One Step Preparation of Sulfonated Solid Catalyst and Its Effect in Esterification Reaction*
One Step Preparation of Sulfonated Solid Catalyst and Its Effect in Esterification Reaction*
KANG Shimin (康世民), CHANG Jie (常杰)**and FAN Juan (范娟)
The Key Laboratory of Fuel Cell Technology of Guangdong Province, School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou 510640, China
A carbon-based sulfonated catalyst was prepared by direct sulfonation and carbonization (in moderate conditions: 200 °C, 12 h) of red liquor solids, a by-product of paper-making process. The prepared sulfonated catalyst (SC) had aromatic structure, composed of carbon enriched inner core, and oxygen-containing (SO3H, COOH, OH) groups enriched surface. The SO3H, COOH, OH groups amounted to 0.74 mmol·g?1, 0.78 mmol·g?1, 2.18 mmol·g?1, respectively. The fresh SC showed much higher catalytic activity than that of the traditional solid acid catalysts (strong-acid 732 cation exchange resin, hydrogen type zeolite socony mobile-five (HZSM-5), sulfated zirconia) in esterification of oleic acid. SC was deactivated during the reactions, through the mechanisms of leaching of sulfonated species and formation of sulfonate esters. Two regeneration methods were developed, and the catalytic activity can be mostly regenerated by regeneration Method 1 and be fully regenerated by regeneration Method 2, respectively.
red liquor solids, sulfonated solid catalyst, carbonization, esterification
1 INTRODUCTION
Carbon-based sulfonated catalyst is becoming a research hotspot recently, which is widely used in biodiesel production [1-4], hydrolysis of cellulose [5], and some other organic synthesis [6, 7]. There are two ways for the synthesis of carbon-based sulfonated catalyst: (1) hydroxyethylsulfonic acid [6], p-toluenesulfonic acid [7] etc. were adapted as the sulfonating agents in hydrothermal conditions, using furaldehyde, glucose etc. as carbon sources; (2) sulfonation of carbon-based precursor with concentrated sulfuric acid (H2SO4), while the precursor was often obtained by carbonization of biomass at high temperatures [3, 4, 8]. However, these sulfonation agents are usually expensive, while the carbonization of biomass for precursor preparation resulted in additional cost. Besides, a few studies on directly incomplete carbonization of low molecular mass compounds (e.g. naphthalene, C10H8) by concentrated H2SO4were reported, but it was found that the sulfonated species on these catalysts were totally lost in the reuse [9]. One possible reason of this instability was due to the low molecular mass of the raw materials, the products of which may be partly dissolved in organic solvents even after carbonization.
Red liquor solid (RLS) is a papermaking byproduct, which is often considered as a low value added material, and the main organic constituent in red liquor is lignosulfonate, a phenolic macromolecular polymer. Besides, biodiesel has received a great deal of attention as an alternative candidate for conventional fossil fuel, and catalytic esterification synthesis of biodiesel by solid acid (e.g. ion exchange resin, molecular sieve, sulfated zirconia) was widely studied [2, 3, 8, 10-14]. Oleic acid is a free fatty acid, and catalytic esterification of oleic acid can be a model process for biodiesel production. The object of this work was to synthesize sulfonated catalyst from macromolecule RLS by one step: the RLS was directly carbonized and sulfonated by concentrated H2SO4, without a carbon-based precursor preparation process at high temperatures. Catalytic effect of the RLS derived sulfonated catalyst for esterification of oleic acid was tested.
2 EXPERIMENTAL
2.1 Materials
The RLS was dried powders of the red liquor, with magnesium lignosulfonate as the main organic constituent. Methyl oleate (standard reagent, purity of 99%) and oleic acid (purity of 85%) were obtained from Aladdin-Reagent Co., Ltd., Shanghai, China. Concentrated H2SO4(95%-98%, by mass) was obtained from Kaixin Chemical Reagent Co., Ltd. from the market. HZSM-5 molecular sieve was obtained from Tianjin Kaimeisite Technology Co. Strong-acid 732 resin was obtained from Shanghai Lingfeng Chemical Reagent Co., which was a strong acidic styrene type cation exchange resin with a diameter of 0.4-0.6 mm. Sulfated zirconia was synthesized according to Yee et al. [12].
2.2 Catalyst preparation
Concentrated H2SO4(100 ml) and 5 g of RLSwere mixed into a 250 ml round-bottomed flask into an oil bath with temperature of 200 °C. The sulfonating reaction was continued for 12 h with a certain mixing speed. After the reaction, the concentrated H2SO4solution was diluted and filtered. The H2SO4recovered after filtration of the solid can be reused for repeated sulfonation. Black precipitate was collected and washed with hot deionized water (~80 °C) until impurities such as sulfate ions were no longer detected in the washing water. The black precipitate was then dried at 75 °C to form the sulfonated catalyst (SC) (about 1.4 g).
2.3 Characterization
The obtained sulfonated carbon catalyst was characterized by X-ray diffraction (XRD) (D8 Advance, Bruker), thermogravimetry (TG) and derivative thermogravimetry (DTG) (TGAQ 5000, TA Instruments Co., USA), Fourier transform infrared spectroscopy (FTIR) (Nexus 670, Nicolet), scanning electron microscopy (SEM) and energy dispersive spectrometer (EDS) (S-3700N, Hitachi, Japan), X-ray photoelectron spectroscopy (XPS) (Kratos AXis Ultra, Shimadzu, Japan), Brunauer-Emmett-Teller (BET) surface area (ASAP 2020 V3.03 H, Micromeritics), and elemental analyzer (elementar Vario EL III, Germany).
The total SO3H + COOH and SO3H + COOH + OH contents were estimated from the exchange of Na+in aqueous NaCl and NaOH solutions, respectively. The densities of SO3H groups were estimated based on the sulfur content determined from sample compositions obtained by elemental analysis and XPS analysis [5].
2.4 Catalytic activity test
Methyl oleate production was performed in a stirred 25 ml round-bottomed flask at 65 °C for 4 h. 0.05 g of solid acid catalyst was added to 1 g of oleic acid and 8 ml of methanol. The yield of methyl oleate was analyzed by the external method through gas chromatography [Shimadzu QP 2010 Plus equipped, with Rxi-5ms column (30 m×0.25 mm×0.25 μm)]. The temperature of the injector was set at 270 °C, the oven temperature was started at 200 °C for 2 min, heated at a rate of 10 °C·min?1to 275 °C, and then held for 5 min. A standard curve was obtained by correlating the peak area with the concentration of a series of methyl oleate solutions, and then the content of methyl oleate after reaction was calculated according to the peak area and the standard curve.
2.5 Catalyst regeneration
Regeneration Method 1: the 3rd time reused catalyst was dipped into 10% H2SO4solution for about 10 h (including sonic oscillation for 1 h) and washed with pure water. Subsequently, the catalyst was dried and resupplied for the next experimental run. The regenerated catalyst was labeled as regenerated SC-1.
Regeneration Method 2: the 3rd time reused catalyst was regenerated according to Refs. [15, 16]. Briefly, it was dipped into 150 °C concentrated H2SO4for 12 h, and then it was washed and, dried, and resupplied for the next experimental run. This regenerated catalyst was labeled as regenerated SC-2.
3 RESULTS AND DISCUSSION
3.1 Characterization of catalyst
The SC exists as solid particles, and the surface topography is shown by SEM spectra image in Fig. 1. The XRD pattern (Fig. 2) shows a broad diffraction peak in a 2θ range of 20°-30°, and the wide-angle pattern matches well with that previously report for non-graphitic carbon [5]. This indicates that the RLS can not be completely graphitized by concentrated H2SO4carbonization at such a low temperature (200 °C). FT-IR spectrum (Fig. 3) shows that the SC owns the aromatic structure (1610, 1420 cm?1), OH group (3410 cm?1), SO3H group (1170, 1040 cm?1) and C O group (1713 cm?1). Compared with the FT-IR spectrum of SC and RLS, the SO3H group on the SC can be derived from

Figure 1 SEM spectra of SC
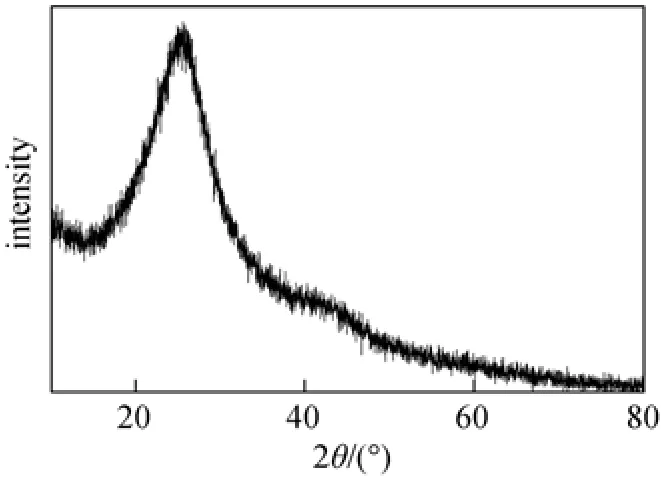
Figure 2 XRD pattern of SC

Figure 3 FT-IR spectrum of SC and RLS
both the RLS and concentrated H2SO4, while the C O group is probably produced by concentrated H2SO4oxidation of OH and CH groups. From the TG and derivative thermogravimetry (DTG) curves (Fig. 4), the sample mass decreased with increasing temperature, and the mass loss before 200 °C is moderate, which maybe caused by the loss of water adsorbed on the SC. As shown in Fig. 5, the results of XPS analysis show the sulphur (S) exists in the forms of SO3H groups and other groups, and the S 2p region in XPS spectrum indicates that about 68% S exists in the form of SO3H group (168 eV). There are two binding energy peaks for the carbon: the peak at 285 eV corresponds to the elemental carbon, which is the substrate of the catalyst; while the small peak at 289 eV corresponds to COOH groups (Fig. 5). Elemental analysis (Table 1), ash test (Table 1), XPS analysis, and cation-exchange experiments (Table 2) reveal that the sample composition is (CH0.68O0.65S0.026)An(A is ash, with a mass content of 0.74%), and the amounts of SO3H, COOH, and phenolic OH groups bonded to the carbon skeleton are 0.74 mmol·g?1, 0.78 mmol·g?1, 2.18 mmol·g?1, respectively.
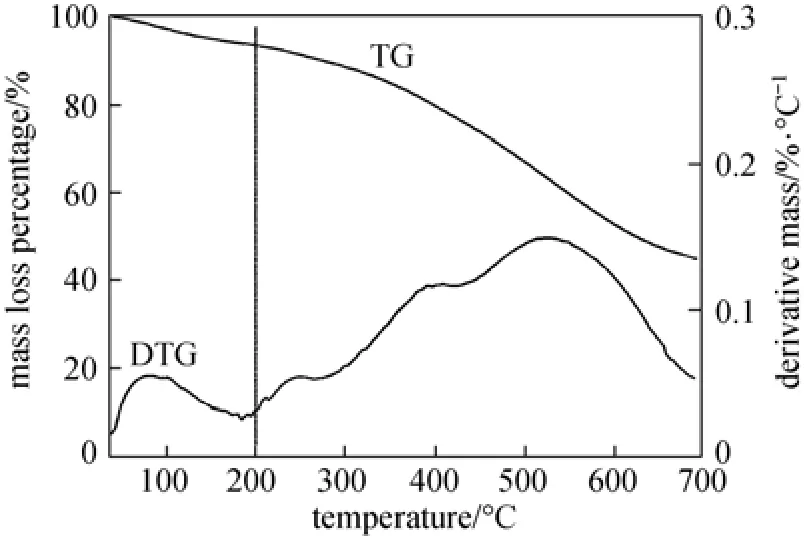
Figure 4 TG and DTG curves of SC
The element content on and near the surface of fresh SC was detected by EDS analysis (Table 3). Compared with the elemental analysis data in Table 1,the results show that the S, O contents on and near the surface are higher than the contents on the whole fresh SC, while the C content on and near the surface is lower than that on the whole fresh SC. These results indicate that the fresh SC is made up of C enriched inner core, and oxygen-containing (SO3H, COOH, OH) groups enriched surface. According to the above discussion, a schematic structure of SC is proposed as shown in Fig. 6.
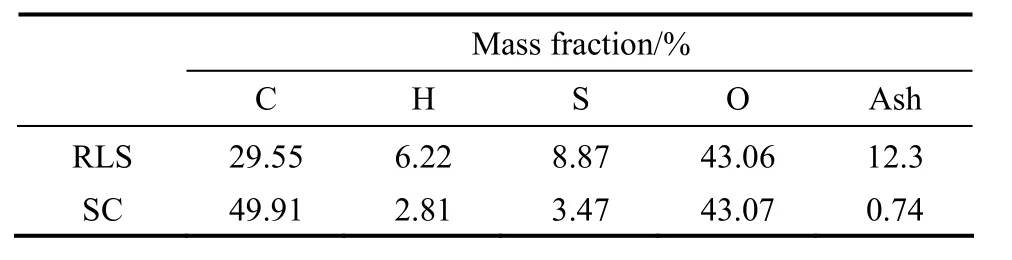
Table 1 Ash content and elementary analysis by elemental analyzer

Table 2 Cation-exchange values and specific surface area
3.2 Catalytic activity
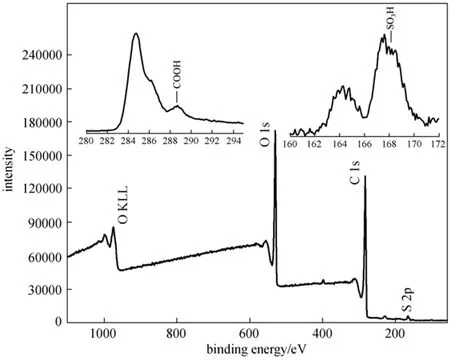
Figure 5 The XPS spectrum of SC
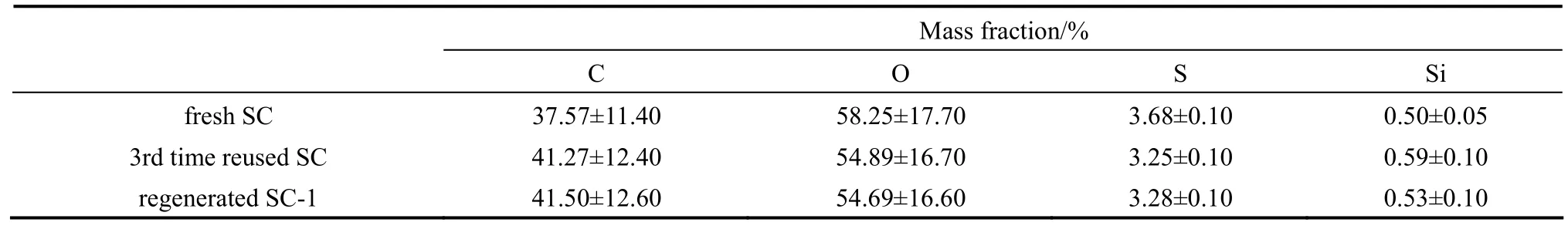
Table 3 Surface elementary analysis of SCs (fresh, 3rd time reused, and regenerated) by EDS
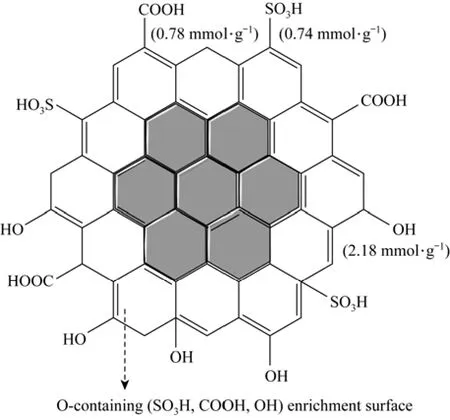
Figure 6 The proposed schematic structure of SC
The catalytic effects for methyl oleate production are shown in Fig. 7. The yield of the control experiments is lower than 1%, while the yield with fresh SC addition reaches about 85%. And the fresh SC shows much higher yield than that of all the other traditional solid acid catalysts (sulfated zirconia, 732 cation exchange resin, and HZSM-5 molecular sieve). Compared with Table 2 and Fig. 7, there seems no relations between catalytic activity and BET specific surface area, cation-exchange capacity for different catalysts. The possible reasons of the strong SC catalytic activity are discussed: (1) The SC possesses three different
acidic functional groups (OH, COOH, SO3H), there may be synergic action among the three acidic functional groups though the SO3H group is often considered as the major catalytic active sites, while those traditional solid acid catalysts usually contain single acidic functional groups; (2) there was a large content of OH group (2.18 mmol·L?1) in the SC, which can incorporate both the two polar reactants (methanol and oleic acid) to the catalyst surface, and then acceleratethe esterification reaction.
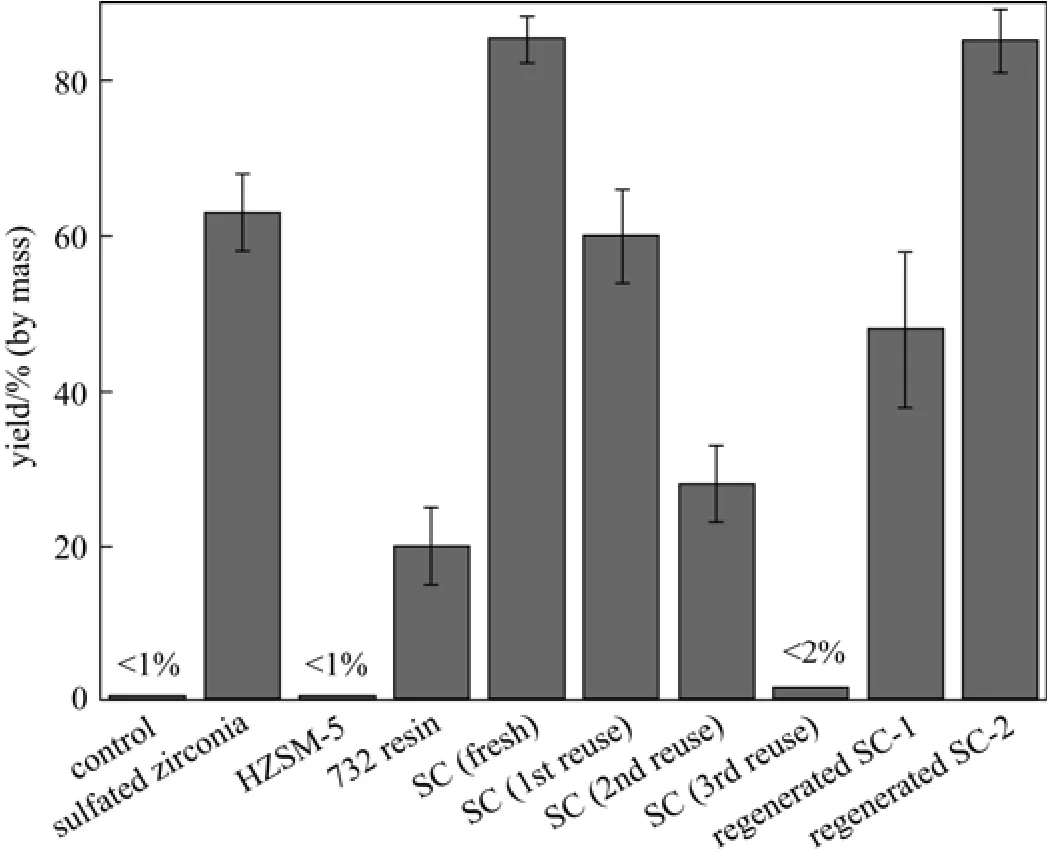
Figure 7 The yield of methyl oleate produced with or without catalysts
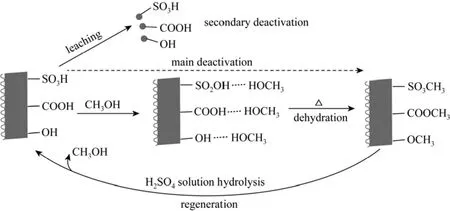
Figure 8 The proposed sulfonated catalyst deactivation and regeneration mechanism, and the deactivation mechanism were a revision according to Fraile et al. [17]
However, the SC catalytic activity decreases with more recycles (Fig. 7), which is coincided with the results of catalysts produced by two procedures (high temperature carbonization, and then sulfonation) reported by Chen and Fang [3], Rao et al. [14], and Fraile et al [17]. The SO3H and the COOH contents decreased from 1.52 to 0.65 mmol·g?1after the 3rd time reuse (Table 2). The reuse experiments caused deactivation should be related to the leaching of SO3H groups from polycyclic aromatic hydrocarbons and/or formation of sulfonate esters according to the former reports [3, 14, 17]. The S content on and near the 3rd time reused SC surface (3.25%, by mass) was 11.6% lower than that on the fresh SC (3.68%, by mass), however, the catalytic activity was almost deactivated after the 3rd time reuse, that indicated the small portion leaching of S was not the main deactivation reasons. These results also indicate that the carbon based sulfonated catalysts produced by direct sulfonation and carbonization from macromolecule polymer may own better stabilities than that from low molecular mass compounds, as compared with the results reported by Hara et al [9].
In regeneration Method 1, 10% H2SO4was used to regenerate the catalyst from the 3rd time reuse. After the catalyst regeneration with 10% H2SO4, the SO3H and COOH contents are back to 1.05 mmol·g?1(Table 2), and more than half of the catalytic activity is recovered, and the catalytic activity is much better than that of 732 cation exchange resin (Fig. 7). On the other hand, the S content on and near the 3rd time reused SC surface after regeneration is only slightly changed (from 3.25% to 3.28%, by mass Table 3). However, few papers on the carbon based sulfonated catalyst regeneration mechanism in this area were reported in the past works. In this paper, the possible SC deactivation and regeneration mechanism are proposed as shown in Fig. 8. Considering the change of cationexchange values (the SO3H and COOH contents) and S contents among different samples (the fresh SC, the 3rd time reused SC, and regenerated SC-1), the main deactivation of the SC should be the formation of sulfonate esters and carbonic esters, similar to the resultsthat reported by Fraile et al [17]. The probable reason for the regeneration results is that the sulfonate methyl esters and carboxylic acid methyl esters on the SC were hydrolyzed, and the SO3H and COOH groups were regained by 10% H2SO4regeneration, as H2SO4is a well know ester hydrolysis catalyst. This deactivation and regeneration mechanism suggests that the carbon based sulfonated catalysts can be deactivated in alcohol involved reactions, e.g. esterification, and these kinds of deactivated catalysts can be simply regenerated by dilute acid treatment.
However, the regeneration Method 1 was not effective enough though most of the catalytic activity can be recovered, so regeneration Method 2 was developed. As shown in Fig. 7, compared with the fresh SC, the regenerated SC-2 showed almost the same catalytic activity. Compared with regeneration Method 1, the regeneration Method 2 showed efficient recovery of catalytic activity but had complicated process conditions. Since the SC is produced from a low-cost raw material with a simple procedure, and can be regeneratable, it can probably compete with commercial catalysts (such as strong-acid 732 cation exchange resin) for the esterification of fatty acids into biodiesel. Further improvement on catalytic stability and simplify of regeneration Method 2 are required before this SC can be considered on an industrial scale.
4 CONCLUSIONS
A carbon based sulfonated catalyst containing SO3H, COOH, OH groups was produced by one step from low value-added RLS in a moderate condition, with a composition of (CH0.68O0.65S0.026)An. Catalytic activity for methyl oleate production was tested, and the fresh catalyst showed higher catalytic activity in esterification of oleic acid compared to traditional solid acid catalysts. The catalyst deactivated gradually after recycles usage, and the catalytic activity of the reused catalyst can be mostly regained by regeneration Method 1 and fully regained by regeneration Method 2, respectively. The deactivation and regeneration mechanisms of catalyst were proposed. Considering the somewhat low leaking degree of the S species from RLS derived sulfonated catalyst, further work on catalyst produced by direct sulfonation and carbonization of some other macromolecular polymers seems promising.
REFERENCES
1 Toda, M., Takagaki, A., Okamura, M., Kondo, J.N., Hayashi, S., Domen, K., Hara, M., “Biodiesel made with sugar catalyst”, Nature, 480, 178 (2005).
2 Lou, W.Y., Zong, M.H., Duan, Z.Q., “Efficient production of biodiesel from high free fatty acid-containing waste oils using various carbohydrate-derived solid acid catalysts”, Bioresour. Technol., 99 (18), 8752-8758 (2008).
3 Chen, G., Fang, B., “Preparation of solid acid catalyst from glucose-starch mixture for biodiesel production”, Bioresour. Technol., 102 (3), 2635-2640 (2011).
4 Shu, Q., Gao, J., Liao, Y., Wang, J., “Reaction kinetics of biodiesel synthesis from waste oil using a carbon-based solid acid catalyst”, Chin. J. Chem. Eng., 19 (1), 163-168 (2011).
5 Suganuma, S., Nakajima, K., Kitano, M., Yamaguchi, D., Kato, H., Hayashi, S., Hara, M., “Hydrolysis of cellulose by amorphous carbon bearing SO3H, COOH, and OH groups”, J. Am. Chem. Soc., 130 (38), 12787-12793 (2008).
6 Liang, X., Zeng, M., Qi, C., “One-step synthesis of carbon functionalized with sulfonic acid groups using hydrothermal carbonization”, Carbon, 48 (6), 1844-1848 (2010).
7 Zhang, W., Tao, H., Zhang, B., Ren, J., Lu, G., Wang, Y., “One-pot synthesis of carbonaceous monolith with surface sulfonic groups and its carbonization/activation”, Carbon, 49 (6), 1811-1820 (2011).
8 Shu, Q., Nawaz, Z., Liao, Y., Zhang, Q., Wang, D., Wang, J., “Synthesis of biodiesel from a model waste oil feedstock using a carbon-based solid acid catalyst: Reaction and separation”, Bioresour. Technol., 101 (3), 5374-5384 (2010).
9 Hara, M., Yoshida, T., Takagaki, A., Takata, T., Kondo, J.N., Hayashi, S., Domen, K., “A carbon material as a strong protonic acid”, Angew Chem. Int. Ed., 43 (22), 2955-2958 (2004).
10 Gan, M., Pan, D., Ma, L., Yue, E., Hong, J. “The kinetics of the esterification of free fatty acids in waste cooking oil using Fe2(SO4)3/C Catalyst”, Chin. J. Chem. Eng., 17 (1), 83-87 (2009)
11 Li, J., Fu, Y.J., Qu, X.J., Wang, W., Luo, M., Zhao, C.J., Zu, Y.G.,“Biodiesel production from yellow horn (Xanthoceras sorbifolia Bunge) seed oil using ion exchange resin as heterogeneous catalyst”, Bioresour. Technol., 108, 112-118 (2012).
12 Yee, K.F., Lee, K.T., Ceccato, R., Abdullah, A.Z., “Production of biodiesel from Jatropha curcas L. oil catalyzed by SO42- /ZrO2 catalyst: Effect of interaction between process variables”, Bioresour. Technol., 102 (5), 4285-4289 (2011).
13 Shibasaki-Kitakawa, N., Honda, H., Kuribayashi, H., Toda, T., Fukumura, T., Yonemoto, T., “Biodiesel production using anionic ion-exchange resin as heterogeneous catalyst”, Bioresour. Technol., 98 (2), 416-421 (2007).
14 Rao, B.V.S.K., Mouli, K.C., Rambabu, N., Dalai, A.K., Prasad, R.B.N., “Carbon-based solid acid catalyst from de-oiled canola meal for biodiesel production”, Catal. Commun., 14 (1), 20-26 (2011).
15 Yang, X., Wan, J., “Preparation of carbon- based solid acid catalyst and its catalytic performance”, Modern Chemical Industry, 31 (10), 34-37 (2011). (in Chinese)
16 Zhao, Y., Wan, J., “Hydrolysis saccharification of OCC by a sulfonated carbon solid-acid catalyst”, Modern Chemical Industry, 30 (9), 40-44 (2010). (in Chinese)
17 Fraile, J.M., García-Bordejé, E., Roldán, L., “Deactivation of sulfonated hydrothermal carbons in the presence of alcohols: Evidences for sulfonic esters formation”, J. Catal., 289, 73-79 (2012).
2012-11-27, accepted 2013-04-17.
* Supported by the State Key Development Program for Basic Research of China (2013CB228104, 2010CB732205), Ph. D Programs Foundation of Ministry of Education of China (20120172110011), and the National High Technology Research and Development Program of China (2012AA051801).
** To whom correspondence should be addressed. E-mail: changjie@scut.edu.cn
 Chinese Journal of Chemical Engineering2014年4期
Chinese Journal of Chemical Engineering2014年4期
- Chinese Journal of Chemical Engineering的其它文章
- PVC Membrane Selective Electrode for Determination of Cadmium(II) Ion in Chocolate Samples
- Determination and Correlation of Solubilities of Four Novel Benzothiazolium Ionic Liquids with6PF?in Six Alcohols*
- An Approach to Formulation of FNLP with Complex Piecewise Linear Membership Functions
- Impacts of Power Density on Heavy Metal Release During Ultrasonic Sludge Treatment Process*
- Determination and Correlation of Solubility for D-Xylose in Volatile Fatty Acid Solvents*
- Effects of Assistant Solvents and Mixing Intensity on the Bromination Process of Butyl Rubber*
