Research advances in immunotherapy combined with DNA damage response inhibitors for liver cancer therapy
Chen Yang, Tao Tao, Yijie Wu, Weijie Wang, Song-Bai Liu
1College of Life Science, North China University of Science and Technology, Tangshan 063210, Hebei, China.
2Jiangsu Province Engineering Research Center of Molecular Target Therapy and Companion Diagnostics in Oncology, Suzhou Vocational Health College, Suzhou 215009, Jiangsu, China.
3Department of Respiratory and Critical Medicine, the Affiliated Infectious Diseases Hospital of Soochow University, Suzhou 215100, Jiangsu, China.
Abstract Cancer immunotherapy utilizes checkpoint inhibitors to amplify the antitumor immune response.The DNA damage repair system plays a crucial role in preserving genome stability, offering therapeutic avenues for tumor treatment by impeding the DNA damage repair pathway.In cancer immunotherapy, checkpoint inhibitors are used to enhance the antitumor immune response.The DNA damage response (DDR) and immune response are inseparably intertwined and have the potential for synergy.Various recent studies have demonstrated that DDR inhibitors (DDRis) can substantially enhance the clinical efficacy of immunotherapy for hepatocellular carcinoma(HCC) by enhancing the immune response of cancer cells.This article primarily describes the immunotherapy of HCC and compiles the research findings and advancements in clinical applications of combined immunotherapy with DDRis.This study provides insight into the application of the DDR pathway in immunotherapy and provides invaluable insights for the clinical advancement of precise and efficacious treatment strategies.
Keywords: Immunotherapy, DNA damage repair, hepatocellular carcinoma
INTRODUCTION
Liver cancer is among the top five most fatal cancers globally; its incidence is increasing annually[1], and it often presents alongside symptoms such as cirrhosis and inflammation[2].Acting as a vital filter in the body’s metabolic processes[3], the liver endures daily oxidative DNA damage, prompting the activation of specific populations of immunosuppressive cells[4]that foster disease progression.The unique anatomical location,intricate organization, and distinct metabolic and immunosuppressive milieu of the liver pave the way for frequent infiltration of metastatic cancers[5], particularly from organs such as the colon.
Given the absence of efficient diagnostic measures, patients with hepatocellular carcinoma (HCC) are typically diagnosed at an intermediate to advanced stage, resulting in a diminished likelihood of surgical resection, heightened postoperative recurrence rates[6], a grim prognosis, and a fundamental loss of opportunities for curative intervention[7].Currently, factors such as obesity, type 2 diabetes mellitus(T2DM), and nonalcoholic fatty liver disease (NAFLD)[8]are supplanting traditional culprits such as viruses and alcohol as principal instigators of HCC.The burden of HCC, the predominant variant of liver cancer,continues to increase steadily[9].
Since the inception of the new century, the fields of surgery, radiotherapy, chemotherapy, molecular targeted therapy, and immunotherapy[10]have experienced significant advancements as quintessential modalities for combating various malignancies.These sophisticated interventions not only arrest but also exterminate neoplastic cells[11], thereby affording a greater number of cancer patients a fighting chance.Timely and precise disease staging is a pivotal determinant in tailoring individualized therapeutic strategies and augmenting patient outcomes.Early diagnosis and accurate staging are essential for determining appropriate treatment options and improving patient prognosis.The Barcelona Clinic Liver Cancer(BCLC)[12]staging system, which categorizes patients with HCC into Stage 0, Stage A, Stage B, Stage C, and Stage D, is currently the most clinically used staging system for HCC worldwide.
Immunotherapy for HCC relies on immune checkpoint inhibitors[13], such as programmed cell death protein 1 (PD-1) inhibitors, programmed cell death 1 ligand 1 (PD-L1) inhibitors, and cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) inhibitors, which promote the antitumor effects of killer immune cells and effectively prolong the survival time of HCC patients, making them the most promising novel therapies in the field of oncology[14].Single-immune drug therapy has limitations in the clinic, so immunotherapies involving immune checkpoint inhibitors (ICIs) in combination with other therapeutic means have been developed.Immunocombination therapies have demonstrated significant efficacy in the treatment of HCC[2]and are rapidly reshaping the landscape of clinical oncology.DNA damage and repair have a significant impact on the interactions between tumors and the immune system[15], and coadministration of these two agents with immunotherapies has the potential to improve the efficacy of HCC treatment.
In this review, we first introduce the current status of immunotherapy for HCC and the therapeutic efficacy of immune checkpoint blockade (ICB)[16]therapy for HCC.Subsequently, we introduce the DDR system[17,18]and the current status of DDRis[19]in liver cancer research, and DDR-related genes can be used as biomarkers for liver cancer immunotherapy[20].Finally, we discuss the rational combination of DDRis in HCC therapy to reveal new options for the application of immunotherapy in HCC, maximize the role of immunotherapy in DNA damage repair mechanisms, and provide ideas and guidance for immunotherapy in HCC.
RESEARCH STATUS OF LIVER CANCER TREATMENT
For the initial and intermediate phases of HCC, surgical excision is the paramount therapeutic modality[21,22], and liver transplantation for liver cancer patients who meet the criteria for liver transplantation can significantly improve the cure rate.Conversely, for patients who present with advancedstage HCC devoid of surgical prospects, therapeutic options include transarterial chemoembolization(TACE), hepatic artery infusion chemotherapy (HAIC), focal ablation[23], and radiotherapy, along with pertinent interventions involving targeted pharmaceutical agents and immune checkpoint inhibitors[24].Studies have shown that the application of targeted drugs and immune checkpoint inhibitors[25]can effectively prolong the life of patients with advanced HCC[26], with instances of complete tumor regression observed following treatment.
TACE for HCC treatment[27]deploys an embolic agent to occlude the tumor artery in conjunction with antineoplastic medications, inducing ischemic and hypoxic conditions in the tumor tissue and eliciting cytotoxic effects to accomplish the objective of managing HCC.According to the START-FIT[28], the use of avelumab, a PD-L1 inhibitor, subsequent to TACE and stereotactic body radiation therapy for locally advanced unresectable HCC, has been shown to be effective.HAIC for HCC[29]implemented 5-fluorouracil and oxaliplatin-based chemotherapeutic protocols for localized HCC perfusion chemotherapy, leading to a significant enhancement in the tumor response rate and patient survival while mitigating the hematologic suppression and gastrointestinal reaction side effects associated with systemic chemotherapy.The combination of TACE with HAIC (TACE-HAIC)[30], leveraging the synergistic attributes of both modalities,has emerged as one of the most efficacious approaches in liver cancer treatment, representing a notable translational therapy for local ablation strategies in managing HCC[31,32].This therapeutic technique directly destroys tumors using physical or chemical methods under imaging guidance, and it is another therapeutic technique with the potential for a local cure for HCC after surgical resection.
CURRENT STATUS OF LIVER CANCER IMMUNOTHERAPY
In addition, radiotherapy for HCC[33]destroys DNA double strands in cancer cells by direct or indirect action of rays, inhibits cell proliferation, and kills tumors completely, and precision radiotherapy with high precision and a low damage dose can significantly improve the local control rate of the tumor.The 3-year local control and overall survival rates for 185 patients with HCC less than 5 cm in diameter[34]treated with stereotactic radiotherapy were 91% and 70%, respectively.The hepatobiliary surgery team of the Cancer Hospital of the Chinese Academy of Medical Sciences[35]reported that the new treatment modality of preoperative neoadjuvant radiotherapy combined with surgery could increase the 5-year survival rate of patients with centralized HCC from 37.2% to 69.1%, which was similar to that of radical surgery for small HCC.In addition, targeted drug therapy[36]for liver cancer is an important component of systemic therapy.The approved first-line therapeutic targeted drugs[37]for liver cancer treatment include sorafenib and lenvatinib, and the second-line therapies are regorafenib, cabozantinib, and nemorubicin.The precision of targeted medications in managing advanced HCC[38]demonstrates superior therapeutic effectiveness and notable patient survival advantages.
From a clinical perspective, the limited efficacy of monoclonal antidrugs in prolonging patient survival[39]is because the liver has an immunosuppressive microenvironment.The progression of HCC is intricately linked to the disturbance of the microenvironment established by hepatocytes and immune cells, along with an imbalance in the immune-inflammatory response within the microenvironment, rendering it to external influences and tolerance development.In addition, the enrichment of tumor immune-associated macrophages (TAMs) and depletion of tumor-infiltrating lymphocytes (TILs)[40,41]in the liver contribute to the weakening of adaptive immune responses in HCC patients, and immune cells, such as regulatory T cells(Tregs), myeloid-derived suppressor cells (MDSCs), and some cytokines, can cause immunosuppression and tumor escape[42], which in turn promotes HCC development and progression, and the depletion of cytotoxic T cells also leads to impaired immune system function to clear malignant cells.Therefore,improving the function of the patient’s immune system through immunotherapy[43]can aid in the treatment of unresectable advanced HCC.Unlike in chemotherapy and targeted therapy, immune cells are targeted to enhance the antitumor immune response, which in turn enhances the body’s own immune response and reactivates immune cells to inhibit tumor development and progression and effectively prolong the survival time of patients, which is important for the systemic treatment of HCC[38,44].
With the successive approval of several immunotherapeutic agents[45]for unresectable HCC, breakthroughs in immunotherapy have revolutionized the treatment paradigm for advanced HCC.PD-1 and PD-L1 checkpoint blockade therapy has been the focus of standard treatment[46]for a variety of malignant tumors[47], and newly identified HCC tissues with CD3-CD49a+CD56+ natural killer (NK) cells express PD-1 on the surface in large numbers[48], which is associated with reduced survival in patients with HCC[49].PD-1 or PD-L1 inhibitors[50]Navulizumab, pembrolizumab, carrelizumab, durvalizumab, and other single agents have been tested in several patient-based clinical trials worldwide, and these agents have shown improved efficacy in second-line regimens[51]for the treatment of patients with HCC who have progressed or are intolerant to prior sorafenib therapy.CTLA-4 belongs to the immune checkpoint signaling pathway[14]and is an inhibitory immune checkpoint expressed on activated T cells.Anti-CTLA-4 therapy increases the abundance of CD4+ and CD8+ T cells and decreases peripheral T-cell clonality in HCC patients.The efficacy and safety of the monoclonal antibody tremelimumab[52,53]in treating HCC have been supported by positive results from clinical studies.
CURRENT STATUS OF IMMUNOLOGIC COMBINATION THERAPY FOR HCC
In clinical treatment, the efficacy of targeted drugs or immune checkpoint inhibitors alone has plateaued,and according to the revised RECIST (version 1.1)[54], the objective response rates (ORRs) of clinically applied monotherapies are all in the range of 15%-20%, which is not yet sufficient to produce meaningful surgical conversion rates; moreover, the ORR of multiple immune combination regimens can reach more than 40%.Various combinations of immune-combination regimens[2]exist, such as immune-combination targeted therapy, immune-combination immunotherapy, immune-combination chemotherapy, and immune-combination local therapy.
Currently, targeted combined immunotherapy is the most common clinical trial protocol, and new breakthroughs in HCC treatment are being made via ICI-based combination therapy modalities.Immunotherapy combined with tyrosine kinase inhibitors (TKIs)[55]is widely used in the clinic, and several tyrosine kinase inhibitors that inhibit blood vessel growth in HCC patients, such as sorafenib and lenvatinib, have been recommended as first-line therapeutic agents for advanced HCC.The results of the phase III clinical trial of karelizumab in combination with the apatinib regimen revealed that, compared with sorafenib alone, karelizumab in combination with the apatinib regimen[56]significantly prolonged the median OS in patients with unresectable or metastatic HCC (22.1vs.15.2 months) and reduced the risk of death by 38%.Multitargeted inhibitors in combination with immune checkpoint inhibitors could be a highly effective and low-toxicity new therapeutic option for patients with refractory tumors to achieve remission or control.
Dual-immunity combination therapy has become a commonly used therapeutic regimen, and combinations of CTLA-4 and PD-L1 inhibitors have shown additive antitumor activity associated with complementary immunostimulatory effects; blockade of the CTLA-4, PD-1, and PD-L1 pathways[5,57]restored the antitumor activity of cytotoxic T lymphocytes (CTLs) in the immune microenvironment of tumors.HIMALAYA STUDY In[52], the STRIDE regimen used a single high-dose Imjudo (tremelimumab) (a CTLA-1 monoclonal antibody) in combination with a regular dose of Imfinzi (durvalumab) (a PD-L1 monoclonal antibody).Tremelimumab and durvalumab are dual immune checkpoint inhibitor therapies used in a phase II clinical study of uHCC[58]that similarly delivered a more efficacious second-line treatment option for patients with advanced HCC, with a 50% improvement in three-year survival compared to the sorafenib monotherapy arm and a 30% reduction in the incidence of grade 3-4 treatment-related adverse events (TRAEs) compared to the control arm, without an increase in serious liver injury or bleeding events, making this combination the first innovative dual-immunity combination therapy approved globally as the first-line treatment for advanced HCC[59].
The CheckMate 040 study[60]evaluated the safety and efficacy of the combination of ipilimumab, a CTLA-4 antibody, and nivolumab, a PD-1 antibody, in patients with advanced HCC treated with sorafenib.It has significant efficacy and a manageable safety profile in advanced HCC patients[61].The results showed a 32%objective remission rate and a median patient survival of 23 months with the combination therapy.Overall,dual-immunization combination therapy was well tolerated in the population, with 37% of patients experiencing grade 3-4 treatment-related adverse events, most commonly itching and rash, and 5% of patients discontinuing treatment due to grade 3-4 adverse events.Based on the results of this study[62], the U.S.FDA granted expedited approval in March 2020 for the use of ipilimumab and nivolumab for the treatment of patients with advanced HCC previously treated with sorafenib.
The IMbrave150 study[63]showed that the “T+A” regimen of atilizumab and bevacizumab demonstrated outstanding efficacy and safety in patients with unresectable HCC.Compared with bevacizumab alone,overall survival was significantly prolonged, the risk of death was reduced by 42%, one-year survival was improved by 12.6%, and progression-free survival was increased by 1.58 times.The “T+A” regimen increases tumor infiltration by CD8+ T cells and exposes cancer cells to more neoantigens, which significantly improves the objective remission rate of treatment.The “A+T” combination[64]has been shown to be effective as a first-line treatment for advanced HCC, significantly prolonging the survival of HCC patients.
Based on breakthroughs in the efficacy of targeted immunotherapy and immunotherapy in advanced uHCC, Xinet al.from Peking Union Medical College in China further explored the clinical efficacy and safety of these triple combinations[65].This study enrolled patients with uHCC who received triple therapy with lenvatinib, PD-1 inhibitors, and TACE or dual therapy with lenvatinib and PD-1 inhibitors and compared the clinical benefit of triple therapy[65]vs.dual therapy for patients with uHCC.Efficacy was assessed by survival and treatment response, and tolerability was evaluated according to the incidence and severity of critical adverse events (AEs).In particular, overall survival (OS) and progression-free survival(PFS) were significantly better in patients receiving the triple combination of lenvatinib, PD-1 inhibitors,and TACE (L-P-T group) than in patients receiving the dual combination of lenvatinib and PD-1 inhibitors(L-P group).The ORR and disease control rate (DCR) were also significantly greater in the L-P group.The triple combination of lenvatinib, PD-1 inhibitors, and TACE may provide greater survival benefits than the dual therapy of lenvatinib and PD-1 inhibitors.
In a study of radiotherapy combined with immunotherapy for advanced HCC[38], HAIC combined with PD-1 inhibitors alongside Lenvatinib[66]achieved an objective remission rate of 96.0% in a relatively short period of time[16], and the surgical conversion rate reached 60.0%, which is a new option for the potential treatment of advanced HCC[67].In addition, local therapy has fewer systemic toxic side effects on organs,and local therapy combined with immunotherapy is expected to improve local immunity while ensuring safety.As a PD-1 inhibitor with a unique structure[68], tirilizumab has become an important choice in combination with an immunologic local therapy regimen, and tirilizumab in combination with lenvatinib,HAIC, and TAE for the treatment of patients[69]with uHCC with portal vein cancer thrombosis (PVTT)[67]has shown an ORR of 60%, which improves the remission rate of patients with intermediate-stage and advanced-stage HCC.
Immunotherapy combined with targeted therapy is a breakthrough combination that offers more definitive and significant efficacy than immunologic monotherapy regimens.Treatment has been available for more than 3 years, and according to the study of immunotherapy in combination with immunotherapy for the clinical treatment of advanced liver cancer, the immunologic ORR of liver cancer is still very limited, and liver cancer patients who can benefit from immunotherapy only account for 1/3 of all liver cancer patients[5].With the continuous development of basic research on liver cancer treatment, additional innovative drugs with new targets and immunotherapies should be applied in clinical treatment to improve the treatment efficacy in liver cancer patients and to overcome the therapeutic challenge of liver cancer treatment.The combination therapies described in the previous section are shown in Table 1.
SYNERGISTIC EFFECTS OF DDRIS AND IMMUNOTHERAPY IN LIVER CANCER
Genomic instability is one of the most common features of tumor cell development, and tumorigenesis originates from aberrant damage to the DNA sequence, which can occur as DNA single-strand breaks, base adducts, base mismatches, DNA double-strand breaks (DSBs), replication fork stalls, or interstrand crosslinks2[82].To maintain the structural integrity of the genome, DNA damage-responsive repair systems are initiated at the same time as DNA damage occurs in the cell[17].Currently, the main types of DNA repair in eukaryotes[18]are nucleotide excision repair (NER), base excision repair (BER), mismatch repair (MMR),single-strand break repair (SSBR), and double-strand break repair (DSBR).NER removes large segments of DNA damage, BER repairs single-base damage, MMR is used to repair base mismatches, and SSBR repairs single-strand breaks.DSBR consists of three mechanisms: nonhomologous end joining (NHEJ),homologous recombination (HR), and microhomology-mediated end joining (MMEJ).The key genes in the five major pathways are shown in Table 2.
Different susceptibility variants associated with different risk factors may occur in different populations[19].The presence of common mutations in the homologous recombination repair and mismatch repair(HRR-MMR) or HRR and base excision repair (HRR-BER) pathways in the DDR pathway has been associated with high tumor mutational burden (TMB), neoantigen load (NAL), and immune regulatory gene expression[88,89], and mismatch repair defects (MMR-D), homologous recombination mutations, and repair gene mutations in the pole mutation pathway are associated with increased expression of the cytotoxicity-related genes PD-1 and PD-L1 as well as elevated NAL, CD4+ and CD8+ TILs[90].DDRassociated genes are potential predictors of treatment with ICIs.
Linet al.divided liver cancer patients into two groups, the DDR high-expression subgroup and the DDR low-expression subgroup, according to the expression level of DDR-related genes.After receiving ICI-type treatment, DDR-high patients who received immunotherapy had a significantly longer survival time than DDR-low patients.In addition, the proportion of activated immune cells was significantly greater in patients with the DDR high-expressing subtype than in those with the other two subtypes in terms of the proportion of immune cells, the level of expression of immune-related genes, the immune status, the enrichment of specific pathways, and inflammatory factors and proteins that have other immune functions(antigen presentation and other functions), all of which also play key roles in response to immunotherapy.
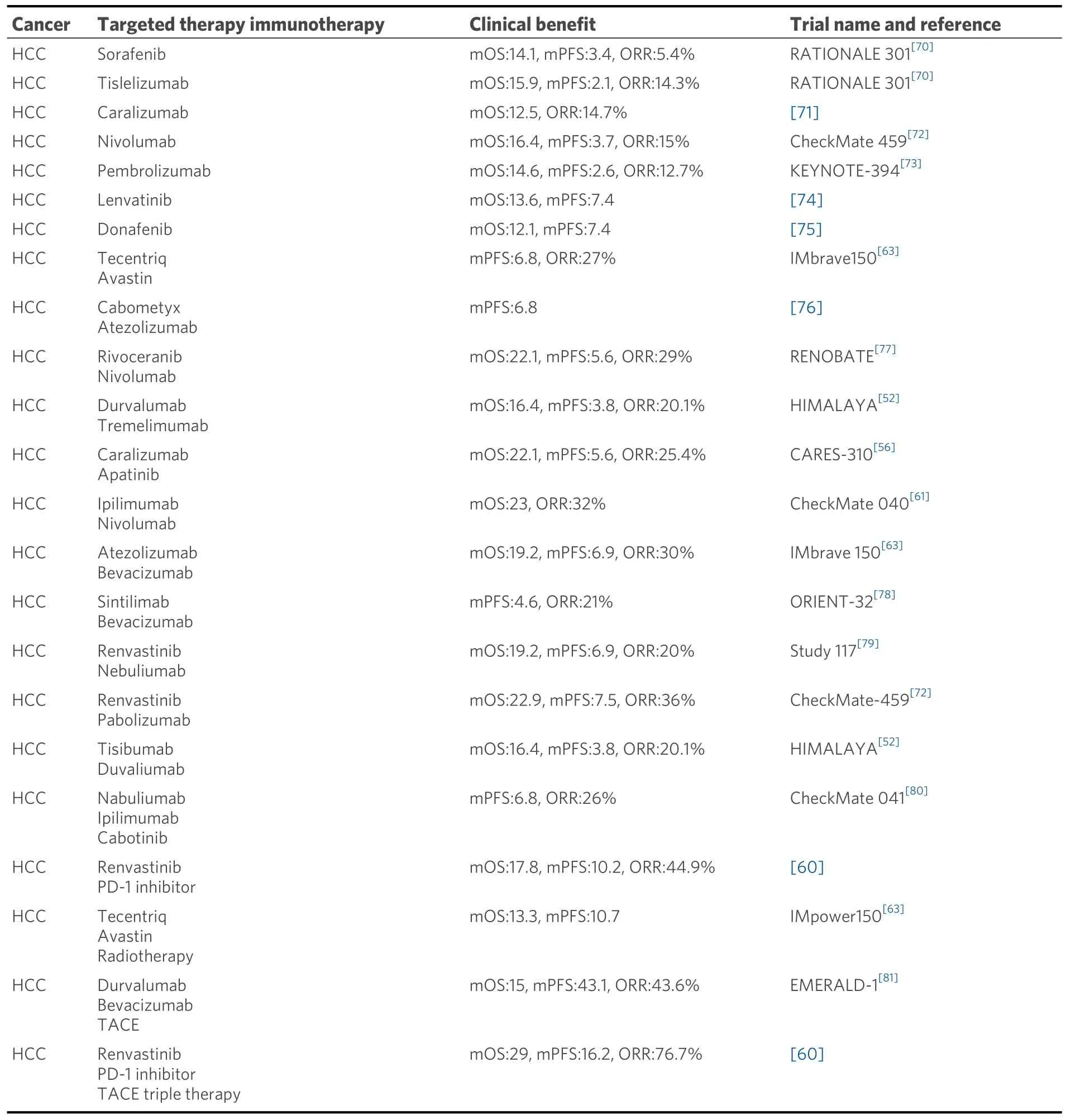
Table 1.Clinical benefit of different combination therapies
High expression of immune checkpoint molecules is associated with superior immunotherapeutic efficacy,and analysis of the immune microenvironment by different DDR types is important.The immune microenvironment is one of the key factors influencing the therapeutic efficacy and clinical benefits of ICIs,and characterization of DDR subtypes can aid in the development of more effective therapeutic targets and biomarkers for immunotherapy in HCC patients[91].In liver cancer treatment, DDR-related genes may be new and more effective biomarkers for screening target populations for ICIs.
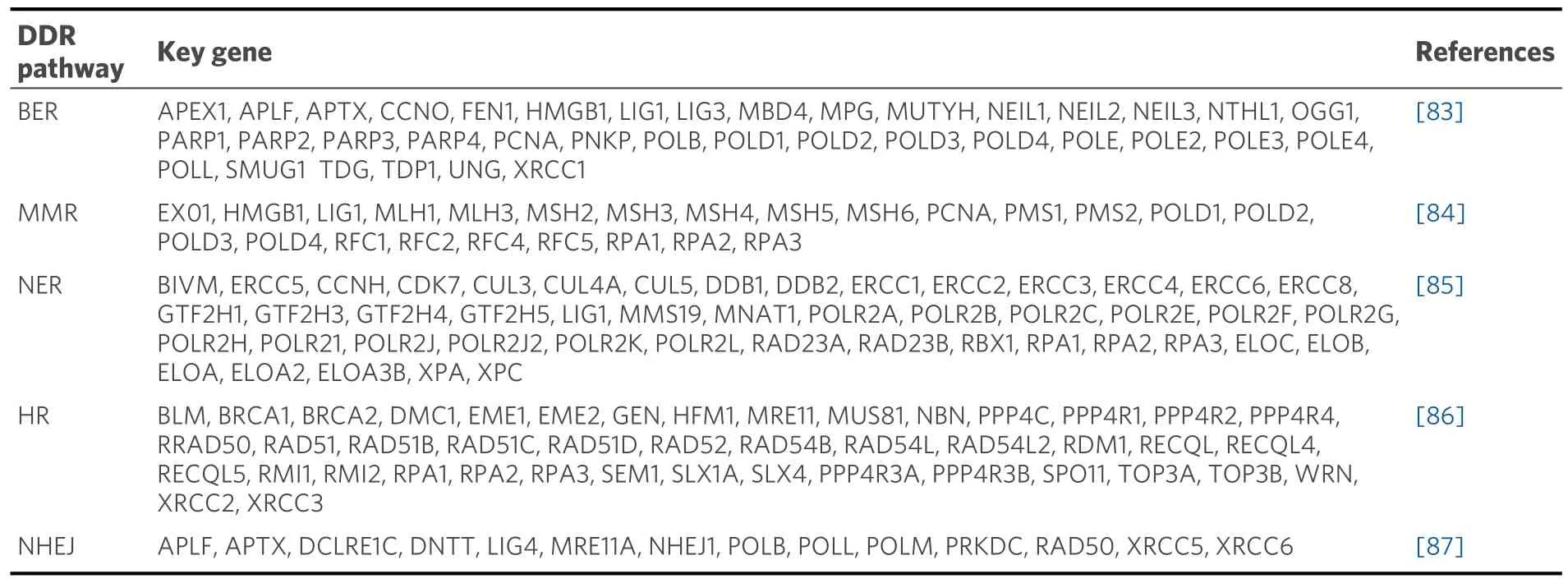
Table 2.Key genes in five major DDR pathways
The number of DDR-targeted drugs in the clinic[19]has rapidly increased to include inhibitors of multiple members involved in the DDR pathway, including polyadenosine diphosphate ribose polymerase (PARP)inhibitors, Ataxia telangiectasia and Rad3-related protein (ATM) inhibitors, DNA-dependent protein kinase(DNA-PK) inhibitors, Ataxia telangiectasia and Rad3-related protein (ATR) inhibitors, checkpoint kinase(CHK1) inhibitors, WEE1 G2 checkpoint kinase (WEE1) and protein kinase,membrane-associated tyrosine 1 (PKMYT1) inhibitors.PARP inhibitors, a class of anticancer drugs that inhibit PARP[92]and target the DDR through a synthetic lethality mechanism via a genetically or functionally defective pathway, have attracted much attention in targeted therapy for HCC.These agents can affect the self-replication of cancer cells and are the first targeted drugs to successfully utilize the concept of synthetic lethality to gain approval for clinical use.These drugs were the first targeted drugs to successfully utilize the concept of synthetic lethality to gain clinical approval.Studies have shown that the inhibition of PARP1 activation inhibits autophagy induced by the platinum-based drug CP, which leads to cell death via mitochondrial apoptosis in HCC HepG2 cells[93].The combination of a lysine methyltransferase 5C (KMT5C) inhibitor and a PARP inhibitor effectively damages HCC cells and inhibits tumor growth in a mouse model of HCC[94].In addition, clinical studies continue to result in the development of new DDRis, such as DNA polymerase θ(POLQ) inhibitors targeting HR-deficient tumors,USP1 inhibitors targeting BRCA1/2-deficient cancers, RAD51 inhibitors, and WRN, a deconjugating enzyme that targets the deconjugating enzyme in microsatellite-unstable cancers[95].
Due to the lack of long-term regular responses to DDRis, primary or acquired resistance can be acquired by such monotherapies.HCC patients are either sensitive or resistant to DDRis[96], largely because of the remaining response and ability to repair SSBs and DSBs, cell cycle regulation and chromatin remodeling pathways, activated oncogenic pathways, and access and utilize cellular material.Limitations of DDRis drugs suggest that overcoming or preventing the development of resistance and expanding the population of potential patients are needed.The combination of DDRis and other treatments is imperative.
Currently, more than 80 clinical trials are evaluating the efficacy of DDR-targeted therapies in combination with immune checkpoint inhibitors[15].Alterations in DNA repair may influence the immune system response to potentially malignant tumors, where the initial immune system response to the tumor is stimulated and the adaptive immune system is recruited to the tumor site, a mechanism that promotes synergistic interactions between defective DNA repair and immune recognition of tumors and opens up new therapeutic avenues[97].Although most clinical trials are still ongoing, some have shown that DDRtargeted therapies in combination with immune checkpoint inhibitors exhibit good tolerability and appreciable anticancer activity[98][Figure 1].Therefore, DDRis are being increasingly considered for combination therapy and may enhance the response rate to immunotherapy by promoting neoantigen release, increasing the tumor mutational load, and enhancing PD-L1 expression[99].PARP inhibitors in combination with immunotherapies such as PD-L1 monoclonal antibodies hold considerable promise[100,101].PARP inhibitors activate DNA damage repair by targeting the STING pathway, inhibiting the tumor microenvironment through the upregulation of interferon, promoting a profound antitumor immune effect,and increasing PD-L1 expression[102].PARP inhibition has been demonstrated to enhance immune checkpoint therapy through the miR-513/PD-L1 pathway in HCC, and the combination of the PARP inhibitors olaparib and anti-PD-L1 effectively enhances the effect of immunotherapy and is beneficial for HCC treatment[103].
ATR is a synthetic lethal target of ATM mutations[104]and is considered one of the most promising synthetic lethal targets after PARP.One study evaluated the ability of the combination of radioimmunotherapy and the ATR inhibitor AZD6738 to control tumor growth and inhibit tumor recurrence by establishing a subcutaneous tumor model in C57BL/6 mice[105].Radioimmunotherapy in combination with the ATR inhibitor AZD6738, which is dependent on activation of the cGAS-STING pathway, increased the infiltration and proliferative capacity of CD8+ T cells in Hepa1-6 tumors in mice and significantly improved the tumor immune microenvironment after radioimmunotherapy.In addition, compared with radioimmunotherapy, triple combination therapy (AZD6738 plus radiotherapy plus anti-PD-L1 treatment)reduced the expression of CD8+ T-cell immune exhaustion markers[105], promoted stronger immune memory and durable antitumor immunity, and thus was able to prevent tumor recurrence in a mouse model.Thus, triple therapy was superior to radioimmunotherapy in terms of antitumor efficacy and mouse survival.These findings suggest that the ATR inhibitor AZD6738 may be a potential synergistic treatment for HCC via radioimmunotherapy.
In addition, René Bernards’ team similarly demonstrated that HCC cell lines treated with AZD6738 or MK-8776 had significantly greater levels of γ-H2AX than did drug-resistant HCC cells and that the ATR-CHK1 signaling pathway is a therapeutic target in HCC[106].In HCC cells resistant to ATR or CHK1 inhibition, DNA replication stress was induced by the use of CDC7 inhibitors; thus, these drugs had significant synergistic effects on ATR and CHK1 inhibitors.The synergistic effect of ATR-CHK1 inhibition and CDC7 inhibition may be due to mitotic catastrophe caused by mitotic abnormalities[106].
PROSPECTS
At present, although first-line treatments for HCC, such as sorafenib, lenvatinib, and donafenib, are available, therapeutic options for patients with advanced HCC are still limited when patients are resistant to targeted therapy.In recent years, with the introduction of immune checkpoint inhibitors in the clinic, more advanced tumors have been treated, and the 2021 update of the National Comprehensive Cancer Network(NCCN)[107]guidelines further emphasized the immunotherapy strategy, namely, moving lenvatinib and sorafenib in the class I recommendation from the previous “preferred regimen” to “other recommended regimens”.The PD-L1 inhibitor atalizumab in combination with bevacizumab (“T+A”)[64]is the only guideline-recommended preferred regimen for first-line treatment and significantly improves overall survival in patients with advanced HCC compared with previous sorafenib treatment.The overall survival of patients with advanced HCC, especially in the Chinese subgroup, reached a median of up to 2 years.In addition, two-drug combination regimens, such as sindilizumab combined with bevacizumab biosimilar[78],apatinib combined with karelizumab[108], and doxorubicin combined with tremelimumab, which are similar in mechanism, have a more limited efficacy rate at present, with a local control rate of approximately 20%-30%.
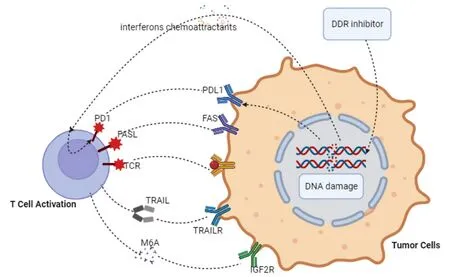
Figure 1.Under the action of DDRis, tumor cells release interferon, and chemotactic substances act as signals to immune cells to promote immune recognition and tumor killing by increasing the immunogenicity of the tumor and recruiting T cells.PD-1 is upregulated at the transcriptional level to activate T cells, and negative costimulatory signals transmitted by PD-1 upon binding to its ligands inhibit T lymphocyte proliferation.In the tumor microenvironment, antitumor immune responses can be maintained by blocking the connection of PD-1 to PD-L1 expressed on tumor or mesenchymal cells.
DDR pathway dysfunction activates the immune response, remodels the immune environment, and facilitates ICI sensitization.The activated cGAS-STING pathway[109]triggers intrinsic immunity and enhances T-cell recruitment and infiltration into tumors, and the ATM/ATR/Chk1 signaling cascade[110]regulates the tumor microenvironment (TME), possibly through the upregulation of PD-L1.Alterations in the tumor DDR pathway due to DDR gene defects or DDRis influence the efficacy of ICIs by affecting immunogenicity, immune cell infiltration, and immune regulatory molecules.Among the advances in DDRi combination immunotherapy, the classic MEDIOLA study[111](olaparib + durvalumab) and TOPACIO study[112](niraparib + pembrolizumab) both showed that PARP inhibitors in combination with ICIs can achieve a 1 + 1 > 2 effect.The combination of PARP inhibitors with an anti-PD-L1 or anti-CTLA-4 antibody[101]enhanced the antitumor immunity induced by ICI therapy and its nonmodel specificity.
The exploration of DDRis combination immunotherapy in patients with HCC continues to be limited by certain limitations.The optimal response to immunotherapy in HCC patients still faces many challenges,and additional studies are needed to validate this theory.In terms of DDRis combinations in HCC immunotherapy[15], expanding research on combinations of DDRis with ICIs could include identifying advantageous populations that are effective for DDRis, stratified screening of tumor types for which the drugs are indicated, and randomized clinical trials to determine whether these combinations are superior to treatment with ICIs alone or to validate them in a larger clinical cohort.
Hepatitis B virus infection, hepatitis C virus infection, high aflatoxin B1 (AFB1) exposure, alcoholic cirrhosis, environmental toxins, diabetes mellitus, and metabolic syndrome are risk factors for HCC[113,114].A large number of molecular pathological epidemiology (MPE)[115]studies have demonstrated that genetic polymorphisms of genes are associated with the risk of HCC[116], e.g., single nucleotide gene polymorphisms related to biological pathways such as oxidative stress, DNA damage repair, and immune response are linked to HCC risk, with variations in genotypes resulting in differing risk levels[117].Ueyamaet al.reported that polymorphisms in the PNPLA3 and JAZF1 genes are associated with an increased risk for the development of HCC, and the PNPLA3 rs738409 gene polymorphism is a major risk factor for the development of HCC[118].
During the course of combination therapy, further subsequent adjustments in the dose administered,duration, and frequency of administration are considered to maximize the survival benefit.The use of natural or synthetic antioxidant drugs[119]before and after chemotherapy reduces free radical-induced oxidative stress, has antioxidant effects, is effective in reducing cancer-related mortality, and reduces side effects caused by drug therapy[120].Recent studies have shown that curcumin can activate the mitochondrial apoptotic pathway to induce apoptosis and inhibit metastasis and invasion of hepatocellular carcinoma cells[121].Resveratrol is rich in stilbene derivatives, which can reduce liver damage caused by alcohol and hepatotoxic drugs, improve lipid metabolism, reduce lipid accumulation, and protect liver cells[122].FDAsupported antioxidant nanotechnology is currently undergoing active investigation in clinical trials and has emerged as a promising therapeutic modality for liver cancer treatment in the foreseeable future[123].
Immunocombination therapy has significantly improved the survival benefit of patients with HCC, but HCC-related immunocombination therapy is still in its infancy, and studies on biomarkers for efficacy prediction, clinicopathological features, selection of immunocombination regimens, and the emergence of clinical resistance are still being explored.The combined application of multiple therapies puts forward greater requirements for the practice of multidisciplinary diagnostic and treatment modes, as well as better tolerance of patients during treatment.In a clinical context, a comprehensive molecular classification of the disease utilizing hepatocellular carcinoma-related molecular markers for risk stratification and prognosis may provide a deeper understanding of the disease’s etiology and pathogenesis.This approach can facilitate the more precise identification of high-risk cohorts, mitigate adverse effects, and furnish a more efficacious target for disease prevention and management.In addition, during the study of the DDR pathway in ICB[20],the connection between NHEJ and the NER pathway and immunotherapy has yet to be explored.The mechanism through which the DDR affects the immune microenvironment has rarely been studied, as has its direct impact on immune cells, and the mechanism through which immune checkpoints emerge in combination with the DDR pathway has yet to be elucidated.
CONCLUSION
HCC treatment has entered the era of immunocombination therapy, various combination modalities have successively achieved considerable efficacy, and treatment regimens of target inhibitors combined with immune checkpoint inhibitors have produced synergistic antitumor effects in liver cancer clinical trials.DDR factors are considered possible immunotherapy biomarkers for predicting the response of HCC patients to ICIs, and the combination of ICIs and DDRis has synergistic effects.Combination therapy involving DDRis and an anti-PD-L1 monoclonal antibody has been effective, but additional DDRis in combination with ICIs are needed in preclinical trials to explore multiple therapeutic options for HCC.
DECLARATIONS
Author contributions
Drafted the original manuscript: Yang C, Tao T, Wu Y
Structured and revised the manuscript: Liu SB, Wang W
All authors have read and agreed to the final version of the manuscript.
Availability of data and materials
Not applicable.
Financial support and sponsorship
This study was supported by the Jiangsu Higher Education Institution Innovative Research Team for Science and Technology (2021), Program of Jiangsu Vocational College Engineering Technology Research Center (2023), Key Technology Program of Suzhou People’s Livelihood Technology Projects (Grant No.SKY2021029), Open Project of Jiangsu Biobank of Clinical Resources (TC2021B009), Project of State Key Laboratory of Radiation Medicine and Protection, Soochow University (No.GZK12023013), Programs of the Suzhou Vocational Health College (Grant No.SZWZYTD202201), and Qing-Lan Project of Jiangsu Province in China (2021, 2022), Project of Jiangsu Province Engineering Research Center of Molecular Target Therapy and Companion Diagnostics in Oncology.
Conflicts of interest
All the authors declare that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable
Copyright
? The Author(s) 2024.
 Journal of Cancer Metastasis and Treatment2024年3期
Journal of Cancer Metastasis and Treatment2024年3期
- Journal of Cancer Metastasis and Treatment的其它文章
- Neutrophils in the tumor microenvironment: role in tumor progression and potential targeted therapeutic strategy
- Research progress of intestinal microbiota in targeted therapy and immunotherapy of colorectal cancer
- ImmuneScore as a novel RNA-based prognostic signature superior to PD-L1 in advanced nonsquamous NSCLC patients receiving chemotherapy combined with immune checkpoint inhibitor therapy
