The Kinetics of the Esterification of Free Fatty Acids in Waste Cooking Oil Using Fe2(SO4)3/C Catalyst
GAN Mengyu (甘孟瑜), PAN Deng (潘登), MA Li (馬利)*, YUE En (岳恩) and HONG Jianbing(洪建兵)
?
The Kinetics of the Esterification of Free Fatty Acids in Waste Cooking Oil Using Fe2(SO4)3/C Catalyst
GAN Mengyu (甘孟瑜), PAN Deng (潘登), MA Li (馬利)*, YUE En (岳恩) and HONG Jianbing(洪建兵)
College of Chemistry & Chemical Engineering, Chongqing University, Chongqing 400044, China
The esterification of free fatty acids (FFA) in waste cooking oil with methanol in the presence of Fe2(SO4)3/C (ferric sulfate/active carbon) catalyst was studied. The effects of different temperature, methanol/FFA mole ratio and amount of catalyst on the conversion of FFA were investigated. The results demonstrated that under optimal esterification conditions the final acid value of the resultant system can be reduced to ~1 (mg KOH)·g-1, which met fully the requirements in post-treatment for efficient separation of glycerin and biodiesel. The kinetics of the esterification were also investigated under different temperatures. The results indicated that the rate-control step could be attributed to the surface reaction and the esterification processes can be well-depicted by the as-calculated kinetic formula in the range of the experimental conditions.
biodiesel, kinetics, esterification, free fatty acids, waste cooking oil
1 INTRODUCTION
Biodiesel consists of long-chain fatty acid methyl ethers (FAME), which can be obtained from renewable feedstock, such as vegetable oils or animal fats. Compared to fossil-based diesel fuels, it possesses many advantages,.. cleaner engine emissions, biodegradable, renewable and superior lubricating property [1], which makes it an excellent substitute or additive to conventional diesel fuels.
However, biodiesel is not yet commercialized all over the world because of the high cost of the raw materials (fresh vegetable oil), resulting in high price of the commercial products in contrast to the petroleum diesel fuel. Waste cooking oil (WCO) is a promising alternative to vegetable oils due to its low price as raw material [2]. In China, WCO is regulated and collected by the specialized agencies in major cities, and it can be considered as a vast resource to mass production of biodiesel [3].
The transesterification method by using alkali or acid as catalyst is the most wildly used technique for producing biodiesel. Currently the alkali catalytic route has received much more attention because of the shorter reaction time and lower cost of catalyst as compared with acid catalytic route. However, the alkali catalyzed route is greatly limited for its high sensitivity to both water and free fatty acids (FFA) in the raw materials. During the process, FFA can react with the alkali catalyst to release soap and water, whilst it does not react with acid catalyst in the acid catalytic route. It has been reported that FFA in the oil or fat used in alkaline transesterification reactions should be restricted down to ~0.5% (by mass), equivalent to ~1 (mg KOH)·g-1[4-6]. Otherwise, the saponification would not only hinder separation of ester from glycerin, but also leads to the low yield as well as the formation rate of FAME. As for WCO with a high concentration of FFA and water, it is not suitable to adopt alkali route for production of biodiesel.
Therefore, a combined acid-alkali two-step catalytic route [7, 8] was developed to synthesize biodiesel from WCO. In the first step the FFA is generally esterified with methanol under sulfuric acid catalysis. When the FFA concentration decreases to less than 0.5% (by mass), sulfuric acid is then removed by phase separation after the system at rest for approx. 30 min. Subsequently the alkali is introduced into the system to further complete the transesterification. However, this route still has some drawbacks: difficult recovery of acid catalyst, corrosiveness of sulfuric acid, and high cost of equipments required for the reaction system [3]. Tremendous efforts have been devoted to remove these disadvantages, and some studies [9-12] suggest that solid acid served as catalyst is turned out to be a good alternative to sulfuric acid in the esterification reaction. Wang. [3] found that a preferred catalytic performance can be achieved by using ferric sulfate substituted for sulfuric acid in the esterification reaction, but the small ferric sulfate particles tended to adhere to the oil, making it difficult to separate from the oil after the reaction for reuse.
In this work the Fe2(SO4)3/C (ferric sulfate/active carbon) was employed as a solid acid catalyst to pre-esterify the FFA in the WCO. By this way the reaction system can be decolored (pigments-adsorption by active carbon), and unlike liquid acid catalyst, solid acid catalyst can be easily separated for reuse. This solid acid route is evidenced to be simply and much versatile for esterification reactions. The kinetics of esterification of FFA in WCO are also investigated using Fe2(SO4)3/C as catalyst.
2 EXPERIMENTAL
2.1 Raw materials
Samples of the WCO were provided by typical Chinese restaurants and filtered to remove impurities and it is mainly consisted of triglycerides (TG) and FFA. FFA acid value of the samples is averaged at 40.06 (mg KOH)·g-1(FFA concentration is approx. 20%, by mass). Samples of active carbon were provided by Chongqing Zhongshan Active Carbon Company.
2.2 Catalyst preparation
Active carbon used as a support in the experiments was pretreated by thorough soaking in saturated ferric sulfate solution at 313.15 K for 24 h, and the resulted slurry was then dried under a temperature of 393.15 K in the thermostatic air-blower-driven drying closet for more than 2 h, the Fe2(SO4)3/C catalyst is then obtained. Before carrying out the reaction, the as-prepared catalysts were pre-activated for over 1 h at a temperature of 393.15 K in the thermostatic air-blower-driven drying closet, and it was grounded to 6.5-19.5 mm (2-6 mesh). Ferric sulfate loaded in the catalyst samples studied in this work was adjusted to about 26.8% (By mass, based on the catalyst mass).
2.3 Characterization of the catalyst
The nitrogen adsorption/desorption isotherms were measured with a porosimeter (ASAP 2010, American Micrometrics) to determine the BET surface area and pore volume.
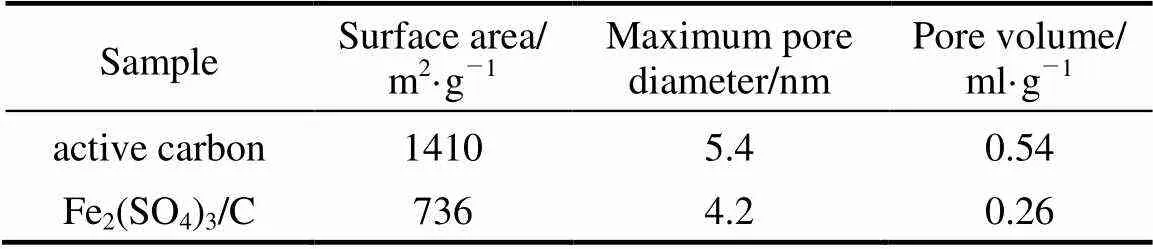
Table 1 Typical physicochemical properties of original materials and samples as-prepared
2.4 Esterification of FFA with methanol
Reactions were performed in 600 ml stainless steel batch reactor (maximum pressure of 68.95 MPa) and equipped with sample withdrawal and heating systems.Esterification was undertaken at the boiling point of methanol under different pressures, with fixed amount of WCO (31.4 g) and different amount of methanol and catalyst. Because of intensive reflux of solvent, the catalyst was fully suspended by vapor bubbles and thus the attrition of active carbon was avoided. The conversion of FFA in the WCO was measured by [3]

whereOLandWCOrefer to the acid value (AV) of oil layer and WCO, respectively. The effects of catalytic activity, catalyst concentrations, molar methanol/FFA ratio and temperature on the conversion were detailed in this work.
3 RESULTS AND DISCUSSION
The esterification of FFA with methanol follows the reaction:

which is catalyzed by solid acid. In this work, R1represents an unsaturated linear chain of 11-17 carbon atoms which varies as the raw materials resource.
3.1 Catalytic activity of Fe2(SO4)3/C
As shown in Fig. 1 the esterification reaction hardly occurred in the absence of catalyst, which was in good accordance with the published results [8]. From Fig. 1 it can also be seen that the conversion maximized at 4 h when catalyzed by sulphuric acid (98%) or Fe2(SO4)3/C (ferric sulfate/active carbon). Its conversion reached to 96% for Fe2(SO4)3/C, 6% higher than that of sulphuric acid. Moreover, the application of Fe2(SO4)3/C solid acid catalyst brought much convenience for recycling and attained a much longer duration than liquid counterparts.

Figure 1 Catalytic activity of Fe2(SO4)3/C solid catalyst and sulphuric acid
(Reaction temperature 368.15 K, mass amount of catalyst 5.5%, methanol/FFA mole ratio 15︰1)
■?non-catalyzed;▲?H2SO4catalyzed;●?Fe2(SO4)3/C catalyzed
3.2 Effects of amount of catalyst on FFA conversion
The mass percent of catalyst used in the reaction varied from 0 to 7.5% based on WCO. It was observed that the esterification reaction seldom took place without catalyst. When 1% of catalyst added, 85.9% of FFA was converted into FAME in 3 h, and the conversion increased with the increments of catalyst, it reached up to as much as 98% when the mass percent of catalyst rose to 3.5%, which was chose as an optimal parameter from the Fig. 2.
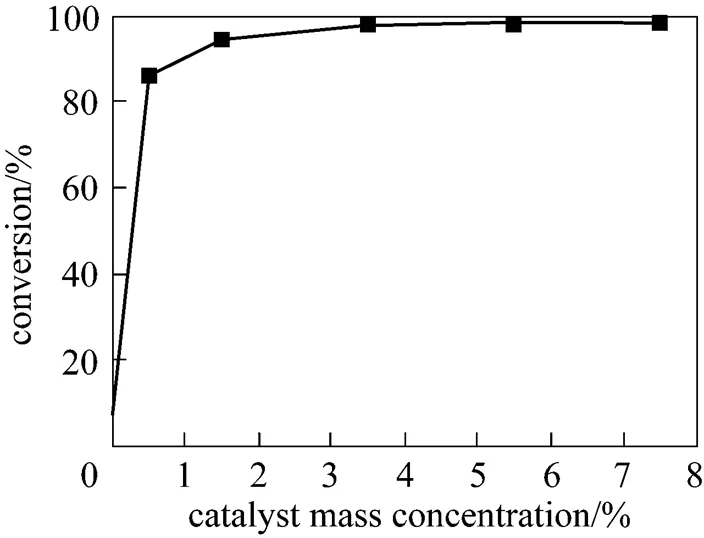
Figure 2 Conversion of FFA. amount of catalyst
(Reaction temperature 368.15 K, reaction time 3 h, methanol/FFA mole ratio 18︰1)
3.3 Effect of methanol/FFA molar ratio on FFA conversion
An excess of methanol was favorable to the esterification of FFA since it can speed the reaction [2]. Fig. 3 showed the effects of methanol/FFA molar ratio on the FFA conversion. The conversion increased with increasing mole ratio. With regard to costs and reactor size as well as the efficient reaction, mole ratio at 18︰1 was adopted for further study.

Figure 3 Conversion of FFA. methanol/FFA molar ratio
(Reaction temperature 368.15 K, reaction time 3 h, mass concentration of catalyst 3.5%)
methanol/FFA molar ratio:■?9︰1;◆?12︰1;▲?15︰1;▼?18︰1;◆?21︰1
3.4 Effect of reaction temperature on FFA conversion
Reaction temperature is one factor that can influence the esterification rate, thus conversion [9]. Fig. 4 indicated the effects of temperature, ranging form 338.15 K to 368.15 K, on FFA conversion, and demonstrated that the reaction rate (conversion) could be rapidly sped up by rising the temperature: the conversion increased up to 98% when esterified at 368.15 K, with final acid value of the resultant product reduced to ~1 (mg KOH)·g-1.
3.5 Reuse of catalyst
The solid catalysts have to be reused for the purpose of process economy. In the present case, catalyst was reused several times following a series of 3 h runs (mass concentration of catalyst 3.5%, methanol/FFA mole ratio 18︰1 and reaction temperature 368.15 K). After each run, the whole reaction mixture and catalyst were centrifuged and separated. The catalyst showed a marked loss of activity with the first recycling, and thereafter a slower gradual loss (Fig. 5). With the increment of the response times, ferric sulfate was desorbed gradually from the surface of active carbon, leading to decrease of catalyst activity.

Figure 4 Conversion of FFA. reaction temperature
(Reaction time 3 h, mass concentration of catalyst 3.5%, methanol/FFA mole ratio 18︰1)
■?338.15 K; ●?348.15 K;▲?358.15 K;▼?368.15 K

Figure 5 Conversion of FFA. reuse number of catalyst
(Reaction time 3 h, amount of catalyst 3.5%, methanol/FFA mole ratio 18︰1, reaction temperature 368.15 K)
3.6 Kinetic model
The kinetics of esterification were established based on the Langmuir-Hinshelwood (LH) mechanism. It mainly consisted of 5 elementary reactions [13-16] (denoting an active site):
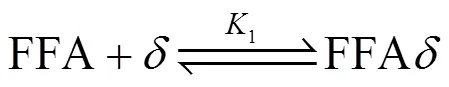




The kinetic model was built on the following assumptions:
(1) The rate of the non-catalyzed reactions can be neglected compared to the catalyzed ones;
(2) The sites at the catalyst surface are the same;
(3) The surface reaction (5) was the rate-control step;
(4) The adsorption and desorption of reactants and products were fast and at equilibrium.
Rate equation in the rate-control step could be depicted as follows:





whereandare the methanol/FFA molar ratio and the conversion of FFA, respectively. For this reason, Eq. (8) reduces to

with






The relationship of the initial rates and methanol/ FFA molar ratios was illustrated in Fig. 6. The esterification was conducted for 3 h with a catalyst mass concentration of 3.5% at a temperature of 368.15 K. The experimental data shown in Fig. 6 is in good accordance with Eq. (16).

Figure 6 Initial rate. methanol/FFA molar ratio
(Reaction temperature 368.15 K, reaction time 3 h, amount of catalyst 3.5%)

Table 2 The kinetic parameters for esterification
The calculated results for the kinetic parameters were summarized in Table 2. It is indicated that the value ofwas approx. 44, which implied that the adsorption capacity of FFA on catalyst surface was approx. 44 times higher than that of methanol.
The influence of temperature on the reaction rate was determined by fitting+and-to the Arrhenius equation:




Figure 7 Effects of temperature on the reaction rate (Arrhenius equation)
■?ln+;●?ln-

Table 3 Energies of activation and frequency factors for the esterification of FFA
Figure 8 displayed a comparison between the conversion of FFA obtained in the experiments and that calculated out from Eq. (11). It demonstrated that the proposed kinetic model was favorably consistent with the experiment results.

Figure 8 Comparison of the experimental conversion and theoretical conversion of FFA
(Reaction temperature 368.15 K, reaction time 3 h, amount of catalyst 3.5%)
△?338.15 K;□?348.15 K;▲?358.15 K;■?368.15 K
4 CONCLUSIONS
Free fatty acids (FFA) in waste cooking oil (WCO) can be effectively eliminated by esterification with methanol, using a 3.5% Fe2(SO4)3/C (ferric sulfate/ active carbon) relative to WCO, optimal parameters (.. methanol/FFA mole ratio: 18︰1, temperature: 368.15 K, catalyst mass concentration: 3.5%) were summed up by experimental data. The Fe2(SO4)3/C catalyst performed much favorable results in contrast to conventional catalysts (.. sulphuric acids). And the kinetic studies of the esterification of FFA were completed as well under reasonable assumptions, which further evidenced to be reliable by the actual results.
1 Alcantara, R., Amores, J., Canoira, L., Fidalgo, E., Franco, M.J., Navarro, A., “Catalytic production of biodiesel from soy-bean oil, used frying oil and tallow”,, 18, 515-527 (2000).
2 Titipong, I., Mangesh, G.K., Ajay, K.D., Narendra, N.B., “Production of biodiesel from waste fryer grease using mixed methanol/ethanol system”,.., 88 (5), 429-436 (2007).
3 Wang, Y., Ou, S.Y., Liu, Z.P., Xue, F., Tang, S.Z., “Comparison of two different processes to synthesize biodiesel by waste cooking oil”,...., 252 (1/2), 107-112 (2006).
4 Freedman, B., Pryde, E.H., Mounts, T.L., “Variables affecting the yields of fatty esters from transesterified vegetable oils”,...., 61, 1638-1643 (1984).
5 Liu, K., “Preparation of fatty acid methyl esters for gas-chromatographic analysis of lipids in biological materials”,...., 71, 1179-1187 (1994).
6 Chongkhong, S., Tongurai, C., Chetpattananondh, P., Bunyakan, C., “Biodiesel production by esterification of palm fatty acid distillate”,, 31 (8), 563-568 (2007).
7 Zhang, Y., Dubé, M.A., McLean, D.D., Kates, M., “Biodiesel production from waste cooking oil (1) Process design and technological assessment”,., 89 (1), 1-16 (2003).
8 Berrios, M., Siles, J., Martin, M.A., Martin, A., “A kinetic study of the esterification of free fatty acids (FFA) in sunflower oil”,, 86 (15), 2383-2388 (2007).
9 Liu, Y.J., Edgar, L., Goodwin, J.G., “Effect of carbon chain length on esterification of carboxylic acids with methanol using acid catalysis”,..., 243 (2), 221-228 (2006).
10 Parida, K.M., Mallick, S., “Silicotungstic acid supported zirconia: An effective catalyst for esterification reaction”,...., 275 (1/2), 77-83 (2006).
11 Juan, J.C., Zhang, J.C., Yarmo, M.A., “12-Tungstophosphoric acid supported on MCM-41 for esterification of fatty acid under solvent-free condition”,...., 267 (1/2), 265-271 (2007).
12 Peters, T.A., Benes, N.E., Holmen, A., Keurentjes, J.T.F., “Comparison of commercial solid acid catalysts for the esterification of acetic acid with butanol”,.., 297 (2), 182-188 (2006).
13 Dossin, T.F., Reyniers, M.F, Marin, G.B., “Kinetics of heterogeneously MgO-catalyzed transesterification”,..., 61 (1/2), 35-45 (2006).
14 Mantri, K., Nakamura, R., “Esterification of long chain aliphatic acids with long chain alcohols catalyzed by multi-valent metal salts”,.., 34 (11), 1502-1503 (2005).
15 Santos, A.M.B., Martinez, M., Mira, J.A., “Comparison study of lewis acid type catalysts on the esterification of octanoic acid and-octyl alcohol”,..., 19 (6), 538-542 (1996).
16 Kuriacose, J.C., “Esterification of alcohols with acetic acid over ferric oxide”,...., 9 (4), 641-650 (1977).
2008-04-29,
2008-09-16.
* To whom correspondence should be addressed. E-mail: mlsys607@126.com
 Chinese Journal of Chemical Engineering2009年1期
Chinese Journal of Chemical Engineering2009年1期
- Chinese Journal of Chemical Engineering的其它文章
- An Improved Fuzzy Predictive Control Algorithm and Its Application to an Industrial CSTR Process*
- Preparation, Characterization and Catalytic Behavior of 12-Molybdophosphoric Acid Encapsulated in the Supercage of Cs+-exchanged Y Zeolite*
- Purification of Sulfuric and Hydriodic Acids Phases in the Iodine-sulfur Process*
- Synthesis and Characterization of Novel Temperature and pH Responsive Hydroxylpropyl Cellulose-based Graft Copolymers
- The Separation of Catechol from Carbofuran Phenol by Extractive Distillation*
- Electrochemical Performance of Nickel Hydroxide/Activated Carbon Supercapacitors Using a Modified Polyvinyl Alcohol Based Alkaline Polymer Electrolyte*
