Hydrophobic Synergism between the Alkyl Tails of Gemini Surfactants during Adsorption and Aggregation
YOU Yi DENG Yong-Shu LI Er-Jun PEI Xiao-Mei ZHAO Jian-Xi
(Institute of Colloid and Interface Chemistry,College of Chemistry and Chemical Engineering,Fuzhou University,Fuzhou 350108,P.R.China)
Hydrophobic Synergism between the Alkyl Tails of Gemini Surfactants during Adsorption and Aggregation
YOU Yi DENG Yong-Shu LI Er-Jun PEI Xiao-Mei ZHAO Jian-Xi*
(Institute of Colloid and Interface Chemistry,College of Chemistry and Chemical Engineering,Fuzhou University,Fuzhou 350108,P.R.China)
The critical micelle concentration(cmc)and the concentration reducing 20 mN·m-1of water surface tension(pC20)by the Gemini surfactant series(m-6-m)and the corresponding monomers(CmTABr)were determined using surface tension measurements.Comparison between the active parameters indicates that the Gemini surfactants have a stronger micellization and adsorption ability than CmTABr,which is attributed to the hydrophobic synergism between the alkyl tails of the Gemini surfactants.The effects of dissymmetry in the Gemini surfactants(12-6-m)on the micellization and adsorption were also discussed.The results showed that the Gemini surfactant with a symmetric structure is more effective in association and adsorption than that with a dissymmetric structure.
Gemini surfactant; Hexamethylene spacer;Hydrophobic synergism between alkyl tails;Surface tension
In the past decade,Gemini surfactants have attracted great interest since these surfactants possess more predominant surface activity than traditional single-chain surfactants[1].This was attributed to the special molecular structure of Gemini surfactants different from conventional surfactants,in which the spacer connecting two hydrophilic headgroups plays a key role[1-4].Summarily,the spacer(a)shields the hydrophobic core of the aggregate from contact with water,(b)constrains the distance between the headgroups,thus imposing non-uniformity in charge distribution at the aggregate surface,and(c)can be partially buried inside the micelle core provided its length and the molecular interactions allows it[1,5].A typical example is alkanediyl-α,ω-bis(dimethylalkylammonium bromide)surfactants.This family of Gemini surfactants is generally referred to as m-s-m and has a flexible polymethylene spacer chain connecting the two quaternaryammoniumheadgroups.For12-s-12 series,an irregular criticalmicelle concentration(cmc)varys with s,i.e.,the cmc increases with s from 2 to 5 then decreases with further increasing s andthe maximum appears at s~5[2],and strong salt effect[6]has been attributed to the above spacer effects.The bending of the long polymethylene spacer chain toward the alkyl tail-side was also found by several groups[3,7-8].
Besides these effects of the spacer chain,we believe that the hydrophobic interaction between the alkyl tails should be strengthened during the adsorption and the aggregation.For example,12-6-12 has a hexamethylene spacer between the two quaternary ammonium headgroups and its stretched length is 0.889 nm calculated by the formula ds=0.127(s+1)nm[3].This value is almost identical to the electrostatic equilibrium distance(ca 0.9 nm[3])between the two headgroups of alkyltrimethylammonium bromides packed on the micelle.This indicates the assumption of the fully stretched hexamethylene spacer is reasonable under the electrostatic repulsion of the two ionic headgroups. This seems to mean that 12-6-12 is a simple dimeric form of C12TABr through a hexamethylene spacer connecting two quaternary ammonium headgroups.However,cmc of 12-6-12(1.12 mmol·L-1at 25℃[7])was found to be one order of magnitude lower than that of C12TABr(16 mmol·L-1at 25℃[9]).This fact indicates that the hydrophobic interaction between the alkyl tails should be strengthened during the aggregation.However,this viewpoint was less stressed so far[10-11].In the present investigation,we compare the self-assembly behavior of a series of Gemini surfactants m-6-m with the corresponding monomers CmTABr in order to reveal the hydrophobic synergism between the alkyl chains of Gemini surfactants.Another series of Gemini surfactants 12-6-m is also introduced to discuss the effect of dissymmetric structure of Gemini molecule on the self-assembly.
1 Experimental
1.1 Materials
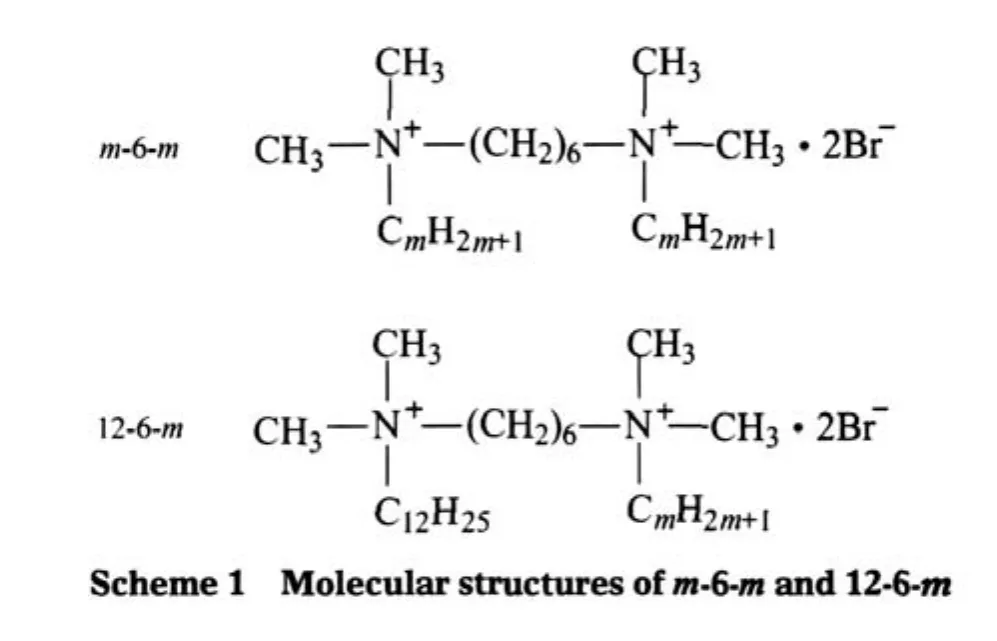
The chemical structures of hexanediyl-α,ω-bis(dimethylalkylammonium bromide)and hexanediyl-α-dimethyldodecylammonium-α-dimethylalkylammonium dibromides,which are referred below as m-6-m(m=8,10,12,14,16)and 12-6-m(m=8,10,14, 16),respectively,are represented in Scheme 1.
The m-6-m series were synthesized by the reaction of N,N,N-alkyldimethylamine with α,ω-dibromoalhexane,which was first reported by Zana et al.[2].The 12-6-m series were synthesized with the method reported by Bai et al.[12].The purity of the m-sm and 12-6-m was checked by elemental analysis and1H NMR spectroscopy.Alkyltrimethylammonium bromide(CmTABr)was purchased from Sigma and used as received.Water used was Milli-Q grade.
1.2 Method
Surface tension of the solutions was measured by du Noüy ring method using tensionmeter(CHAN DCA-315,America). The circumference of the ring is 5.930 cm.The ratio of the outside radius to the radius of the ring cross section(R/r)is 53.1218.From the semilogarithmic plot of surface tension versus surfactant concentration,cmc,the minimum surface tension at the cmc(γcmc)and the concentration required to reduce 20 mN· m-1of water surface tension(pC20)can be obtained.
2 Results and discussion
2.1 Surface activity of symmetric m-6-m series
As mentioned in Introduction,the Gemini surfactant m-6-m looks like simple dimer of CmTABr through a hexamethylene spacer since the stretched length of this spacer chain is close to the electrostatic equilibrium distance between the two headgroups of CmTABr packed on the micelle.In this section,we compare the surface activity of m-6-m with that of the corresponding monomer surfactants CmTABr.
A typical surface tension plot of symmetric 12-6-12 is shown in Fig.1.The break point characterizes cmc,which reflects the ability of surfactant forming micelles.γcmcand pC20characterize the effectiveness and the efficiency of surfactant in surface tension reduction,respectively[9].The maximum surface excess of surfactant Γmaxat the cmc can be calculated by Gibbs formula:

from which the minimum area per molecule(Amin)at the interface is calculated by following formula:

where NAis Avogardo constant.These parameters are listed in Table 1.
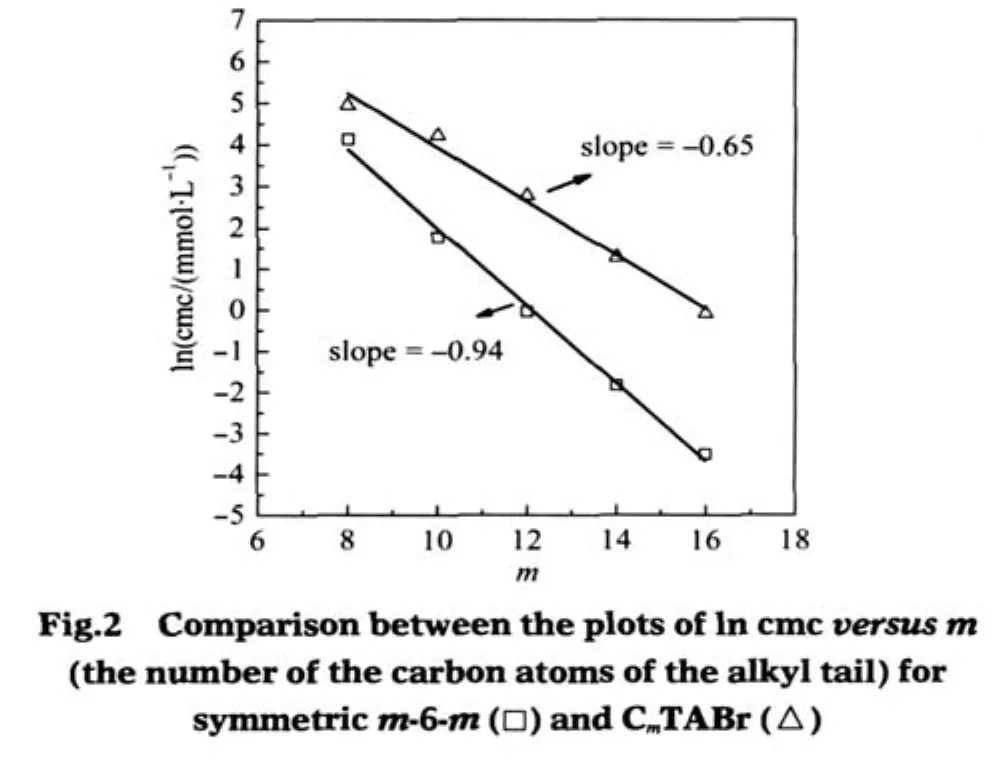
Fig.2shows the plots of ln cmc versus m(the numberofthe carbon atoms of the alkyl tail)for symmetric m-6-m and CmTABr, which exhibit good linear variation.This linear relationship has been known to satisfy the following formula[9]
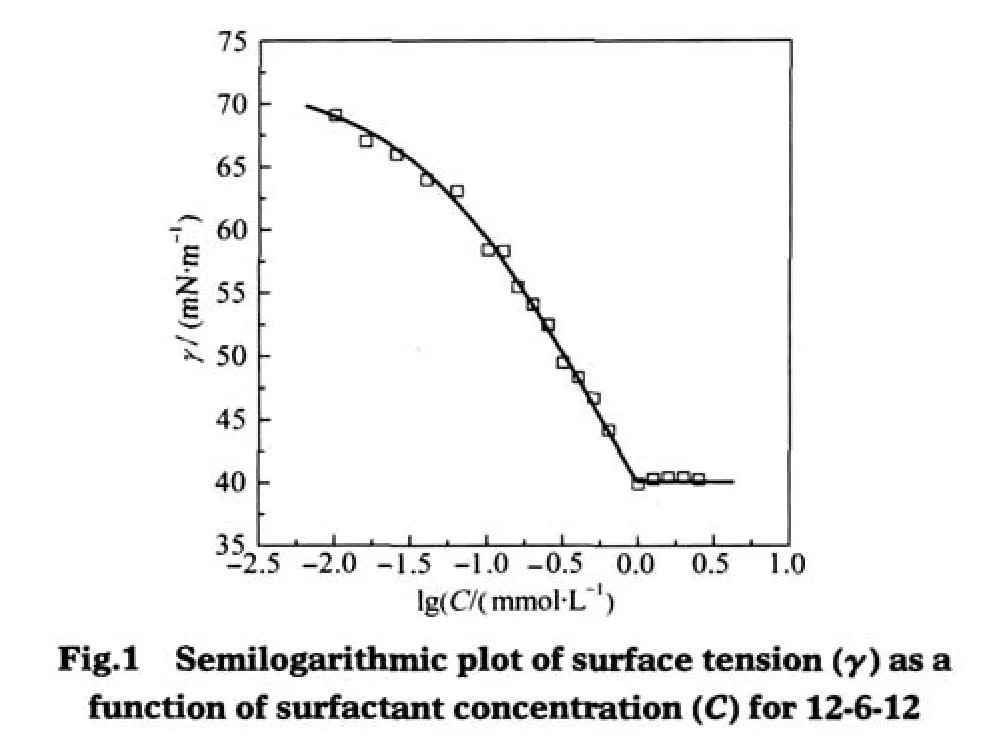
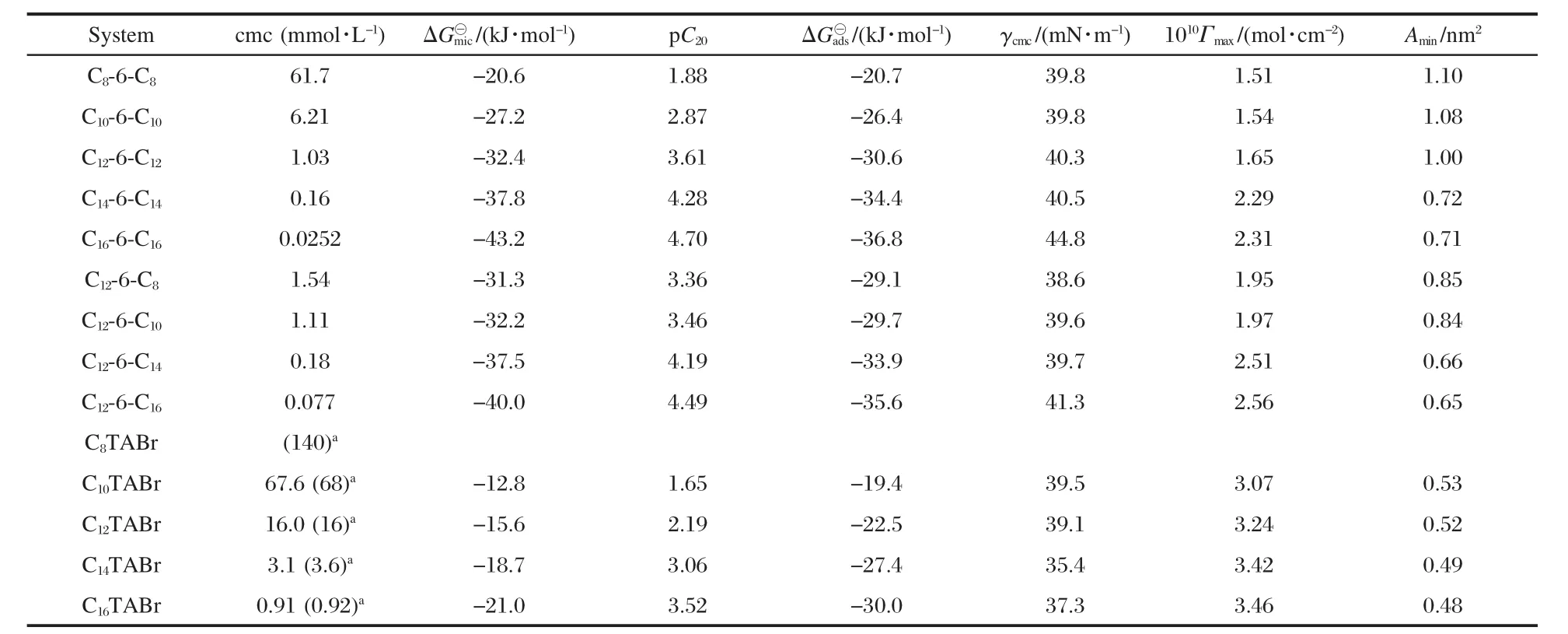
Table 1 Activity parameters of symmetric m-6-m,dissymmetric 12-6-m,and CmTABr at 25℃
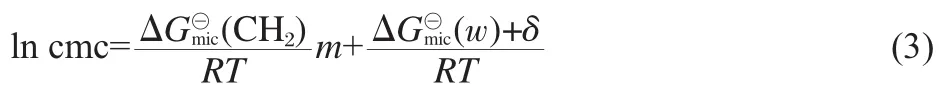
where ω is the moles of H2O per cubic decimeter for pure water at absolute temperature T(ω=55.5 mol·L-1at 25℃),△G?micis the standard free energy of micellization,δ is a constant at given temperature and represents as a difference between△G?mic(CH3) and△G?mic(CH2).The slope reflects the contribution of a methylene group in the alkyl chain to micellization.As seen from Fig. 2,the negative value of the slope(-0.94)of m-6-m is greatly larger than that(-0.65)of CmTABr,indicating that the alkyl chains of the Gemini surfactants have larger tendency to aggregation than that of the single-chain CmTABr.This clearly shows that though the spacer chain length of m-6-m is close to the electrostatic equilibrium distance between the two headgroups of CmTABr packed on the micelle,the Gemini structure of m-6-m molecule makes the hydrophobic effect of their alkyl chains remarkable in the micellization.
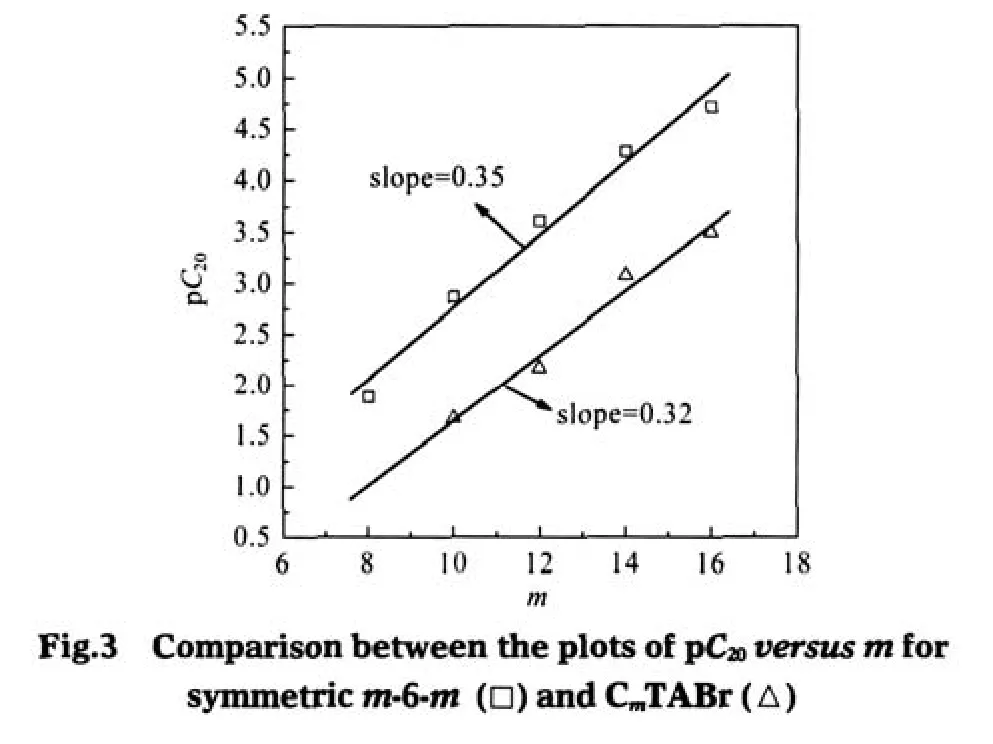
The pC20that characterizes the efficiency of surfactant in surface tension reduction[9]satisfies the similar formula

where K2represents the increase in the free energy when the hydrophilic groups are moved into the interface.The results in Fig. 3 also give out the same information,i.e.,a slope(0.35)for m-6-m is relatively larger than that(0.32)for CmTABr,indicating the stronger hydrophobic effect between the alkyl chains of the Gemini surfactants than the corresponding monomers in the adsorption.

where K0is the degree of counterion association.The K0of 12-6-12 and C12TABr have been known to be 0.67[2]and 0.77[14],respectively.Both the values are used to calculate△G?micof m-6-m and CmTABr series regardless of the length of the alkyl tail. The data listed in Table 1 show the obviously larger negative values of△G?micin comparison with those of CmTABr,indicating stronger tendency of the Gemini surfactants for micellization.
The standard free energy of adsorption at the surface pressure π,follows the formula:

At π=52 mN·m-1(corresponding surfactant concentration is C20),△G?adscan relate to the pC20and the Amin:
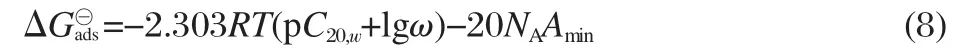
The calculated△G?adsalso shows the larger negative values for m-6-m than those for CmTABr(Table 1).
2.2 Effect of dissymmetric structure of 12-6-m
Similar to symmetric m-6-m,the dissymmetric 12-6-m contains a hexamethylene spacer linking the two quaternary ammonium headgroups but an alkyl tail is fixed at the length of 12 carbon atoms and another has variable length from 8 to 16 carbon atoms.As can be seen from Fig.4,the plots of both ln cmc and pC20versus m exhibit two linear regions with different slopes separating at m=12.At m<12,the ln cmc and pC20have almost no change with increasing m,meaning a very small contribution of increasing the number of carbon atoms in the short chain to both the micellization and the adsorption.In other words,the micellization and the adsorption of these compounds are mainly driven by the hydrophobic effect between the long alkyl chains. At m>12,the slopes of the straight lines for both the ln cmc and the pC20plots are smaller than those of the corresponding monomers,indicating that for the combination of two alkyl tails with different lengths(dissymmetric Geminis)the contribution of increasing methylene group to the micellization and to the adsorption is relatively smaller.This was also reflected in the binary mixture of traditional single-chain surfactants to some extent, for which two surfactants having the same lengths of the alkyl chains was found to have the synergism larger than those having different lengths of the alkyl chains[15].

Compared the results of Fig.2 and Fig.3 with Fig.4,one can realize that the Gemini surfactants having the same lengths of alkyl chains(symmetric structure)can play a better role of the molecular structure in the aggregation and the adsorption than those having the different lengths of alkyl chains(dissymmetric structure).
2.3 Structure of the adsorption layer
As can be seen from Table 1,the average area(Amin)of symmetric 10-6-10 or 12-6-12 occupied at the air/water interface is about twice Aminof the corresponding monomer C10TABr or C12TABr.However,the Aminof 14-6-14 and 16-6-16 are greatly smaller than the expected twice values.This clearly shows the enhanced hydrophobic interaction between the alkyl tails of 14-6-14 and 16-6-16 and again indicates that Gemini structure of the amphiphilic molecule is favorable to the synergism between the alkyl tails though its spacer chain is close to the electrostatic equilibrium distance between the two headgroups of CmTABr packed on the micelle.
The smaller Amincorresponds to more tight arrangement of the adsorbed molecules at the air/water interface.In general,the tight and uniform arrangement of surfactant molecules at the air/water interface can result in smaller γcmc[9].However,it is surprised that 14-6-14 and 16-6-16 have rather large γcmcin comparison with that of C14TABr or C16TABr.We recall our previous investigation where the effect of added salt on the adsorption and aggregation of 12-6-12 was found to be much stronger than on that of C12TABr[6].This was attributed to the concentrated charge effect in the headgroups of 12-6-12,resulting in highly sensitive response to salt.This fact indicates that even though for 12-6-12,the hydrophobic interaction between the alkyl tails within the molecule may have been put the two quaternary heads close to some extent.That is to say that the real area of the head of 12-6-12 is perhaps smaller than the area calculated by the stretched length of the hexamethylene group.Therefore the apparent area that is twice the area of the corresponding monomer C12TABr is the result of the increased electrostatic repulsion between the ionic headgroups of 12-6-12.The gap between the occupied molecules at the air/water interface thus allows water molecules to fill,which results in the relatively high γcmc.In other words,the non-uniform arrangement of Gemini molecules at the air/water interface should be the reason leading to larger γcmc.Obviously,this phenomenon is certainly more pronounced for 14-6-14 and 16-6-16 since they have stronger hydrophobic interaction between the alkyl tails.Perhaps,this can interpret why Gemini surfactants cannot generally enhance the effectiveness in surface tension reduction.
As seen from Table 1,all the Aminof 12-6-8,12-6-10,12-6-14, and 12-6-16 are smaller than that of 12-6-12.This phenomenon should also relate to the mechanism discussed above.Lange and Schwuger[15]examined the binary mixture of traditional singlechain surfactants and suggested that two surfactants having the same lengths of the alkyl chains could produced the stronger synergism than those having different lengths of the alkyl chains.This means that the present dissymmetric series has a relatively weaker synergism between the two alkyl tails compared with symmetric 12-6-12 and thus smaller concentrated charge effect in the headgroups of dissymmetric series.The decreased electrostatic repulsion between the headgroups results in smaller area of the adsorbed molecule at the air/water interface.
3 Conclusions
In this paper,we report a comparative study on the surface activity of symmetric Gemini surfactant series m-6-m with that of the corresponding monomers CmTABr.Since the length of the hexamethylene spacer of m-6-m is close to the distance between the two headgroups of alkyltrimethylammonium bromides packed on the micelle,m-6-m can be considered as the simple dimer of CmTABr in appearance.However,the experimental results show that the surface activity of m-6-m is obviously higher than that of CmTABr.This fact verifies the existence of the hydrophobic synergism between the alkyl tails of Gemini surfactants very well.Comparatively,the symmetric Gemini surfactants are more favorable for their association and adsorption than the dissymmetric Gemini surfactants.
1 Zana,R.;Xia,J.D.Gemini surfactants.New York:Marcel Dekker Inc.,2004 and references therein
2 Zana,R.;Benrraou,M.;Rueff,R.Langmuir,1991,7:1072
3 Danino,D.;Talmon,Y.;Zana,R.Langmuir,1995,11:1448
4 Zana,R.J.Colloid Interface Sci.,2002,248:203
5 Camesano,T.A.;Nagarajan,R.Colloids Surf.A,2000,167:165
6 You,Y.;Zhao,J.X.;Jiang,R.;Cao,J.J.Colloid Polym.Sci., 2009,287:839
7 Alami,E.;Beinert,G.;Marie,P.;Zana,R.Langmuir,1993,9: 1465
8 Chen,X.D.;Wang,J.B.;Shen,N.;Luo,Y.H.;Li,L.;Liu,M.H.; Thomas,R.K.Langmuir,2002,18:6222
9 Rosen,M.J.Surfactants and interfacial phenomena.3rd ed.New York:John Wiley&Sons,2004:208-230
10 Ali,M.S.;Suhail,M.;Ghosh,G.;Kabir-ud-Din,M.K.Colloids Surf.A,2009,350:51
11 Fan,Y.;Li.Y.;Cao,M.;Wang,J.;Wang,Y.;Thomas,R.K. Langmuir,2007,23:11458
12 Bai,G.Y.;Wang,J.B.;Wang,Y.L.;Yan,H.K.;Thomas,R.K. J.Phys.Chem.B,2002,106:6614
13 Zana,R.Lamgmuir,1996,12:1208
14 Zana,R.J.Colloid Interface Sci.,1980,78:330
15 Lange,H.;Schwuger,J.Kolloid-Z.Z.Polymere,1971,243:120
Gemini表面活性劑烷烴尾鏈在吸附和聚集過程中的疏水協(xié)同效應(yīng)
游 毅 鄧永淑 李二軍 裴曉梅 趙劍曦*
(福州大學(xué)化學(xué)化工學(xué)院,膠體與界面化學(xué)研究所,福州 350108)
以表面張力法測定了系列Gemini表面活性劑m-6-m以及對應(yīng)單體表面活性劑CmTABr的臨界膠束濃度(cmc)和降低水表面張力20 mN·m-1需要的濃度(pC20).比較這些參數(shù)表明m-6-m膠束化和在界面吸附的能力均強(qiáng)于CmTABr,這被歸結(jié)為Gemini表面活性劑烷烴尾鏈間的疏水協(xié)同效應(yīng).與不對稱Gemini表面活性劑12-6-m比較,對稱的Gemini結(jié)構(gòu)更有利于表面活性劑的聚集和吸附.
Gemini表面活性劑;六亞甲基聯(lián)接鏈;烷烴尾鏈?zhǔn)杷畢f(xié)同效應(yīng);表面張力
O648
Received:January 13,2010;Revised:March 23,2010;Published on Web:May 13,2010.
*Corresponding author.Email:jxzhao.colloid@fzu.edu.cn;Tel:+86-591-22866338.
The project was supported by the National Natural Science Foundation of China(20673021,20873024)and Professor Foundation of Fuzhou University,China(2008-XY-5).
國家自然科學(xué)基金(20673021,20873024)和福州大學(xué)教授基金(2008-XY-5)資助項目
?Editorial office of Acta Physico-Chimica Sinica

