v/v). The developed chromatoplates were sprayed with vanillin-sulphuric acid reagent, heated at 100 °C for 5 min. Unsaponifiable matter was also analyzed by gasliquid chromatography (GLC) using the following conditions, Column: HP-1 methyl siloxane. Capillary column: length 30 m; diameter 530 μm; thickens 2.56 μm; temperature 250 °C; detector temperature 300 °C; injector temperature 250 °C; carrier gas: N2, flow rate 30 mL/min; H2, flow rate 30 mL/min; air, flow rate 300 mL/min; detector: flame ionization detector; oven program: initial temperature 60 °C at rate 10 °C/min andfinal temperature 280 °C.
The fatty acids fraction (0.5 g) ofB. capitataleaves was subjected to methylation and was analyzed adopting GLC conditions. Analysis of the fatty acid methyl esters was carried out by direct comparison of retention times of each of the separated compounds with those of certain available authentic samples with the following conditions, column: capillary column HP-innowax polyethylene glycol; length 30 m, diameter 530 μm, film thickness 1 μm; oven: temperature program: rate, 2 °C/min; initial temperature 60 °C; final temperature 280 °C; pressure 12.28 psi; flow rate 13.8 mL/min; detector: flame ionization detector; temperature 300 °C; carrier gas: N2flow-rate 30 mL/min; H2flow rate 30 mL/min; air flow rate 300 mL/min.
2.1.6. Investigation ofα-tocopherol
The solvent free petrolum ether extract (1.0 g) of the leaves was saponified with equal volume of 10% (v/w) methanolic KOH. Saponification was allowed to proceed for 2 to 3 h at room temperature. After dilution with water, the unsaponifiable fraction was extracted by careful shaking of 50 mL with ether containing 0.1% butylated hydroxy toluene. The ether extract was washed with water until washing were neutral to pH paper. The ether was evaporated under stream of nitrogen gas and the extract was weighed[13,14]. Alpha-tocopherol was investigated using HPLC. Mobile phase: methanol: acetonitrile (10:90, v/v); flow rate: 1 mL/min; detector: UV 292 nm; injector volume: 100 μL; column: Biobasic C18(4.6 mm×250 mm, 5 μm) with guard column C18.
2.1.7. Investigation of free sugars and polysaccharide content
2.1.7.1. Study of the free sugars
A known weight of the vacuum dried powdered plant sample (100 g) was extracted under reflux with 20 mL ethanol (80 %) in water bath (at 70 °C) for one hour. The extract was dried, weighed and kept for chromatographic analysis[15].
Paper chromatographic analysis of free sugars was carried out. The residue of the sample and reference sugars were dissolved in 10% isopropanol/water, then spotted on Whatman No. 1 sheets. The spots were developed adopting the descending technique for 18 h usingn-butanol: acetic acid: water (4:1:5, v/ v/v, upper layer). The chromatogram was visualized by spraying with aniline phthalate reagent and heating in oven for 5 min at 110 °C.
HPLC was also used for analysis of free sugars. Ten milligram of the residue from the leaves, as well as individual authentic reference sugars were separately homogenized with acetonitrile: water (76:24, v/v). The extract was filtered through a Whatman filter paper No. 1 and microfilter (0.45 μm) partitioned three times with ethyl acetate and stored in a vial. HPLC analysis was used to determine sugars in the extracts. The analysis was performed on a model HP1050 HPLC equipped with UV detector. Separation and determination were performed on APS coloumn (4.6 mm×200 mm). The mobile phase was the same as that used in the extraction. UV detector was 192 nm/2 mL flow rate.
2.1.7.2. Investigation of polysaccharide
The isolation and identification of polysaccharides were carried out according to Karawyaet al[16].
Acid hydrolysis was used for the investigatio of polysaccharide[17]. The powder of the isolated polysaccharide ofB. capitataleaves (100 mg) was heated in 2 mL of 0.5 molar sulphuric acid in a sealed tube for 20 h on a boiling water bath. A flocculent precipitate was noticed at the end of hydrolysis. This was filtered off and the filtrate was freed of SO4-by precipitation with barium carbonate.
Paper chromatographic investigation was also carried out. The hydrolysate was concentrated under vacuum at a temperature not exceeding 40 °C to a syrupy consistency. It was diluted with 10% isopropanol in water to about 10 mL. The prepared hydrolysate of the polysaccharide was chromatographed over PC using solvent systemn-butanol: acetic acid: water (4:1:5, v/v/v, upper layer). The chromatograms were air-dried and visualized by spraying with aniline phathalate and heating in an oven for 5 min at 110 °C.
HPLC analysis of the hydrolysate of polysaccharides was carried out according to Gertez[18].
2.1.8. Extraction and isolation of flavonoids
Twenty five grams of the dry bioactive methanol extract ofB. capitataleaves were diluted with distilled water and successively extracted with chloroform, ethyl acetate andn-butanol. Then-butanol soluble fraction (10 g) was chromatographed on 250 g of silica gel glass column (130 cm× 3.5 cm) and eluted with chloroform and methanol in a gradient elution technique to afford many fractions and each fraction was 50 mL, the fractions were screened by PC using sheets of Whatman filter paper No. 1 and two solvent systemsn-butanol: acetic acid: water (4:1:5, v/v/v, upper layer) and acetic acid: water (15:85, v/v). Similar fractions were collected to afford three fractions. Fractions (10-25) were eluted with chlorform: methanol (9:1, v/v) F1 (0.4 g), fractions (35-45) were eluted with chlorform: methanol (8:2, v/v) F2 (1.7 g) and fractions (52-75) were eluted with chlorform: methanol (6:4, v/v) F3 (2.2 g). Each fraction was subjected to a column chromatography to afford the isolated compounds (1, 2, 3, 4, 5 and 6). The isolated compounds were finally purified by passing over Sephadex LH20 in methanol. Glycosides were hydrolyzed to their aglycones and sugars[19,20].
2.2. Biological study
2.2.1. In-vitro determination of the antioxidant activity
The antioxidant activity of the different successive extracts (petroleum ether, ether, chloroform, methanol and 50% aqueous methanol extracts) ofB. capitataleaves was assessed using DL-α-tocopherol (Sigma Chemical Co., USA, St. Louis) as standardaccording to the β-carotene bleaching method[21]. One millilitre of β-carotene (Sigma) solution (0.2 mg/mL in chloroform) was added to round bottom flask (100 mL) containing 0.02 mL of linoleic acid and 0.2 mL Tween 20. The mixture was then dosed with 0.2 mL of 80% methanol (as control), or 50 mg/L of DL-α-tocopherol (as standard) or the corresponding plant extract. After evaporation to dryness under vacuum at room temperature, 50 mL of oxygenated distilled water (distilled water in which oxygen was passed for 15 min) was added and the mixtures were shaken to form liposome solutions. The mixtures were then subjected to thermal autoxidation at 50 °C for 2 h. The absorbance of the solutions were measured at 470 nm immediately after their preparation (t=0 min) and at the end of the experiment (t=120 min) using UVPC spectrophotometer. All samples were assayed in triplicates. Antioxidant activity (AA) was calculated as percent inhibition relative to control using the equation of Al- Saikhanet al[22].
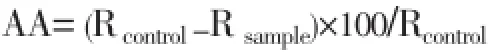
Where, Rcontroland Rsamplewere the bleaching rates of βcarotene in reactant mixture of the control and samples, respectively.
2.2.2. Assessment of antiinflammatory activity
White female albino rats of 150 g average body weight were used in the anti-inflammatory test. Rats were housed individually in stainless steel cages at room temperature. Carrageenan, type IV (Sigma, USA) was used for induction of acute inflammation in rats. Non polar leaf extract was prepared from mixture of equal volumes of petroleum ether and ether extracts and were emulsified in water using gum acacia. Polar extract was prepared from mixture of equal volumes of methanol and 50% aqueous methanol extracts. The experiment was done using one dose level of either polar or non polar leaf extracts. Rats were fasted for 16 h before starting the experiment and divided into three groups, each comprised of six rats. Groups 1 and 2 were used as test groups where polar and non polar extracts of the leaves were given orally to rats of each group separately (500 mg/kg body weight), the third group served as control where no extracts were given. After one hour of the oral administration, rats of all groups were injected into the subplanter surface of the right hind paw with 0.1 mL carrageenan (1% w/v in 0.9% NaCl)[19]. Paw thickness was measured using vernier caliper immediately before the injection of carrageenan and after 30 min, 1, 1.5, 2, 3, and 4 h of carrageenan injection. The mean inflammation thickness of the hind paw of rats given the different plant extracts were calculated and compared with that of the control inflamed rats. Statistical analysis was carried out using student’st-test. Animal procedure was performed in accordance with the Ethics Committee of the National Research Centre, Cairo, Egypt, and followed the recommendations of the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85-23, revised 1985).
3. Results
The results of GC/MS analysis of the volatile constituents ofB. capitataleaves revealed the identification of fourty seven compounds from sixty five representing 72.3% of the total volatile constituents of the leaves. The major compound was decanol (28.69%). The identified oxygenated compounds constituted 64.58% while the identified non oxygenated compounds were 35.41%. The oxygenated compounds were represented by aldehydes as decanal (28.69%), hexanal (0.64%) and 4-decanal (0.59%). Ketones as cyclohexanone 3-methyl was 4.47%. The identified alcohols were phyllocladanol (4.47%), decanol (1.57%), undecanol (1.03%), dodecanol (0.52%) and octadecanol (0.33%). Monoterpenes as isomethone 2-ethyl was 0.33%. Identified sesquiterpenes were longipinanol (0.55%), cidrol (0.32%) and liguloxide (0.38%) while identified monoterpene esters were αterpinyl acetate (0.35%), gerenyl tigalate (0.98%), allyl decanoate (0.35%), and linalool butyrate (0.11%).
The yield of lipoidal matter extracted from the leaf was 3.3%. The lipoidal matter was freely soluble in ether, chloroform and acetone, but insoluble in ethanol (96%). The percentage of the hydrocarbons identified in USM ofB. capitataleaves was 48.39% where the main hydrocarbon was octacosane (13.2%). The percentage of sterols inB. capitataleaves were 25.05% (from which campesterol and β-sitosterol were identified as 1.23% and 11.32%, respectively), while the only identified triterpenoidal compound was β-amyrin representing a percentage of 2.4%.
GLC analysis of the total fatty acid fractions ofB. capitataleaves showed the presence of myristic pentadecyclic, stearic, arachidic, erucic, lignoceric, nervonic, caprilic, palmitic, heptadecanoic and linoleic acids. Nervonic and erucic acids, the monounsaturated omega-9 fatty acid, were the major fatty acids and had been identified in the leaves ofB. capitatain the percentage of 5.4% for each of them. The percentage of saturated fatty acids in the leaves was 6.67% while that of the unsaturated fatty acids was 21.09 %, lignoceric acid (3.45%) was the major identified saturated fatty acids.
Alpha-tocopherol an oil soluble vitamin was identified in the USM ofB. capitataleaves in a concentration of 667 mg/100 g plant.
The percentage of the free sugars isolated from the leaves ofB. capitatawas found to be 7.5%. Qualitative PC analysis of the free sugars ofB. capitataleaves was confirmed by quantitative HPLC investigation which revealed that the percentage of the identified sugars was 44.23%. The percentage of rhamnose, galactose, galactournic acid, xylose, arabinose and glucuronic acid in the leaves were 39.93%, 1.10%, 0.72%, 0.71%, 0.05% and 1.40%, respectively.
The percentage of the polysaccharide isolated from the leaves ofB. capitatawas found to be 0.25%. The isolated polysaccharide was odorless, soluble in water, insoluble in ethanol, ether and chloroform, gave positive Molish’s test and did not reduce Fehling’s and Barafoed’s solutions. They gave negative test for protein and left no ash on ignition. Thetest with alcoholic KOH solution indicated its mucilaginous nature. Chromatographic investigation of the hydrolysate by different tools of chromatographic separation was carried out. Qualitative PC analysis of the polysaccharide hydrolysates ofB. capitataleaves was confirmed by quantitative HPLC analysis which revealed the presence of rhamnose in the percentage of 20.22%, while galactose, xylose and fructose were present in a percentage of 2.22%, 0.16% and 2.06%, respectively. Glucuronic acid was present in the leaves in a percentage of 1.27%.
Six flavonoidal compouds were isolated from the aqueous methanol extract ofB. capitataleaves using different chromatographic techniques. All isolated compounds have been finally purified though Sephadex LH-20. Sugar linkages have been elucidated through acid hydrolysis. Structure elucidation was confirmed through UV with methanol and complex shift reagents,1H NMR,13C NMR, EI-MS, HMBC and HMQC and comparison with authentic samples. The compounds were identified as apigenin, tricin 7-O-rutinoside, isorhamnetin 3-O-rutinoside, Kaemferol 3-O-β-rutinoside, luteolin 7-O-β-D-glucoside and quercetin 3-O-rhamnoglucopyranoside (rutin).
The antioxidant activity of the successive extracts ofB. capitataleaves is complied in Table 1. The results showed a potent antioxidant activity of both methanol and petroleum ether extracts of the leaves (78.85% and 71.33%, respectively). The 50% aqueous methanol and the ether leaves extracts also showed a moderate antioxidant activity. The anti-inflammatory activity of polar and non polar extracts ofB. capitataleaves (Table 2) was studied in acute inflammation model in rats using the hind paw edema method. The results showed that both non polar and polar extracts possess a significant anti-inflammatory activity all over the different monitored times of the test; the maximum effect was 51% and 41%, respectively after four hours from carrageenan injection.

Table 1 Percentage of the antioxidant activity of different tested extracts of B. capitata leaves.
4. Discussion
Egypt is characterized by the presence of a great number of palms abundantly distributed all over the country. Palms possess many biological activities and economical importance. In this study the antioxidant and anti-inflammatory activity of the leaves ofB. capitataBecc palm, as well as the study of the phytoactive constituents in the bioactive extracts and fractions were carried out.
The results of the antioxidant activity of the different successive extracts of the leaves revealed that the methanol extract possess the highest antioxidant activity (78.55%), followed by the petroleum ether extract (71.33%). There are numerous types of antioxidants in plants; the most important ones are tocopherols, ascorbate, thiols, β-carotenes and phenolic compounds such as flavonoids, chromones and lignans. They play an important antioxidant role in the body[23]. In the present study, the antioxidant activity of the polar extract of the leaves ofB. capitatamay be attributed to the presence of the flavonoids, tricin 7-O rutinoside, isorhamnetin 3-O rutinoside, kaempferol 3-O rutinoside, rutin, luteolin 7-O-glucoside and apigenin, which were isolated and purified by chromatographic tools and identified by different spectral analysis. Reviewing the literature some authors reported a linear relationship between the antioxidant capacities and the total phenolic content of medicinal herbs[24]. The major antioxidant constituents of phenolics were found to be flavonoid in nature[25-27].
As a matter of fact the antioxidant extracts may possess a protective effect towards diseases in which free radicals are involved such as cardiovascular diseases, diabetes, chronic inflammatory diseases and cancer[28].
It is also important to note that the petroleum ether extract possess a remarkable antioxidant activity (71.33%) which may be due to the presence of sterols such as campesterol and β sitosterol and the oil soluble vitamin, α-tocopherol, which were all identified by different chromatographic analysis. It has been reported previously that sterols may have an antioxidant activity through acting as hydrogen donor[29].
Tocopherols are parts of minor components of main interest. It is present in the unsaponifiable fractions of many plant samples. Their importance in biological studies makesdetermination of tocopherols and related compounds of major interest. They are found in fat products of vegetable origin. Tocopherols are effective as antioxidant due to the free phenolic hydroxyl groups. The presence of α-tocopherol in the nonpolar extracts in the current study rendered them antioxidant activity. The plant kingdom offers a large range of phenolic compounds, among which α-tocopherol is best known as one of the most efficient naturally occurring liposoluble antioxidants. Alpha-tocopherol is commonly present in plant leaves since α-tocopherol biosynthesis takes place inside the chloroplast membranes of the plant[30]. It is an accepted fact that α-tocopherol shows excellent biological activity as a free radical scavenger and for this reason there is an agreement that it could serve as a therapeutic drug against free radicals involved diseases[28].
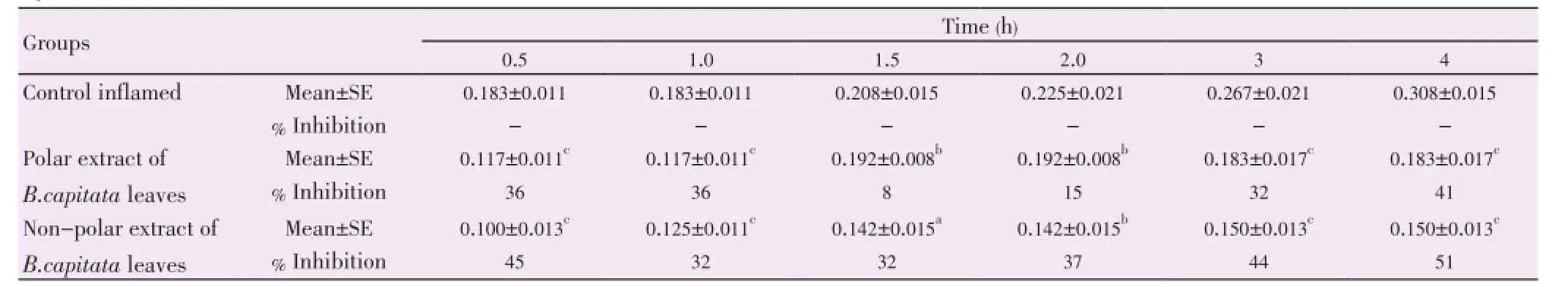
Table 2 Mean thickness (cm) of the hind paw of control and tested rats given different extracts of B. capitata leaves at different time intervals from carrageenan injection.
The results of the acute inflammation test showed that both polar and non polar extracts of the leaves ofB. capitatapossess a significant anti-inflammatory activity all over the tested times. The anti-inflammatory activity of the polar extract may be attributed to the presence of many flavonoids in the bioactive extract which were detected from the phytochemical part. It has been reported previously that most of flavonoids which exhibit a remarkable antioxidant also possess an anti-inflammatory activity[31]. The non polar bioactive extract of the leaves showed also anti-inflammatory activity. Phytochemical examination of this extract revealed the presence of β-sitosterol and β-amyrin which have been previously reported to possess a remarkable anti-inflammatory activity since they block the inflammatory enzyme modifying the prostaglandin path way. It was also shown that β-sitosterol inhibited either myeloperoxidase and adenosine deaminase activity or IL-1β and tumor necrosis factor alpha level thereby reduced inflammation. Triterpene such as β-amyrin was reported to inhibit inflammation via activation of cannabinoid receptors and by inhibiting the production of cytokines and expression of nuclear factor κB and cyclooxygenase 2[32-34]. Alpha-tocopherol was shown to possess lower anti-inflammatory activity than tocotrienol rich fraction however it still possess an anti-inflammatory effect[35]. Guneset al.[36] reported anti-inflammatory activity of α-tocopherol. So, both sterol and α-tocopherol may act synergistically as antiinflammatory within the non polar extract ofB. capitataleaves.
B. capitataleaf extracts were shown to possess variable antioxidant effect, the most promising was methanol extract. Both polar and non polar extracts were proved to have antiinflammatory activity, the non polar extract was superior in this respect. The bioactivity of the extracts was ascribed to the presence of flavonoids, sterols and α-tocopherol.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
The authors thank the National Research Centre, Cairo, Egypt for financing this work. The present research is a part of a project entitled “Phytochemical and Biological Studies of Some Biologically Active Palms Growing in Egypt”granted and totally funded by National Research Centre, Cairo, Egypt (Fifth Plan of National Research Centre, Grant No. 1/8/5).
Comments
Background
Egypt is characterized by great number of palms abundantly distributed all over the country which possess many important biological uses. The discovery of new natural bioactive compounds nowadays is needed to minimize the severe side effects of synthetic drugs, soB. capitataleaves might be a good source of such compounds.
Research frontiers
In this work, the anti-inflammatory and antioxidant activities of the leaves ofB. capitataBecc. palm as well as the study of the phytoactive constituents of the bioactive extracts were carried out.
Related reports
Some Palmae have many economic uses and biological activities such as saw palmetto which is a species from the genusSabalof palmae which is used to relief local irritation of mucous membranes of respiratory, digestive and reproductive tract.
Innovations and breakthroughs
Very few works were reported concerningB. capitataBecc. The present work studied the biological activities and phytochemical constituents ofB. capitataBecc. cultivated in Egypt for the first time. The study revealed the promising anti-inflammatory and antioxidant activities of the different extracts of the leaves with variable degrees.
Applications
The bioactive fractions and constituents that could be isolated from the extracts ofB. capitataleaves could be used as complementary agents in diseases related to inflammation and elevated reactive oxygen species after studying their safety.
Peer review
This work has an added value since the authors evaluated for the first time the biological activity of the successive extracts ofB. capitataleaves palm cultivated in Egypt. Alsothe research dealt with isolation and identification of the bioactive constituents of the extracts.
[1] Thomas R, De Franceschi D. Palm stem anatomy and computeraided identification: the Coryphoideae (Arecaceae). Am J Bot 2013; 100(2): 289-313.
[2] Bjorholm S, Svenning JC, Baker WJ, Skov F, Balslev H. Historical legacies in the geographical diversity patterns of New World palm (Arecaceae) subfamilies. Bot J Linnean Soc 2006; 151(1): 113-125.
[3] García S, Heinzen H, Hubbuch C, Martínez R, De Vries X, Moyna P. Triterpene methyl ethers from Palmae epicuticular waxes. Phytochemistry 1995; 39: 1381-1382.
[4] Williams CA, Harborne JB, Clifford HT. Flavonoid patterns in the monocotyledons. Flavonols and flavones in some families associated with the Poaceae. Phytochemistry 1971; 10: 1059-1063.
[5] Harborne JB, Williams CA, Greenham J, Moyna P. Distribution of charged flavones and caffeylshikimic acid in Palmae. Phytochemistry 1974; 13: 1557-1559.
[6] Markham KR. Techniques of flavonoids identification. London: Academic Press; 1982, p. 36-51.
[7] Egon S. Thin-layer chromatography: a laboratory handbook. 2nd ed. Berlin: Springer-Verlag; 1969.
[8] Mabry TJ, Markham KR, Thomas MB. The systematic identification of flavonoids. New York: Springer Verlag; 1970.
[9] Macleod AJ, Cave SJ. Volatile flavour components of eggs. J Sci Food Agric 1975; 26: 351-360.
[10] Adams RP. Identification of essential oils by ion trap mass spectroscopy. New York: Academic Press; 1989.
[11] Great Britain Scottish Home and Health Department. British pharmacopoeia 1993 : amendment no. 1. London: HMSO; 1993.
[12] Moreda W, Camino MCP, Cert A. Analysis of neutral lipids: unsaponifiable. In: Nollet LML, editor. Handbook of food analysis: physical characterization and nutrient analysis. 2nd ed. New York, USA: CRC Press; 2004, p. 313-348.
[13] Epler KS, Sander LC, Ziegler RG, Wise SA, Craft NE. Evaluation of reversed-phase liquid chromatographic columns for recovery and selectivity of selected carotenoids. J Chromatogr 1992; 595(1-2): 89-101.
[14] Luque-García JL, Luque de Castro MD. Extraction of fat-soluble vitamins. J Chromatogr 2001; 935 (1-2): 3-11.
[15] Harborne JB. Phytochemical methods a guide to modern techniques of plant analysis. 3rd ed. London, UK: Chapman and Hall; 1998, p. 235.
[16] Karawya MS, Wassel GM, Baghdadi HH, Ammar NM. Mucilages and pectins of Opuntia, Tamarindus and Cydonia. Planta Medica 1980; 40: 68-75.
[17] Chrums SC, Stephan AM, van der Bijl P. Methylation and hydrolysis studies of a gum from Opuntia megacantha Lehmaniana. J South Afr Chem Inst 1973; 26: 45-52.
[18] Stephen AM, Phillips GO, Williams PA. Food polysaccharides and their applications. 2nd ed. New York, USA: CRC press; 2006
[19] Lanhers MC, Fleurentin J, Mortier F, Vinche A, Younos C. Antiinflammatory and analgesic effects of an aqueous extract of Harpagophytum procumbens. Planta Med 1992; 58: 117-123.
[20] Harborne JB, Mabry TJ, Mabry H. The flavonoids. London: Chapman & Hall; 1975.
[21] Phang CW, Malek SNA, Ibrahim H, Wahab NA. Antioxidant properties of crude and fractionated extracts of Alpinia mutica rhizomes and their total phenolic content. Afr J Pharm Pharmacol 2011; 5: 842-852.
[22] Al-Saikhan MS, Howard LR, Miller JC, Jr. Antioxidant activity and total phenolics in different genotypes of potato (Solanum tuberosum L.). J Food Sci 1995; 60: 341-343.
[23] Smirnoff N. Antioxidants and reactive oxygen species in plants. Oxford, UK: Wiley-Blackwell; 2005.
[24] Deng GF, Lin X, Xu XR, Gao LL, Xie JF, Li HB. Antioxidant capacities and total phenolic contents of 56 vegetables. J Funct Foods 2013; 5(1): 260-266.
[25] Sowndhararajan K, Kang SC. Free radical scavenging activity from different extracts of leaves of Bauhinia vahlii Wight & Arn. Saudi J Biol Sci 2013; 20(4): 319-325.
[26] Olennikov DN, Chirikova NK, Okhlopkova ZM, Zulfugarov IS. Chemical composition and antioxidant activity of Tánara ótó (Dracocephalum palmatum Stephan), a medicinal plant used by the North-Yakutian nomads. Molecules 2013; 18(11): 14105-14121.
[27] Kumar S, Gupta A, Pandey AK. Calotropis procera root extract has the capability to combat free radical mediated damage. ISRN Pharmacol 2013; doi: 10.1155/2013/691372.
[28] García MS. Emerging role of natural antioxidants in chronic disease prevention with an emphasis on vitamin E and selenium. In: Morales-González JA, editor. Oxidative stress and chronic degenerative diseases-a role for antioxidants. Rijeka, Croatia; InTech; 2013.
[29] Sengupta A, Ghosh M. Protective role of phytosterol esters in combating oxidative hepatocellular injury in hypercholesterolemic rats. Pak J Biol Sci 2013; 16(2): 59-66.
[30] Voll LM, Abbasi AR. Are there specific in vivo roles for alphaand gamma-tocopherol in plants? Plant Signal Behav 2007; 2(6): 486-488.
[31] Czaplińska M, Czepas J, Gwo?dziński K. [Structure, antioxidative and anticancer properties of flavonoids]. Postepy Biochem 2012; 58(3): 235-244. Polish.
[32] Yang C, Chen ZY, Wong SL, Liu J, Liang YT, Lau CW, et al. β-Sitosterol oxidation products attenuate vasorelaxation by increasing reactive oxygen species and cyclooxygenase-2. Cardiovasc Res 2013; 97(3): 520-532.
[33] Liz R, Zanatta L, dos Reis GO, Horst H, Pizzolatti MG, Silva FR, et al. Acute effect of β-sitosterol on calcium uptake mediates anti-inflammatory effect in murine activated neutrophils. J Pharm Pharmacol 2013; 65(1):115-122.
[34] da Silva KA, Paszcuk AF, Passos GF, Silva ES, Bento AF, Meotti FC, et al. Activation of cannabinoid receptors by the pentacyclic triterpene α, β-amyrin inhibits inflammatory and neuropathic persistent pain in mice. Pain 2011; 152(8): 1872-1887.
[35] Ng LT, Ko HJ. Comparative effects of tocotrienol-rich fraction, α-tocopherol and α-tocopheryl acetate on inflammatory mediators and nuclear factor kappa B expression in mouse peritoneal macrophages. Food Chem 2012; 134(2): 920-925.
[36] Gunes T, Bozok S, Kestelli M, Yurekli I, Ilhan G, Ozpak B, et al. α-Tocopherol and ascorbic acid in early postoperative period of cardiopulmonary bypass. J Cardiovasc Med (Hagerstown) 2012; 13(11): 691-699.
10.12980/APJTB.4.2014C1192
*Corresponding author: Sahar Youssef Al-Okbi, Professor of Biochemistry, Ph.D., Food Sciences and Nutrition Department, National Research Centre, Dokki, El-Buhouth Street, 12622, Cairo, Egypt.
Tel: 00201003785152
Fax: +(202)33370931
E-mail: S_Y_alokbi@hotmail.com
Foundation Project: Supported by National Research Centre, Cairo, Egypt (Fifth plan of National Research Centre, Grant No: 1/8/5).
Article history:
Received 12 Mar 2014
Received in revised form 23 Mar, 2nd revised form 4 Apr, 3rd revised form 17 Apr 2014
Accepted 12 May 2014
Available online 28 Jun 2014
Methods:Different successive extracts of B. capitata Becc. leaves were prepared with selective organic solvents and screened for their anti-inflammatory activities in tested animals and invitro antioxidant effect. An extensive phytochemical investigation of the bioactive extracts through paper chromatography, thin layer chromatography, column chromatography, gasliquid chromatography (GLC), high pressure liquid chromatography and spectral analysis. GCMass, ultraviolet, hydrogen and carbon nuclear magnetic resonance, electron ionization-mass spectrometry, heteronuclear multiple bond correlation and heteronuclear multiple quantum correlation were carried out.
Results:Results showed that different extracts possess promising antioxidant effect and significant anti-inflammatory activity with variable degrees. The results of the phytochemical investigation of the bioactive extracts revealed the presence of volatile substances, lipoidal matter, α-tocopherol, free sugars, polysaccharides and flavonoidal compounds.
Conclusions:B. capitata leaf extracts were shown to possess variable antioxidant effect, the most promising was methanol extract. Both polar and non polar extracts were proved to have anti-inflammatory activity, the non polar extract was superior in this respect. The bioactivity of the extracts was ascribed to the presence of flavonoids, sterols and α-tocopherol.
 Asian Pacific Journal of Tropical Biomedicine2014年6期
Asian Pacific Journal of Tropical Biomedicine2014年6期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- A retrospective evaluation of the quality of malaria case management at twelve health facilities in four districts in Zambia
- Study of the efficacy of a Wheaton coated bottle with permethrin and deltamethrin in laboratory conditions and a WHO impregnated paper with bendiocarb in field conditions
- Pharmacognostical study and establishment of quality parameters of aerial parts of Costus speciosus-a well known tropical folklore medicine
- Hepatocurative potential of Vitex doniana root bark, stem bark and leaves extracts against CCl4-induced liver damage in rats
- In vitro α-amylase inhibitory activity and in vivo hypoglycemic effect of methanol extract of Citrus macroptera Montr. fruit
- Antimicrobial activity of some essential oils against oral multidrugresistant Enterococcus faecalis in both planktonic and biofilm state
