Removal of Cu2+from Aqueous Solutions Using Na-A Zeolite from Oil Shale Ash*
BAO Weiwei (包維維), LIU Lu (劉璐), ZOU Haifeng (鄒海峰),**, GAN Shuc a i (甘樹才),**, XU Xuechun (徐學純), JI Guijuan (季桂娟), GAO Guimei (高桂梅)and Zheng Keyan (鄭克巖)
1College of Chemistry, Jilin University, Changchun 130012, China
2College of Earth Science, Jilin University, Changchun 130026, C hina
Removal of Cu2+from Aqueous Solutions Using Na-A Zeolite from Oil Shale Ash*
BAO Weiwei (包維維)1, LIU Lu (劉璐)1, ZOU Haifeng (鄒海峰)1,**, GAN Shuc a i (甘樹才)1,**, XU Xuechun (徐學純)2, JI Guijuan (季桂娟)1, GAO Guimei (高桂梅)1and Zheng Keyan (鄭克巖)1
1College of Chemistry, Jilin University, Changchun 130012, China
2College of Earth Science, Jilin University, Changchun 130026, C hina
Na-A zeolite was synthesized using oil shale ash (OSA), which is a solid by-product of oil shale processing. The samples were characterized by various techniques, such as scanning electron microscopy, X-ray diffraction and Brunauer Emmet Teller method. The batch isothermal equilibrium adsorption experiments were performed to evaluate the ability of Na-A zeolite for removal of Cu (II) from aqueous solutions. The effects of operating parameters, such as concentration of copper solutions, adsorbent dosages, pH value of solutions and temperature, on the adsorption efficiency were investigated. The equilibrium adsorption data were fitted with Langmuir and Freundlich models. The maximum adsorption capacity of Na-A zeolite obtained from the Langmuir adsorption isotherm is 156.7 mg·g?1of Cu (II). The increase of pH level in the adsorption process suggests that the uptake of heavy metals on the zeolite follows an ion exchange mechanism. The batch kinetic data fit the pseudo-second order equation well. The thermodynamic parameters, such as changes in Gibbs free energy (ΔG), enthalpy (ΔH) and entropy (ΔS), are used to predict the nature of the adsorption process. The negative ΔG values at different temperatures confirm that the adsorption processes are spontaneous.
oil shale ash, zeolite, copper removal, adsorption isotherm
1INTRODUCTION
A serious problem of environmental pollution in recent years is due to heavy metals in aqueous waste streams from many industries such as electroplating, metal finishing and metallurgical industries, coal combustion, chemical manufacturing, tanneries and battery manufacturing. Cu (II) is a widely used material owing to its excellent physical and mechanical properties, such as electrical and thermal conductivities, and good corrosion resistance. However, the presence of excessive copper in water may cause toxic and harmful effects to the living organisms, such as cancer of lungs, stomach upset, headache, dizziness, and respiratory distress. Therefore, it is of great significance to remove excess Cu (II) from wastewater.
Chemical precipitation, ion exchange, solvent extraction, reverse osmosis membrane filtration, electrodialysis and adsorption are the commonly used processes for removal of heavy metals. but these methods are sometimes restricted because of technical and/or economical constraints. There is a growing interest in using industrial or agricultural solid wastes as adsorbents for removal of metals from aqueous solutions [1-4]. The advantage is to save disposal costs while alleviating potential environmental problems.
Oil shale ash (OSA) is usually formed in two processes: retort oil shale to produce shale oil and fuel gas and burn oil shale to generate electricity. OSA is consists of a wide variety of acidic, basic and amphoteric oxides, and may have high porosity since it is produced at high temperature. Being readily available and inexpensive, OSA is considered as an economic alternative to conventional adsorbents. It has been reported that OSA could be effective for removal of heavy metals, dyes, and pesticides from wastewater [5, 6]. However, OSA shows lower adsorption capacity unless it is treated or activated chemically [7-9]. Shawabkeh et al. [7, 8] synthesized zeolites using OSA with alkaline hydrothermal treatment and used them to remove cadmium and lead from water through ion-exchange processes. Their results showed that the zeolites had good removal efficiency for cadmium, lead and copper. Fernandes-Machado and Miotto [10] conversed OSA into Na-A and Na-X zeolites by two different methods, but the adsorption capacity was not evaluated.
In this study, Na-A zeolite is synthesized using OSA by fusion method and used as adsorbent for the removal of Cu (II) from aqueous solutions. The effects of operating parameters, such as concentration of copper solutions, adsorbent dosages, pH value of solutions and temperature, on the adsorption efficiency are investigated. The equilibrium adsorption data are fitted with Langmuir and Freundlich models. The thermodynamic parameters, such as changes in Gibbs free energy (ΔG), enthalpy (ΔH) and entropy (ΔS), are used to predict the nature of adsorption process.
2EXPERIMENTAL
2.1Adsorbent materials and reagents
All chemicals were analytical grade reagents supplied by Beijing Chemical Reagent Research Institute.Oil shale was collected from Heilongjiang province of China. It was crushed to particle sizes less than 2 mm and burned in a Muffle furnace at 700 °C to remove all incorporated hydrocarbons. The chemical compositions of oil shale ash are SiO2(62.70%), Al2O3(25.57%), Fe2O3(5.38%), CaO (3.61%) and MgO (0.33%).
2.2Preparation of Na-A zeolite
Na-A zeolite was synthesized using OSA by fusion method. Since the Si/Al molar ratio in the Na-A zeolite is 1, aluminium oxide should be added in the reaction mixture for desired Si/Al molar ratio. Firstly, OSA well-mixed with sodium hydroxide and aluminium oxide powder in a certain mass ratio was poured into a graphite crucible and heated in muffle furnace at 600 °C for 2 h. The mass ratio of NaOH/OSA was varied from 1.0 to 2.0. The fused mass was cooled and poured into 250 ml beaker filled with distilled water, with the mass ratio of NaOH/H2O of 0.1. Then, the mixture was treated by ultrasonic for 3 h, and aged under static conditions at room temperature and ambient pressure for 12 h. The slurry was crystallized at 100 °C in an oven for 24 h. Finally, the resulting mixture was separated by filtration, and washed with distilled water until the pH value of the filtrate reached 9-10, and then dried at 105 °C in air for 24 h.
2.3Batch study
Batch exchange experiments were conducted by placing 50 mg of the Na-A zeolite in 100 ml glass bottles containing 50 ml solutions with various concentrations (50-200 mg·L?1) of Cu (II) in the pH value range of 1.0-6.0 at different temperatures. The solution pH value was adjusted to the desired value with hydrochloric acid solution. The solution was agitated at the rate of 100 per minute using a mechanical shaker to reach equilibrium. After filtration, the concentration of Cu (II) in the aqueous phase was determined by flame atomic absorption spectrometer (AAS, Varian 220FS, USA).
2.4Column experiments
The fixed-bed columns were made of perspex tubes with 3.0 cm in internal diameter and 30 cm in height. The column was filled to a height of 10 cm with the known mass of adsorbent. The metal ion solution containing 100-150 mg·L?1of Cu (II) was fed to the column at a constant flow rate of 10 ml·min?1. The pH of the solutions was maintained constant at 6.0. For packing the column, the supporting medium, glass wood, was filled hydraulically. The solution leaving the bottom of the column was collected at various time intervals and the samples were analyzed.
2.5Apparatus
X-ray diffraction (XRD) patterns of the samples were examined by D/Max-IIIC (Rigaku, Japan) with CuKαradiation. Scanning electron microscopic (SEM) analysis was taken using a field-emission scanning electron microscope (JEOL JSM-6701F, Japan). N2adsorption/desorption isotherm was measured using a surface analyzer (ASAP 2010, Micromeritics, USA). The surface area and the pore size distributions were measured using the Brunauer Emmet Teller (BET) method and the Barrette Joynere Halenda (BJH) cumulative pore volume method [11, 12].
3RESULTS AND DISCUSSION
3.1Characterization of adsorbent
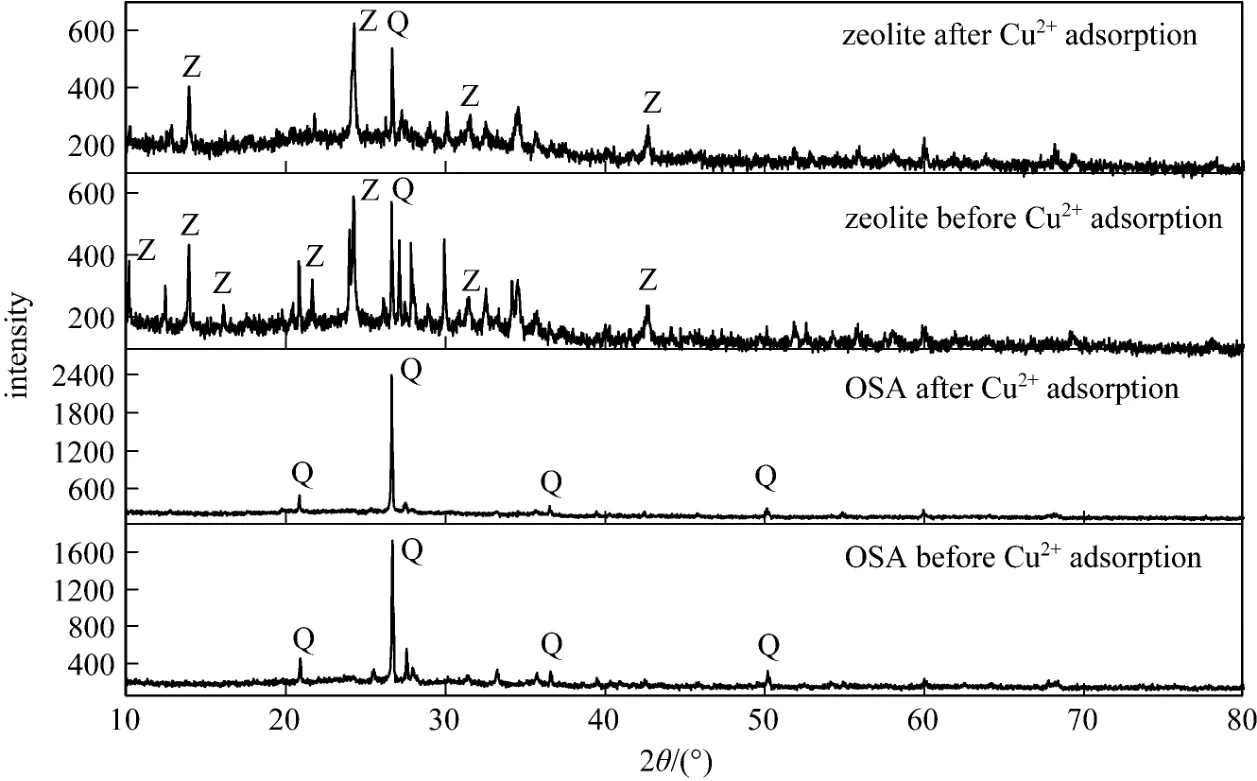
Figure 1XRD patterns of the OSA and products (Z: Na-A zeolite; Q: quartz)
Figure 1 shows the XRD patterns from the OSA and synthesized sample. The major crystalline phasesin the OSA are quartz, clay minerals and so on. After alkaline fusion, the glass phase on the surface of OSA particles transforms from quartz into more reactive species. The XRD pattern of the final product indicates the appearance of diffraction peaks of zeolite crystal, which could be easily indexed as Na-A zeolite according to the references [10, 13]. After the adsorption of copper ions, the intensity of diffraction peaks changes slightly, revealing that the adsorption of metal ions does not alter the structure of adsorbent. The relative crystallinity (rC) is calculated as follows, with the sample of commercial Na-A zeolite as standard.
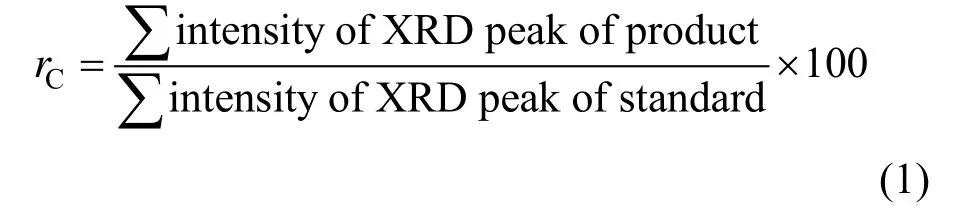
According to XRD observation, Na-A zeolite crystallinity increases with the mass ratio of NaOH/OSA. At the mass ratio of 1.6, the crystallion Na-A zeolite content reaches 63.0%, while at higher ratio, the crystallinity decreases.
Typical morphologies of OSA and synthesized zeolite were determined by scanning electron microscopy (SEM). As presented in Fig. 2, the morphologies of particles display a great difference. A high magnification image [Fig. 2 (a)] shows that the OSA is composed of a number of crystalline flakes with lateral sizes less than 1 μm. After adsorption, the surface morphology presents much asperity and more coarse grains [Fig. 2 (b)]. SEM image also shows that synthesized zeolite is cube with the edge length of 2-3 μm [Fig. 2 (c)]. After adsorption of Cu (II) ions, the surface becomes rough [Fig. 2 (d)].
Particle size is a significant factor to influence the removal efficiency. The particle size of the synthesized zeolite was determined by dynamic light scatter (DLS) measurement. As shown in Fig. 2 (e), the synthesized zeolite has a particle size distribution range from 0.7 μm to 4.1 μm with an average diameter of 2.0 μm.
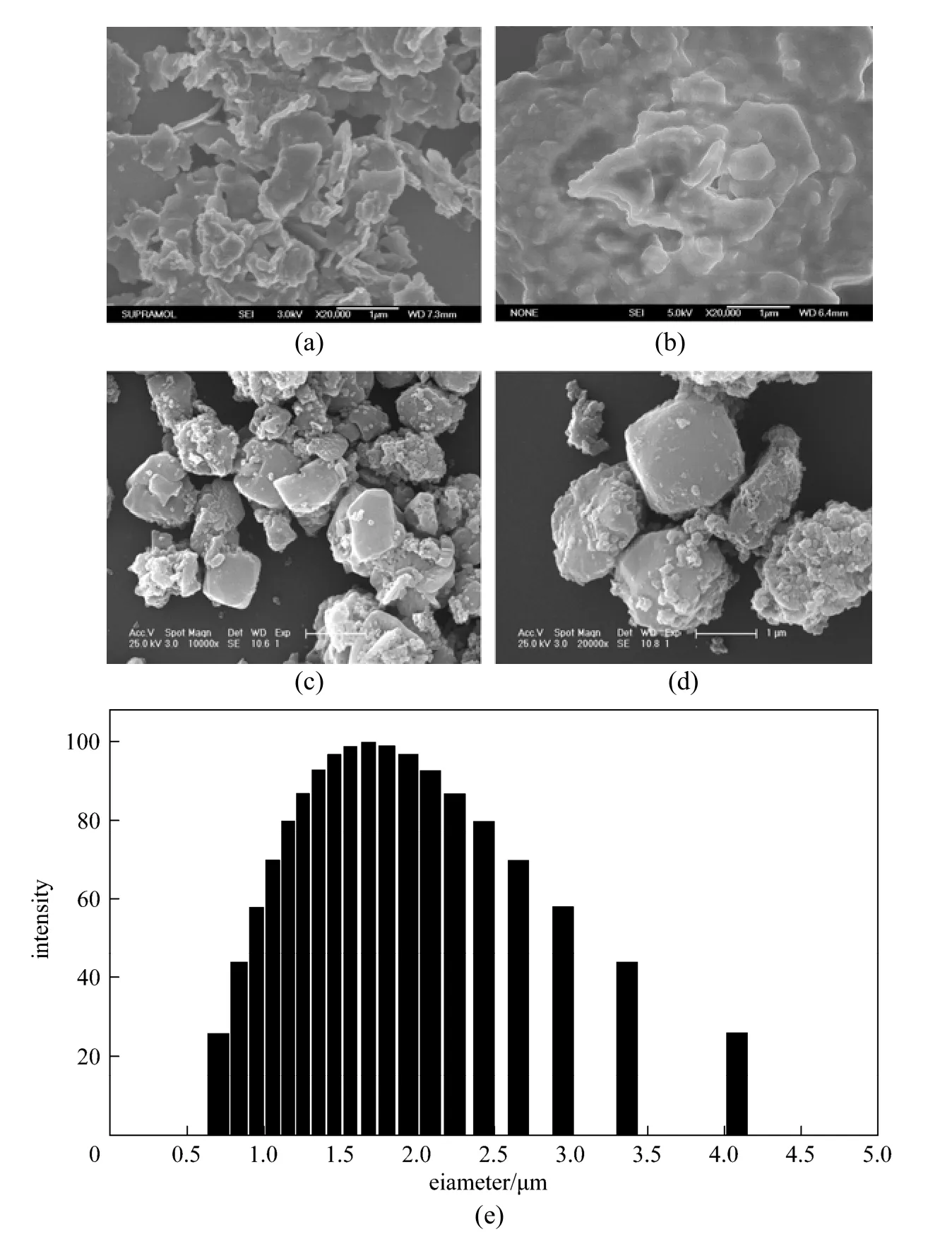
Figure 2SEM image of OSA before and after Cu2+adsorption (a, b) , Na-A zeolite (c, d) and DLS patterns of the Na-A zeolite (e)
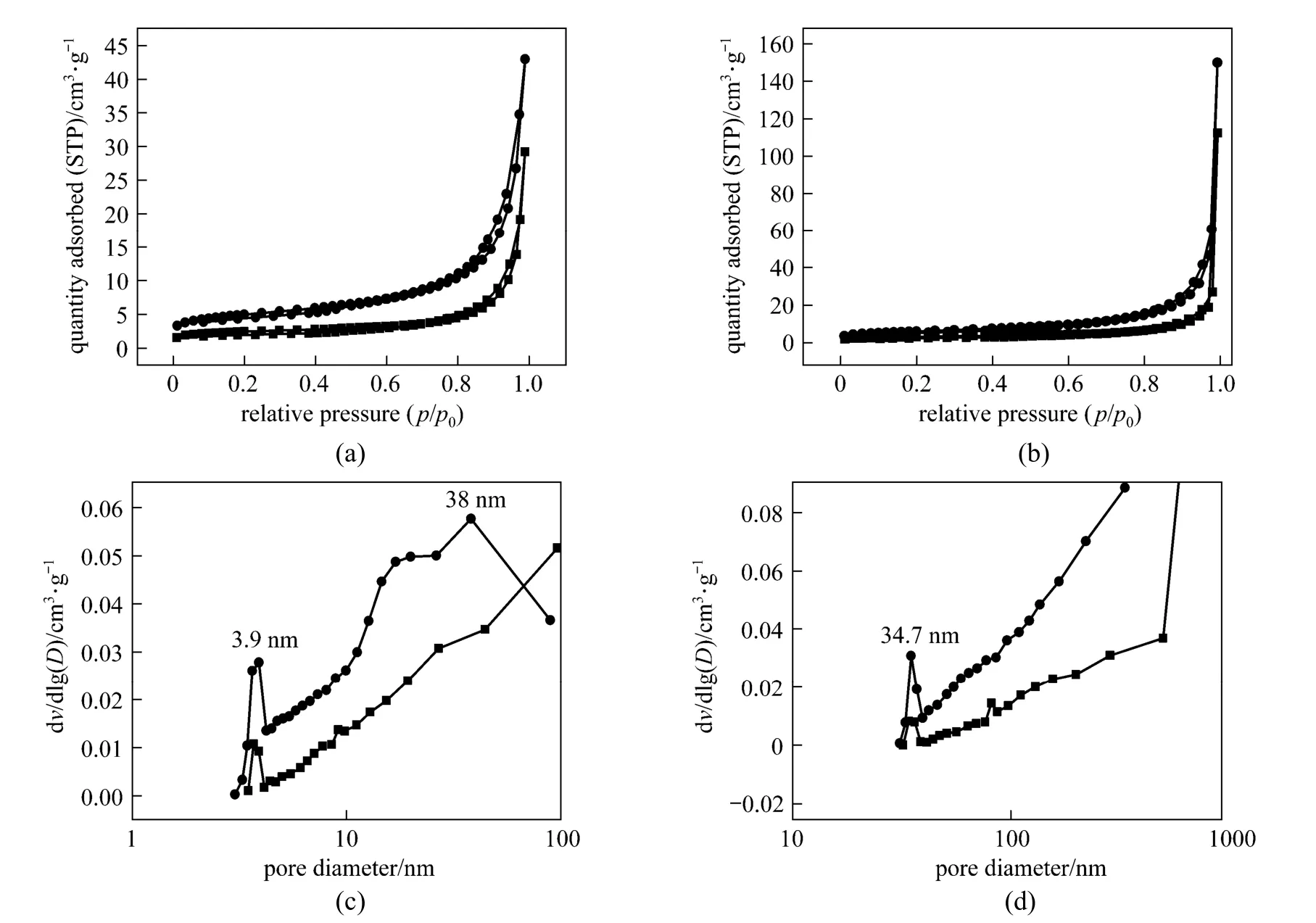
Figure 3The N2adsorption-desorption isotherms and pore size distributions (PSD) of OSA and zeolite before (a, c) and after (b, d) Cu2+adsorption
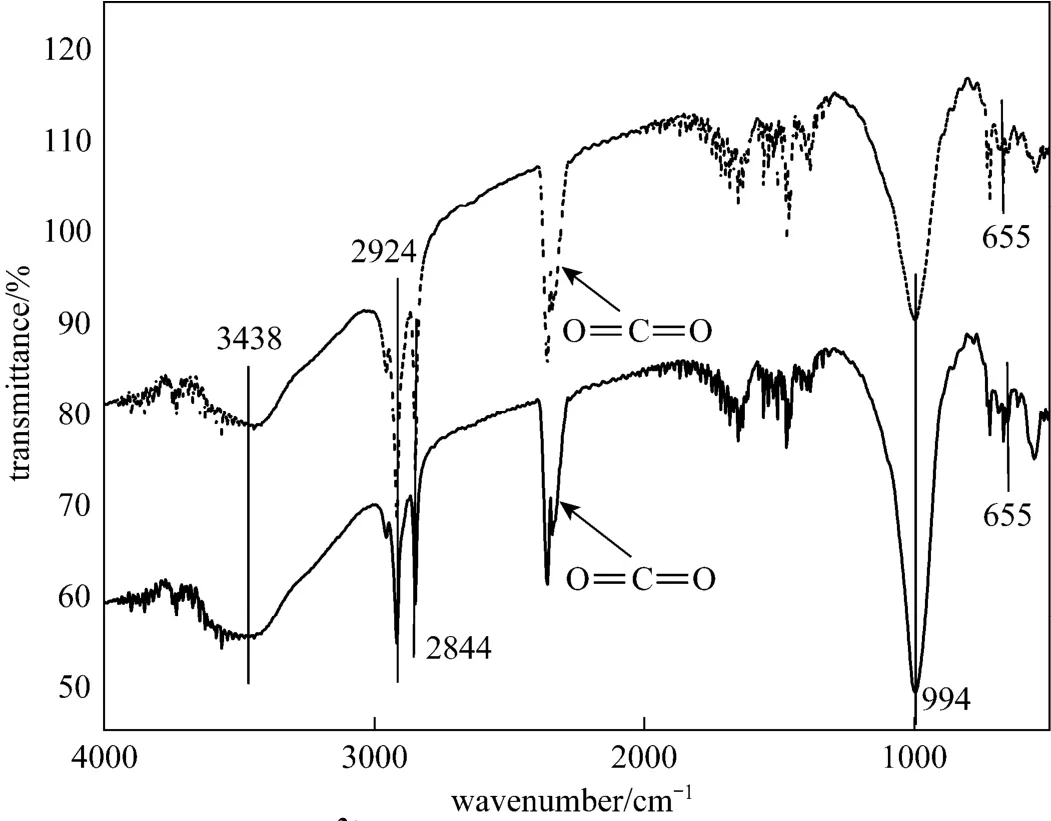
Figure 4The FTIR of zeolite before and after Cu2+adsorption zeolite; Cu-zeolite
The N2adsorption-desorption isotherms and pore size distributions (PSD) of the zeolite and OSA are shown in Fig. 3. The nitrogen adsorption isotherms of all samples exhibit type IV characteristics, indicating that the zeolite and OSA are typical mesoporous materials [12], which is also revealed from the PSD profile [Fig. 3 (c)-(d)]. Further, the BET analysis shows that the surface area of the zeolite (17.6 m2·g?1) is much higher than that of OSA (8.6 m2·g?1), so that the zeolite has potential application in adsorption. After adsorption of Cu (II) ions, the surface area of adsorbent decreases (OSA 5.5 m2·g?1, zeolite 10.9 m2·g?1), since copper ions are adsorbed on the surface or through the pore of adsorbents.

Figure 5Effect of adsorbent dose on removal (a) and adsorption capacities (b) of Cu2+(sorbent concentration 150 mg·L?1, 293 K and pH 6)
Figure 4 shows the FT-IR spectra of the zeolites before and after adsorption of Cu (II) ions. There is no difference between the two curves. The brand peak at 3438 cm?1is attributed to OH stretching vibration. The Si OSi and Si OAl vibrations are in the range 1200 cm?1to 400 cm?1.
3.2Adsorption mechanism
The removal of heavy metal cations on zeolites has been extensively studied. Most researchers considered it as ion exchange between metal cations in the solution and cations in the zeolite framework [14-16]. The removal of heavy metal ions is attributed to different mechanisms of ion-exchange processes as well as adsorption process. During the ion-exchange process, metal ions move through the pores of zeolites and channels of the lattice, and replace exchangeable cations (mainly sodium). The diffusion is faster through the pores and is retarded when the ions move through the smaller channels. In the later case the metal ion uptake may be mainly attributed to ion-exchange reactions in the microporous minerals of the zeolite samples.
3.3Effect of adsorbent dose
To evaluate the effect of adsorbent dose on the removal efficiency of Cu (II), the experiments were carried out with varying dose from 1 g·L?1to 6 g·L?1, and the results are shown in Fig. 5 (a). The synthesized zeolite exhibits much higher removal efficiency than OSA, and the removal efficiency increases with the increase of adsorbent dose. For the zeolite or OSA of 2 g·L?1, the removal percentages of Cu (II) are 97.5% and 52.2%, respectively. However, the amount of adsorption on the unit mass of zeolite and OSA (q) decreases as adsorbent dose increases, as shown in Fig. 5 (b). For solutions with C0=150 mg·L?1, the adsorption amount decreases from 129.5 mg·g?1to 24.9 mg·g?1and from 45.8 mg·g?1to 24.5 mg·g?1, respectively, as the amount of zeolite and OSA increase from 1 g·L?1to 6 g·L?1. The decreases of adsorption amount may be due to the particle aggregation resulted from high adsorbent concentration. Such aggregation may reduce the total surface area of adsorbent and increase the diffusion length [17].
3.4Effect of acidity
The pH value of the solution has a significant impact on the removal of heavy metals, because it determines the surface charge of adsorbent, the degree of ionization and speciation of adsorbate. Fig. 6 illustrates the effect of pH value on the adsorption of Cu (II) ions. The removal capacity is little at pH value below 2.0 and then increases with the pH value, reaching a plateau value at pH 4.0. That is, the removal efficiency increases slightly as pH value increases from 4.0 and 6.0. The explanation is as follows. At low pH value, strong competition exists between protons and Cu (II) ions for adsorption sites, so the removal efficiency is low. As the pH value increases, linked H+is released from the active sites, and the amount of adsorbed Cu(II) ions increases. However, when the initial pH value of the solution is adjusted to a value higher than 6.0, Cu (II) ions are precipitated because of the higher concentration of OH?ions in the medium. For this reason the experiments are not conducted for pH≥6.0 [8, 18].
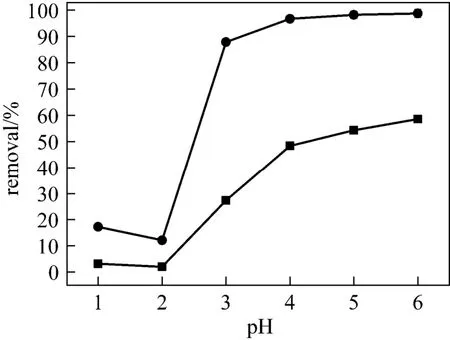
Figure 6Effect of pH on adsorption of Cu2+onto adsorbent (adsorbent dose 1 g·L?1, sorbent concentration 150 mg·L?1, and 293 K)
3.5Equilibrium isotherms
Equilibrium data, commonly known as the adsorption isotherms, are the main requirements to investigate the adsorption mechanism. Two traditional adsorption isotherm models of Langmuir [19] and Freundlich [20] are used to describe the equilibrium between adsorbed Cu (II) ions on the adsorbent. The Langmuir isotherm model can be expressed as
Its linear form is
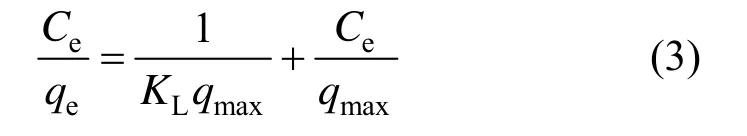
where Ceis the equilibrium concentration of Cu (II) ions (mg·L?1), qeis the equilibrium capacity of Cu (II) ions on the adsorbent (mg·g?1), qmaxis the maximum adsorption capacity (mg·g?1) of adsorbent, and KLis the Langmuir adsorption constant (L·mg?1). qmaxand KLcan be calculated from the linear plots of Ce/qeversus Ce[Fig. 7 (a)].
The Freundlich isotherm is an empirical equation. It can be used to describe heterogeneous systems. The non-linear form of Freundlich model is

Its linear form is
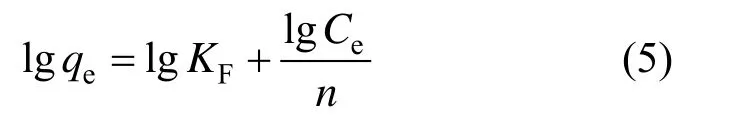
where KFis the indicator of sorption capacity (mg·g?1) and n is a constant for adsorption intensity. KFand 1/n can be determined from the linear plots of lg qeversus lg Ce[Fig. 7 (b)].
The model parameters of Langmuir and Freundlich isotherms for the adsorption of Cu (II) ions on the adsorbent are summarized in Table 1, indicating that the data fit the Langmuir isotherm better than the Freundlich one. The highest value of qmaxobtained at 293 K is 156.7 mg·g?1. Table 2 compares the adsorption capacities of various ash samples for Cu (II) from this and other studies. The results in this work are better. The adsorption capacities depend on the operating conditions and the source of adsorbent. The results in Table 2 indicate that the synthesized zeolite in this study has a good adsorption capacity for all the metal types commonly present in wastewater.
3.6Kinetic study
Kinetics is a significant aspect in the evaluation for sorption as a unit operation. In order to obtain adsorption kinetics, two common models are used to fit the experimental data. The first-order rate model is [26]
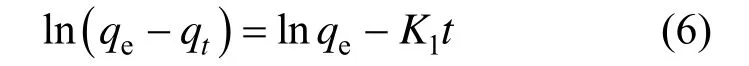
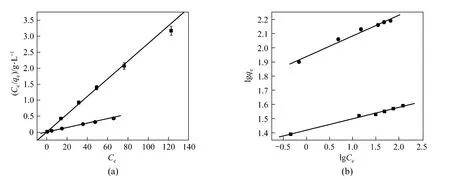
Figure 7Langmuir (a) and Freundlich (b) plots for the adsorption of Cu2+on the adsorbent (adsorbent dose OSA 2 g·L?1, zeolite 1 g·L?1, 293K and pH 6)
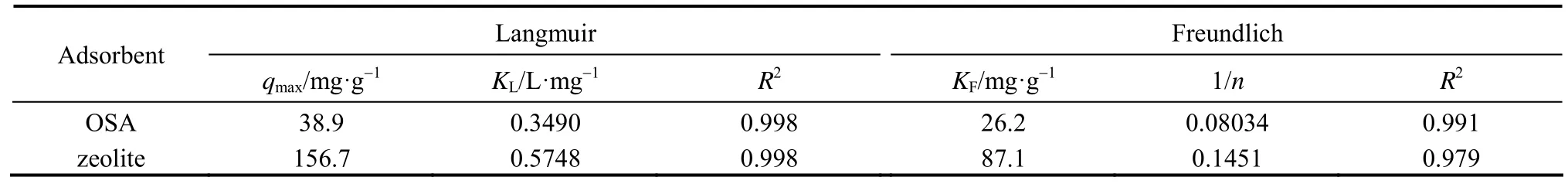
Table 1Langmuir and Freundlich isotherm constants and correlation coefficients
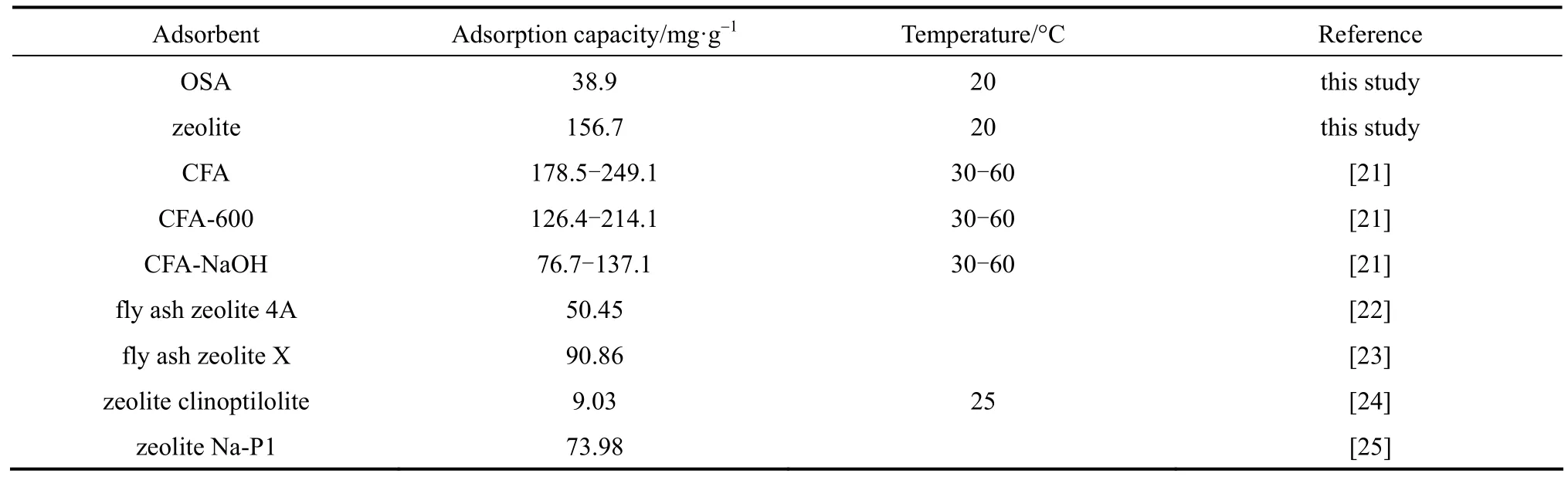
Table 2Adsorption capacities of Cu2+on various adsorbents
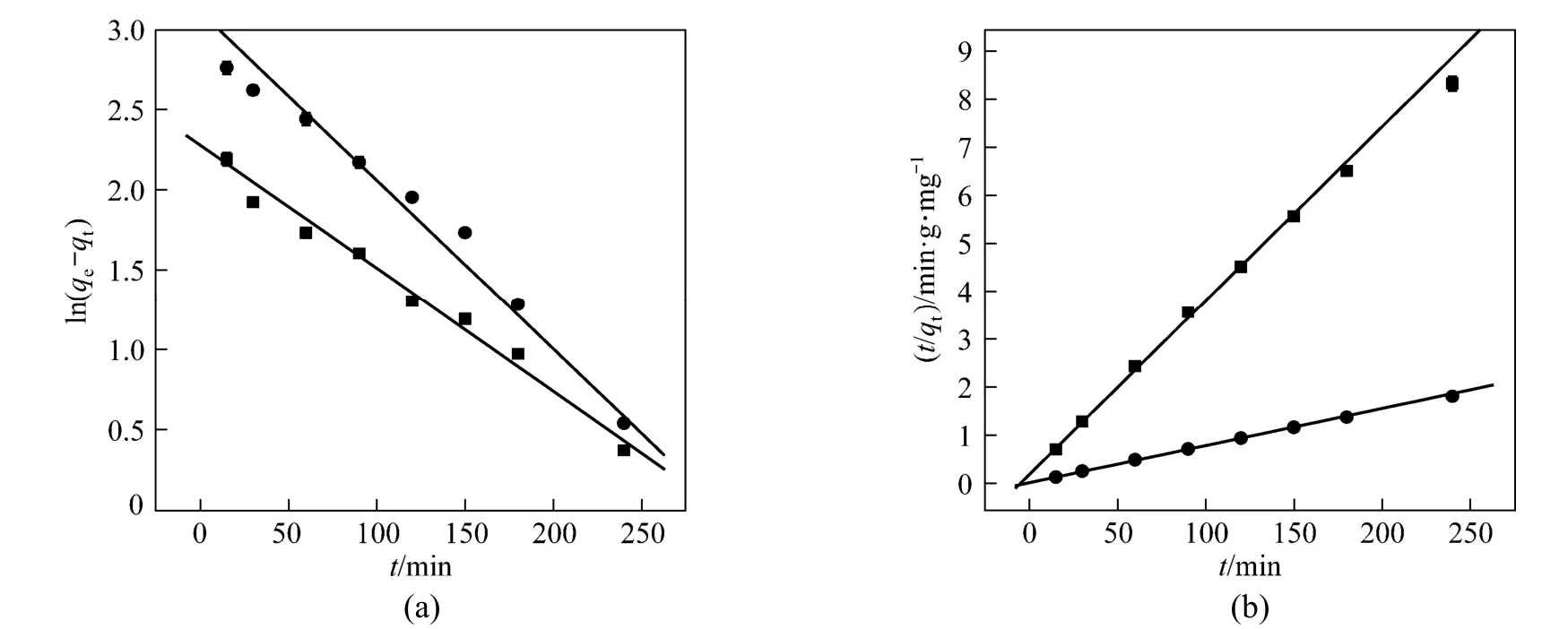
Figure 8Pseudo-first-order (a) and pseudo-second-order (b) kinetics for Cu2+adsorption on the adsorbent● zeolite; ■ OSA
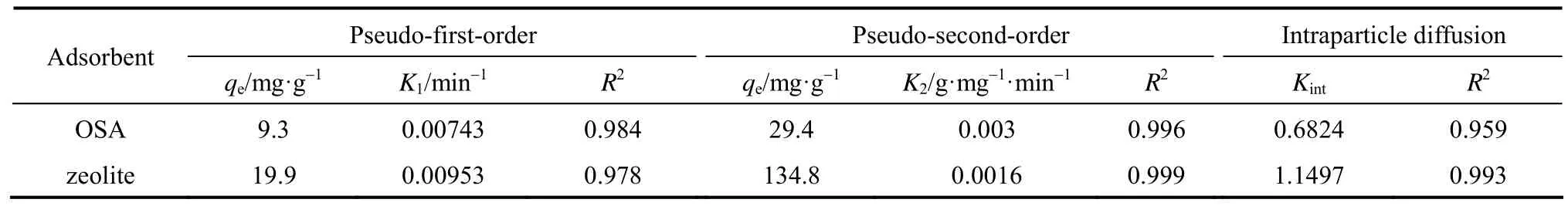
Table 3A comparison for rate constants of pseudo-first-order, pseudo-second-order and intraparticle diffusion kinetic models calculated from experimental data
where qtand qeare the amount of Cu (II) ions adsorbed (mg·g?1) at time t (min) and at equilibrium, respectively, and K1is the rate constant of the pseudo- firstorder adsorption process (min?1). The straight line plots of ln(qe?qt) against t are used to determine the rate constant K1and correlation coefficient R2(Fig. 8 a). The values of constants are shown in Table 3.
The pseudo-second-order model is expressed as [27]
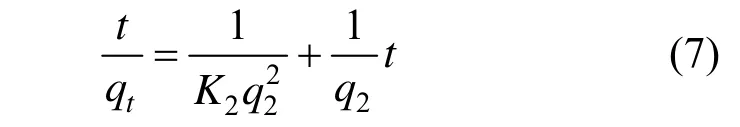
where K2is the constant of pseudo-second-order rate (g·mg?1·min?1), q2is the amount adsorbed at equilibrium, and qtis the amount adsorbed at time t. The equilibrium adsorption amount (q2) and the pseudosecond-order rate parameters (K2) can be obtained from the slope and intercept of plot of t/qtversus t [Fig. 8 (b)]. The corresponding values are presented in Table 3.
The intraparticle diffusion model is presented by the following equation:

where kintis the intraparticle diffusion rate constant (mg·g?1·min?1/2). If the intraparticle diffusion is involved in the adsorption process, the plot of square root of time against the uptake (qt) will be a linear relationship and the intraparticle diffusion is the controlling step if this line passes through the origin.
Table 3 shows the adsorption kinetic parameters of pseudo-first-order, pseudo-second-order and intraparticle diffusion models. The correlation coefficient R2indicates that the second-order kinetic equation agrees the data better. It is more likely that the rate-limitingstep is chemical adsorption and the adsorption behavior may involve valency forces through sharing electrons between transition metal cations and adsorbent. The intraparticle diffusion model shows that intraparticle diffusion may be involved in the adsorption process but it is not the controlling factor.
3.7Thermodynamic study
The adsorption of Cu2+ions was investigated at 293, 303, 313 and 323 K. The removal efficiency of Cu2+ions increases with temperature, indicating that higher temperature is beneficial to the adsorption. The thermodynamic parameters of the adsorption process are calculated from experiments at different temperatures using the following relations [28]
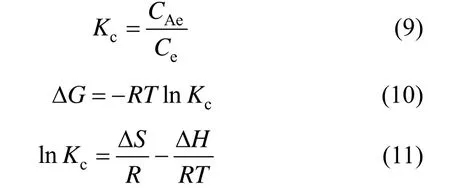
where Kcis the equilibrium constant, CAeis the concentration of Cu(II) ions on the adsorbent at equilibrium, Ceis the equilibrium concentration of copper ions in the solution (mg·L?1), ΔG, ΔH and ΔS are changes in free energy, enthalpy and entropy, respectively, which are calculated from the linear relation ln Kcversus 1/T (Fig. 9). Table 4 summarizes the thermodynamic values for the process of heavy metal removal by the synthesized zeolite. The values for ΔG, ΔH and ΔS indicate the spontaneity of the cationexchange process. The positive values of ΔH confirm the endothermic nature of Cu (II) removal, as supported by the values of the equilibrium constants and previous studies with other natural adsorbents [29, 30].
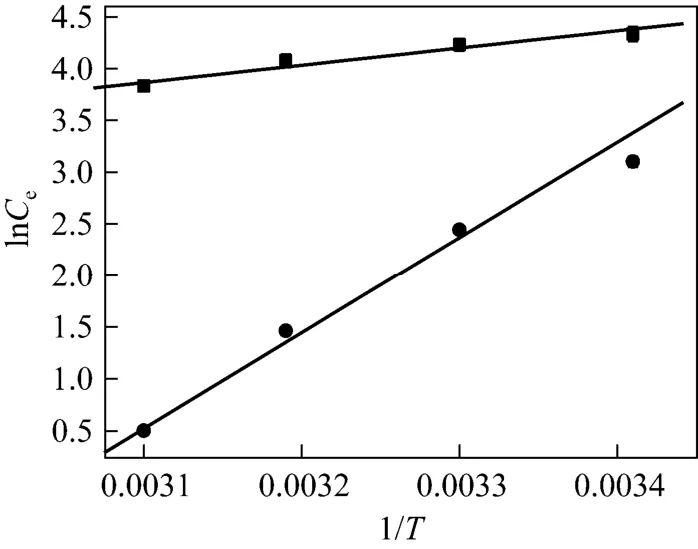
Figure 9Thermodynamic plots for Cu2+adsorption on the adsorbent● zeolite; ■ OSA
3.8Column experiment
Fixed bed columns are usually used as industrial adsorber. Experiments were performed to understandthe adsorption behavior in fixed bed columns. Breakthrough curve between concentration ratio (Ceff/C0, Ceffis the metal concentration in the effluent) and time is used for the calculation of column capacity. Fig. 10 shows the breakthrough curves for Cu (II) adsorption onto OSA and zeolite from the metal ion solutions of concentration 100 mg·L?1and 150 mg·L?1. Compared to the batch experiments, the trend in the column is similar, but the adsorption capacities are less, approximately 30% and 60% for Cu (II) adsorption on the OSA and zeolite, that is, 15.0 and 75.0 mg·g?1, respectively. The lower adsorption capacity of adsorbent in the column experiments may be due to following reasons. In the fixed bed, the adsorbent is packed in the column and the surfaces of solid particles are in contact with each other, reducing the solid-solute interaction. Further, channeling may occur in the fixed bed column, resulting in poor solid-metal ion contact and less residence time [31].
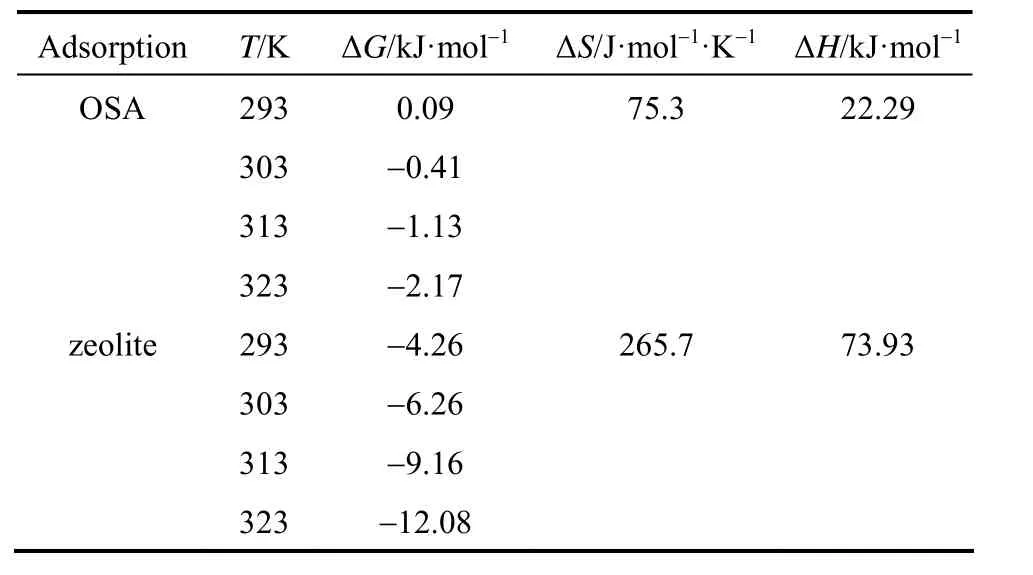
Table 4Values of thermodynamic parameters for the adsorption of copper ions on adsorbent
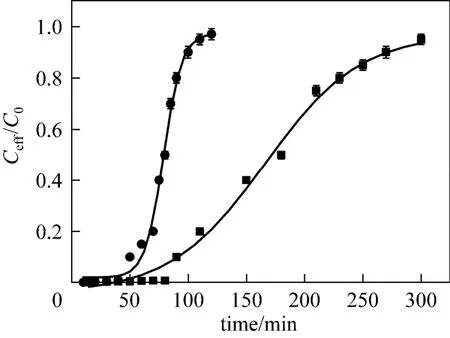
Figure 10Breakthrough curves for adsorption of Cu(II) onto OSA and zeolite● zeolite; ■ OSA
3.9Competition among cations
To mimic industrial wastewater, we used one adsorbent (prepared zeolite) to carry out an adsorption experiment in the presence of Cu2+, Ni2+and Zn2+. The sorption of these metals on the zeolite is as follows: Cu (II) 34.6 mg·g?1(69.1%), Ni (II) 15.0 mg·g?1(30.1%), and Zn (II) 11.0 mg·g?1(22.1%). It should benoted that the adsorption capacity of the zeolite for copper metal ions is lower under the competitive condition. The competitive adsorption ability of heavy metal ions is determined by the hydrated ionic radii and the hydration energies of the heavy metal species.
3.10Adsorbent regeneration
The regeneration of adsorbent is extremely important in a wastewater treatment process. To evaluate the regeneration of the synthesized zeolite, three consecutive adsorption-desorption cycles were conducted with 50 ml of 150 mg·L?1Cu (II) solution in the adsorption step and 50 ml of 5% (by mass) NaCl in the desorption cycle as desorption agent. The regeneration efficiency reached 94.56%, 92.88% and 88.19% for Cu2+at the first, second and third cycle, respectively. The data for regeneration indicate that the prepared zeolite presents not only high removal efficiency but also good reusability for the treatment of wastewaters containing heavy metal ions.
4CONCLUSIONS
Na-A zeolite was successfully synthesized from OSA by fusion process and used as the adsorbent for the removal of Cu (II) from aqueous solutions. The as-prepared zeolite exhibits much higher removal efficiency than OSA, and the maximum adsorption capacity from the Langmuir adsorption isotherm is found to be 156.7 mg·g?1for Cu (II). The kinetics of Cu (II) adsorption onto the adsorbent fits the pseudo-second order model well. The determined free energy change (ΔG) and enthalpy change (ΔG) illustrate that Cu (II) ion sorption on the adsorbent is a spontaneous process of endothermic nature. The synthesized zeolite can be generated, maintaining almost the same Cu (II) adsorption capacity in three cycles. The results in this study show that the synthesized zeolite based on OSA is a good adsorbent for removal of copper ions from wastewater.
REFERENCES
1 Nascimento, M., Soares, P.S.M., Souz, V.P.D., “Adsorption of heavy metal cations using coal fly ash modified by hydrothermal method”, Fuel,88, 1714-1719 (2009).
2 Mata, Y.N., Blázquez, M.L., Ballester, A., González, F., Munoz, J.A.,“Sugar-beet pulp pectin gels as biosorbent for heavy metals: Preparation and determination of biosorption and desorption characteristics”, Chem. Eng. J.,150, 289-301 (2009).
3 Babel, S., Kurniawan, T.A., “Low-cost adsorbents for heavy metals uptake from contaminated water: A review”, J. Hazard. Mater.,B97, 219-243 (2003).
4 Bhatnagar, A., Sillanpaa, M., “Utilization of agro-industrial and municipal waste materials as potential adsorbents for water treatment—A review”, Chem. Eng. J.,157, 277-296 (2010).
5 Gharaibeh, S.H., Abu-El-Sha’r, W.Y., Al-Kofahi, M.M., “Removal of selected heavy metals from aqueous solutions using a solid by-product from the Jordanian oil shale refining”, Environ. Geol.,39(2), 113-116 (1999).
6 Al-Qodah, Z., Shawaqfeh, A.T., Lafi, W.K., “Adsorption of pesticides from aqueous solutions using oil shale ash”, Desalination,208, 294-305 (2007).
7 Shawabkeh, R., Al-Harahsheh, A., Hami, M., Khlaifat, A., “Conversion of oil shale ash into zeolite for cadmium and lead removal from wastewater”, Fuel,83, 981-985 (2004).
8 Shawabkeh, R., “Equilibrium study and kinetics of Cu2+removal from water by zeolite prepared from oil shale ash. Process”, S af. Environ.,87, 261-266 (2009).
9 Shawabkeh, R., Al-Harahsheh, A., Al-Otoom, A., “Copper and zinc sorption by treated oil shale ash”, Sep. Purif. Tech nol.,40, 251-257 (2004).
10 Fernandes-Machado, N.R.C., Miotto, D.M.M., “Synthesis of Na-A and Na-X zeolites from oil shale ash”, Fuel,84, 2289-2294 (2005).
11 Leofanti, G., Padovan, M., Tozzol, G., Venturelli, B., “Surface area and pore texture of catalysts”, Catal. Today,41, 207-219 (1998).
12 Gao, G.M., Liu, D.R., Zou, H.F., Zou, L.C., Gan, S.C., “Preparation of silica aerogel from oil shale ash by fluidized bed drying”, Powder. Technol.,197, 283-287 (2010).
13 Ye, Y., Zeng, X., Qian, W., Wang, M., “Synthesis of pure zeolites from supersaturated silicon and aluminum alkali extracts from fused coal fly ash”, Fuel,87, 1880-1886 (2008).
14 Erdem, E., Karapinar, N., Donat, R., “The removal of heavy metal cations by natural zeolites”, J. Colloid Interf. Sci.,280, 309-314 (2004).
15 El-Kamash, A.M., Zaki, A.A., Abed El Geleel, M., “Modeling batch kinetics and thermodynamics of zinc and cadmium ions removal from waste solutions using synthetic zeolite A”, J. Hazard. Mater.,B127, 211-220 (2005).
16 Wang, S., Ariyanto, E., “Competitive adsorption of malachite green and Pb ions on natural zeolite”, J. Colloid Interf. Sci.,314, 25-31 (2007).
17 Chang, Y., Liu, H., Zha, F., Chen, H., Ren, X., Lei, Z., “Adsorption of Pb(II) by N-methylimidazole modified palygorskite”, Chem. Eng. J.,167, 183-189 (2010).
18 ?zer, A., ?zer, D., ?zer, A., “The adsorption of copper (II) ions on to dehydrated wheat bran (DWB): Determination of the equilibrium and thermodynamic parameters”, Process. Biochem.,39, 2183-2191 (2004).
19 Liu, Z., Zhang, F.S., “Removal of lead from water using biochars prepared from hydrothermal liquefaction of biomass”, J. Hazard. Mater.,167, 933-939 (2009).
20 Pimentel, P.M., Melo, M.A.F., Melo, D.M.A., Assun??o., A.L.C., Henrique, D.M., Silva Jr., C.N., González, G., “Kinetics and thermodynamics of Cu(II) adsorption on oil shale wastes”, Fuel. Process.Techonol.,89, 62-67 (2008).
21 Hsu, T.C., Yu, C.C., Yeh, C.M., “Adsorption of Cu2+from water using raw and modified coal fly ashes”, Fuel,87, 1355-1359 (2008).
22 Hui, K.S., Chao, C.Y.H., Kot, S.C., “Removal of mixed heavy metal ions in wastewater by zeolite 4A and residual products from recycled coal fly ash”, J. Hazard. Mater.,B127, 89-101 (2005).
23 Apiratikul, R., Pavasant, P., “Sorption of Cu2+, Cd2+, and Pb2+using modified zeolite from coal fly ash”, Chem. Eng. J.,144, 245-258 (2008).
24 Erdem, E., Karapinar, N., Donat, R., “The removal of heavy metal cations by natural zeolites”, J. Colloid Interf. Sci.,280, 309-314 (2004).
25 Lee, M.G., Ahn, B.J., Roddick, F., “Conversion of coal fly ash into zeolite and heavy metal removal characteristics of the products”, Korean J. Chem. Eng.,17(3), 325-331 (2000).
26 Chiou, M.S., Li, H.Y., “Adsorption behavior of reactive dye in aqueous solution on chemical cross-linked chitosan beads”, Chemosphere,50, 1095-1105 (2003).
27 Wang, Y., Mu, Y., Zhao, Q.B., Yu, H.Q., “Isotherms, kinetics and thermodynamics of dye biosorption by anaerobic sludge”, Sep. Purif. Technol.,50, 1-7 (2006).
28 Pehlivan, E., Arslan, G., “Removal of metal ions using lignite in aqueous solution-low cost biosorbents”, Fuel. Process. Technol.,88, 99-106 (2007).
29 Panayotova, M.I., “Kinetics and thermodynamics of copper ions removal from wastewater by use of zeolite”, Waste Manage.,21, 671-676 (2001).
30 Bosco, S.M.D., Jimenez, R.S., Carvalho, W.A., “Removal of toxic metals from wastewater by Brazilian natural scolecite”, J. Colloid Interf. Sci.,281, 424-431 (2005).
31 Amarasinghe, B.M.W.P.K., Williams, R.A., “Tea waste as a low cost adsorbent for the removal of Cu and Pb from wastewater”, Chem. Eng. J.,132, 299-309 (2007).
10.1016/S1004-9541(13)60529-7
2012-08-22, accepted 2012-12-03.
* Supported by the National Innovative Projects with Cooperation in terms of Production, Study and Research (OSR-05), and the National Science and Technology Major Projects (2008ZX05018-005).
** To whom correspondence should be addressed. E-mail: zouhf@jlu.edu.cn; gansc@jlu.edu.cn
 Chinese Journal of Chemical Engineering2013年9期
Chinese Journal of Chemical Engineering2013年9期
- Chinese Journal of Chemical Engineering的其它文章
- Soft Sensor for Inputs and Parameters Using Nonlinear Singular State Observer in Chemical Processes*
- A New Tuning Method for Two-Degree-of-Freedom Internal Model Control under Parametric Uncertainty*
- Halloysite Nanotube Composited Thermo-responsive Hydrogel System for Controlled-release*
- Recent Advances in Separation of Bioactive Natural Products*
- A Novel γ-Alumina Supported Fe-Mo Bimetallic Catalyst for Reverse Water Gas Shift Reaction*
- Experimental and Theoretical Studies of CO2Absorption Enhancement by Nano-Al2O3and Carbon Nanotube Particles
