Engineering of ovarian tissue for ovarian dysfunctions: A review
Aliya Zhylkybekova ,Gulbakit K.Koshmaganbetova ,Myltykbay S.Rysmakhanov ,Nurgul Abdullayevna Abenova ,Nadiar Maratovich Mussin ,Asset Askerovich Kaliyev ,Mahdi Mahdipour,Amin Tamadon
1Department of Evidence-Based Medicine and Scientific Management,West Kazakhstan Marat Ospanov Medical University,Aktobe,Kazakhstan
2Department of Surgery and Urology No.2,West Kazakhstan Medical University,Aktobe,Kazakhstan
3Department of Surgery and Transplantation,Aktobe Medical Center,Aktobe,Kazakhstan
4Department of Internal Diseases,West Kazakhstan Marat Ospanov Medical University,Aktobe,Kazakhstan
5Stem Cell Research Center,Tabriz University of Medical Sciences,Tabriz,Iran
6Department of Reproductive Biology,Faculty of Advanced Medical Sciences,Tabriz University of Medical Sciences,Tabriz,Iran
7Department for Scientific Work,West-Kazakhstan Marat Ospanov Medical University,Aktobe,Kazakhstan
ABSTRACT This review explores tissue engineering as a potential solution for reproductive health issues in women caused by genetic or acquired diseases,such as premature ovarian failure or oophorectomy.The loss of ovarian function can lead to infertility,osteoporosis,and cardiovascular disease.Hormone replacement therapy is a common treatment,but it has limitations and risks.The review focuses on two main approaches in tissue engineering: scaffold-based (3D printing,electrospinning,decellularization) and scaffold-free (stem cell transplantation,organoid cultivation).Both approaches show promise in preclinical studies for creating functional ovarian tissue.Challenges include vascularization,innervation,long-term function,and safety.Despite these challenges,tissue engineering offers a potential avenue for restoring fertility and hormone balance in women with ovarian dysfunction.
KEYWORDS: Female gonads;Tissue engineering;Estrogen;Reproductive system
1.Introduction
The female reproductive system is tasked with the production of gametes,referred to as eggs or ova,the synthesis of specific sex hormones,and the nurturing of fertilized eggs during their maturation process in preparation for delivery.Within this context,the ovary plays a pivotal role,as it serves as the site where the production of eggs takes place,with the ultimate potential for these eggs to undergo fertilization upon encountering male gametes,commonly known as sperm[1].
Ovarian dysfunction can manifest due to various etiological factors,encompassing genetic anomalies such as the suppression of α-Klotho expression within ovarian granulosa cells[2],chromosomal aberrations such as Turner syndrome (X-chromosome monosomy)[3],autoimmune conditions including autoimmune polyendocrine syndrome type Ⅰ (also known as APECED,autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy) associated with 21st chromosome autoimmunity[4],cancer treatments[5],autoimmune disorders like lymphocytic oophoritis,antioocyte antibodies,antibodies to steroid-producing cells,thyroid autoimmunity,and adrenal gland autoimmunity[6],as well as surgical interventions such as oophorectomy[7].Diagnosis hinges upon the identification of amenorrhea occurring before the age of 40,coupled with elevated follicle-stimulating hormone (FSH)levels within the menopausal range[8].Elevated FSH is a marker of decreased ovarian function,as the ovaries are less responsive to its regulatory effects.
Insufficient production of female sex hormones manifests as menstrual irregularities,infertility,an increased incidence of Alzheimer's disease,urogenital atrophy,cardiovascular disease,decreased bone density,and reduced quality of life[9,10].Hormone replacement therapy (HRT) is the primary treatment for female gonadal insufficiency[9].Although existing hormone regimens are effective in suppressing menopausal symptoms,they are inadequate for achieving normal uterine volume,endometrial thickness,and uterine blood flow.This is because physiological replacement of estrogen and progesterone is necessary[11].Moreover,the dose required to treat vasomotor symptoms may differ from the dose needed to protect the cardiovascular system,bones[12],or muscles[13].Additionally,long-term use of hormone replacement therapy drugs is linked to a high risk of breast and endometrial cancer[14].
Regenerative medicine and tissue engineering are potential alternative treatments for premature ovarian failure[15].Tissue engineering offers a promising option by creating functional ovarian tissue that can produce hormones in response to endogenous signals.Tissue engineering involves combining cells,biomaterials,and signaling factors to create functional tissues or organsin vitroorin vivo[16].In the last decade,researchers have conducted extensive research on creating the artificial ovary[17] using various techniques to restore female reproductive gonad function (Table 1)[18,19].The goal of ovarian tissue engineering is to generate a 3D structure that mimics the native tissue architecture and function,including the ability to produce estrogen and progesterone[20].
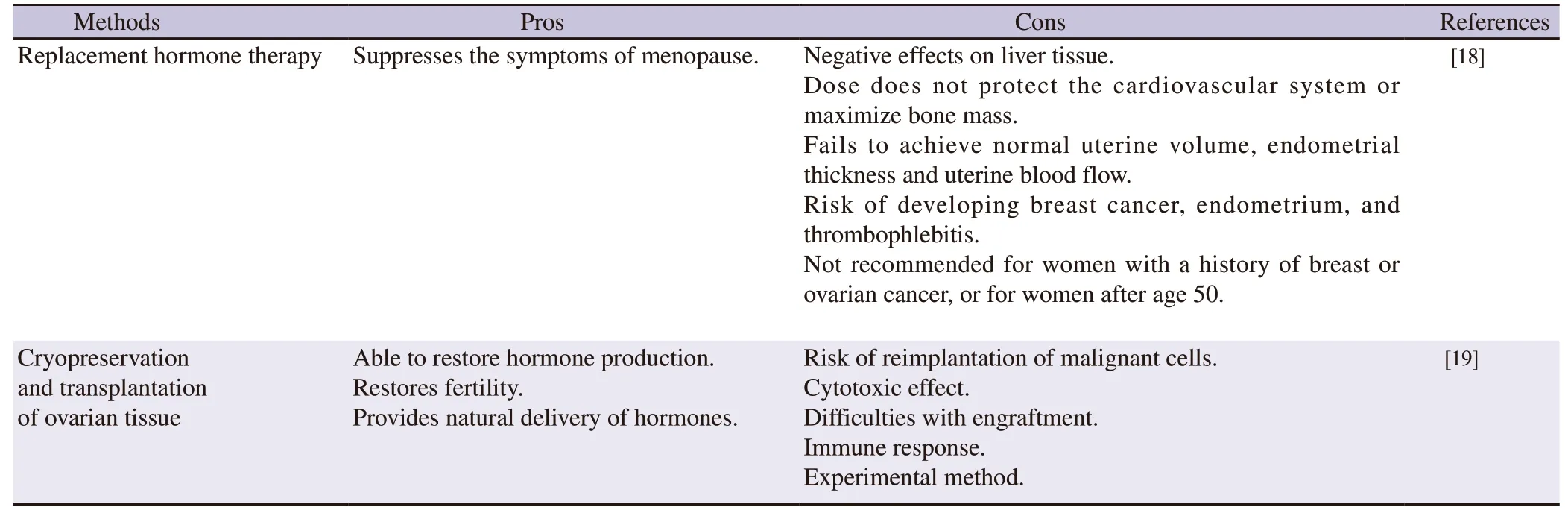
Table 1.Pros and cons of current clinical techniques used to restore female reproductive gonad function.
In recent years,several techniques have been developed to engineer ovarian tissue,including scaffold-based[21] and scaffoldfree[22] approaches.Scaffold-based strategies involve using natural or synthetic scaffolds to support the growth and differentiation of ovarian cells[23],while scaffold-free methods rely on the formation of multicellular aggregates or organoids[24].Both approaches have shown promising results in preclinical studies,such as the successful transplantation of tissue-engineered ovaries into mice and the restoration of fertility[24].The choice between scaffold-based and scaffold-free methods depends on the specific tissue's requirements.Scaffold-based approaches offer structural support,mimicking the native extracellular matrix,suitable for complex tissues but may raise concerns about scaffold material[25].Scaffold-free techniques rely on natural cell interactions,preserving the microenvironment,yet may struggle with structural integrity[26].
However,several challenges still need to be addressed before ovarian tissue engineering can become a clinical reality.These challenges include the need for vascularization and innervation of the engineered tissue,ensuring its long-term function and safety,and regulatory approval[27].Vascularization and innervation play critical roles in the growth and maturation of ovarian follicles,as they provide oxygen,nutrients,and signaling molecules necessary for follicular development and function,as well as regulate the release of hormones and neurotransmitters from the follicles[28,29].Imaging techniques that can non-invasively assess the vascularization and innervation of developing follicles in the human ovary,such as Doppler ultrasound and magnetic resonance imaging (MRI),can aid in the design and optimization of tissue engineering strategies for the ovary by enabling the monitoring of tissue perfusion and neurovascular integration[30].
This review aims to summarize the available techniques for tissue engineering of the ovary,including their advantages and limitations.The review focused on the current state of the field,recent advances,and future directions for ovarian tissue engineering for the replacement of sex hormones and the restoration of fertility in women with ovarian dysfunction.
2.Methods
We conducted a search of four electronic databases,Scopus,PubMed,Web of Science and ClinicalTrial.gov to identify scientific publications related to tissue engineering techniques for replicating female sex hormones.We used the following MeSH terms: ovary,tissue engineering,female gonads,tissue engineering,stem cell,estrogen,and their combinations using Boolean operators (AND,OR).
The eligibility criteria for including articles in this review were as follows: 1) primarily focus on tissue engineering techniques related to replicating female sex hormones or addressing female gonadinsufficiency;2) the search included only full-text research articles;3) all articles should have been published from January 2018 to June 2023 and 4) English published papers and theses were included.The exclusion criteria for including articles in this review were reviews and articles that: 1) were duplicated previously;2) had irrelevant publication types and 3) had irrelevant intervention.
We screened the titles of the articles for relevance,followed by the analysis of abstracts.Next,we evaluated the full-text articles that met the search criteria.Additionally,we examined and added references from the selected articles that matched the research topic.We included studies published in peer-reviewed journals that met the following inclusion criteria: a) studies concerning regenerative medicine techniques for female gonad insufficiency,and b) both animal and human studies.Two authors,AZh and GK,performed a thorough analysis of the titles,abstracts,and full-texts of all identified articles to determine their eligibility in accordance with the inclusion criteria.Instances of divergent opinions were resolved through deliberative discussions guided by an AT.
We analyzed the resulting research articles using VOSviewer software (v.1.6.8,2018)[31],which can analyze the semantic contents of publication titles,keywords,and abstracts,and then relate them to citation count data.The software produced a bubble map that revealed the most frequent compounds that have been studied for replicating female sex hormones,thus allowing us to identify key topics and areas of research focus within the field.
3.Scaffold-free ovarian tissue engineering
3.1.Stem cells transplantation
Studies investigating the effects of stem cells on the function of female reproductive glands are primarily conducted using animal models.For these experiments,stem cells that are capable of self-renewal and multilineage differentiation are utilized[32].It is believed that stem cells migrating to the damaged ovary have antiinflammatory effects,participate in immunoregulation,and secrete important cytokines that contribute to anti-apoptosis and antifibrosis,ultimately leading to improved ovarian function[33-35].Furthermore,research suggests that stem cell therapy leads to an increase in the total number of follicles,which in turn increases the levels of sex hormones to physiological levels[36].
Ovarian mass and index are significantly increased,the regular estrus cycle is restored,and fertility is resumed following stem cell transplantation[37].Moreover,stem cell therapy increases angiogenesis,thereby improving the microenvironment of ovarian tissue[38].A pilot human clinical trial was conducted for bone marrow-derived mesenchymal stem cell transplantation[39].The results indicated that patients experienced reduced menopausal symptoms,resumed estrogen production,and resumed menstruation seven months after mesenchymal stem cell engraftment[39].There is a list of clinical trials that used stem cells for the treatment of ovarian diseases (Table 2).
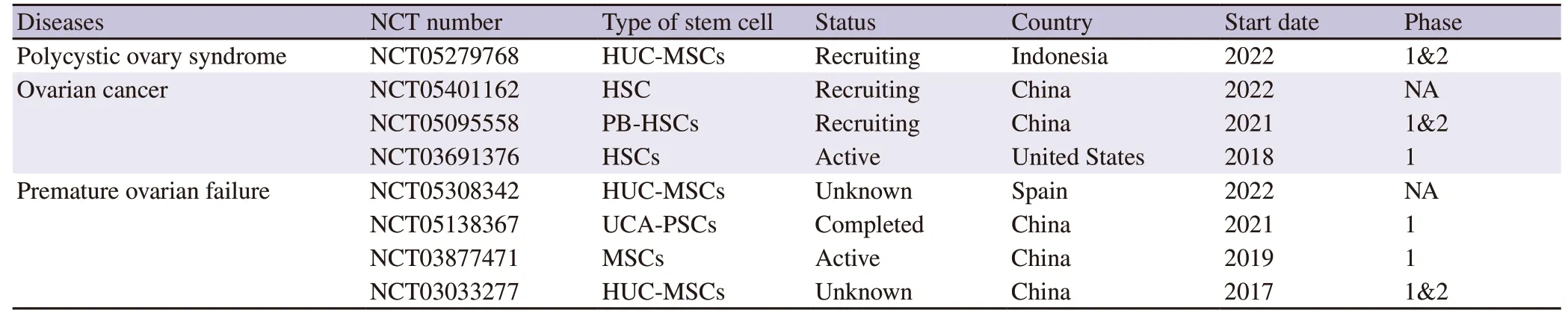
Table 2.Clinical trials for stem cell therapy of ovarian diseases.
3.2.Ovarian tissue organoids
Ovarian tissue organoids represent a promising approach to model the complex physiology of the human ovary and to develop tissue engineering strategies for ovarian dysfunction[24].Organoids are three-dimensional (3D) structures consisting of self-organizing cells that recapitulate the architecture and function of a specific tissue or organ[40].Ovarian tissue organoids can be generated from pluripotent stem cells,adult stem cells,or primary cells derived from ovarian tissue[41].They can contain multiple cell types,including granulosa cells,theca cells,and oocytes,and exhibit follicle-like structures with functional hormone production[22].Organoids have several advantages,such as the ability to study ovarian development and diseasein vitro,perform drug screening and toxicity assays,and potentially generate patient-specific tissue for transplantation[24,42,43].Researchers have generated an ovarian organoid model derived from female germline stem cells (FGSCs) of transgenic mice using a 3D culture system.The resulting ovarian organoids contained follicles and secreted hormones,and healthy offspring were obtained from mature cellsvia in vitrofertilization[24].Ovarian cancer organoids taken from patients can be used to study the pathogenic mechanisms of DNA isolation and histological analysis,as well as their response to various drugs,which could aid in the treatment of malignant ovarian diseases[44].However,challenges associated with organoid technology include the reproducibility and scalability of the cultures,the need for defined culture conditions and media,and the lack of vascularization and innervation,which can limit the size and function of the organoids.
4.Scaffold-based ovarian tissue engineering
4.1.Ovarian follicles in hydrogels
Ovarian follicles embedded in hydrogels offer a promising approach for developing tissue engineering strategies to treat ovarian dysfunction[45].Hydrogels are hydrophilic polymer networks that mimic the extracellular matrix of a particular tissue or organ,providing mechanical support and allowing for the diffusion of nutrients and oxygen[46].Encapsulating ovarian follicles in hydrogels can support their growth and maturationin vitro,potentially leading to the development of functional ovarian tissue[47].The use of hydrogels has several advantages,such as the ability to tune the mechanical properties and bioactivity of the hydrogel,promote the survival and proliferation of the follicles,and create a 3D microenvironment that mimics the native ovarian tissue[47].
In recent years,researchers have been developing a framework for packaging oocytes and follicles to create a favorable environment for maintaining cell viability and to increase transplant survival by reducing the damage caused by ischemia and oxidative stress.In 2012,researchers conducted the first experiment on transplanting a biodegradable framework made of alginate hydrogel with isolated follicles and ovarian cells[34].Recovery rates in experiments involving human follicles were found to be proportional to the percentage of alginate used.Samples without the addition of alginate had no follicles,in contrast to samples with high alginate content[48].Subsequent studies focused on the development of scaffold matrices created using the decellularization method.These scaffolds consist of tissue-specific extracellular matrix (ECM) and can maintain the microarchitecture and biological signals of the original tissue[49].
However,challenges associated with this approach include the need for appropriate selection of the hydrogel material and crosslinking density,ensuring sufficient oxygen and nutrient diffusion,and adequate vascularization and innervation to promote follicle growth and maturation.
4.2.Reconstruction of ovarian scaffold by 3D printer
Reconstruction of ovarian scaffolds using 3D printing technology is a promising approach for developing tissue engineering strategies to treat ovarian dysfunction[50].3D printing allows for the creation of customized structures with precise geometry,porosity,and mechanical properties,making it an ideal approach for reconstructing ovarian scaffolds.The use of 3D printing has several advantages,such as the ability to create complex structures that can mimic native ovarian tissue,control the porosity and surface area of the scaffold,and create a suitable microenvironment for the growth and differentiation of ovarian cells[51].The scaffold can be made from a variety of biocompatible materials,including natural polymers such as collagen and fibrin,and synthetic polymers such as polycaprolactone (PCL) and poly lactic-co-glycolic acid (PLGA)[17].Natural[49,50-64] and synthetic[65-74] biomaterials which are available for this purpose are categorized (Figure 1).
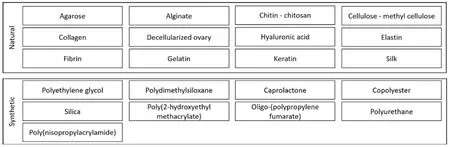
Figure 1.Biomaterials have capabilities to be used as bio-inks for ovarian tissue reconstruction.
In an experiment,the survival and proliferation ability of Chinese hamster ovary cells through the nozzles of a thermal inkjet was successfully demonstrated.Soy agar and collagen gel were used as biopaper[57].The viability of cells printed by the thermal inkjet printer was 89%,with only 3.5% apoptotic cells observed after printing.The ability of cells to restore transient pores in the cell membrane was observed within 2 hours after printing[75].However,the challenges associated with this approach include the need to optimize printing parameters and materials to ensure biocompatibility and scaffold stability,the need to promote cell infiltration and vascularization of the scaffold,and the need to ensure proper innervation for follicular maturation.
Establishing functional vascular networks within engineered tissues demands biomimetic innovations[76],precise scaffold design[77],and growth factor strategies[78] to ensure metabolic support.Innervation requires a deep understanding of neurobiology and the promotion of neural connectivity,while maintaining long-term tissue functionality necessitates the careful selection of biocompatible materials[79] and rigorous safety testing.However,the potential of tissue engineering to overcome these challenges is substantial,with cutting-edge technologies like 3D bio-printing and stem cell therapies offering transformative solutions.As researchers continue to bridge these gaps through interdisciplinary collaboration,tissue engineering's significance in healthcare advancement becomes increasingly evident,promising lasting benefits for patients globally.
4.3.Reconstruction of ovarian microenvironment by electrospinning
Electrospinning is a promising approach for developing ovarian microenvironments that can support follicle growth and maturation[17,80].Electrospinning is a technique that uses an electric field to generate nanofibers from a polymer solution or melt,creating a 3D microenvironment that can mimic the extracellular matrix of a particular tissue or organ.Ovarian microenvironments can be created by electrospinning fibers from biocompatible polymers,such as poly(lactic acid) (PLA) or poly (lactic-co-glycolic acid) (PLGA),which can be functionalized with extracellular matrix proteins,growth factors,and other bioactive molecules to promote cell adhesion,proliferation,and differentiation[81,82].The use of electrospinning has several advantages,such as the ability to create scaffolds with high surface area-tovolume ratios and controllable porosity,to promote cell infiltration and vascularization of the scaffold,and to create a suitable microenvironment for the growth and differentiation of ovarian cells.However,the challenges associated with this approach include the need to optimize the electrospinning parameters and materials to ensure biocompatibility and scaffold stability,the need to promote appropriate vascularization and innervation for follicular maturation,and the need to ensure the proper spatial distribution of cells within the scaffold.Nonetheless,ongoing research is focused on addressing these challenges and improving the use of electrospun ovarian microenvironments for the development of functional ovarian tissuein vitroand potentially for transplantationin vivo.
4.4.Reconstruction of ovarian microenvironment by decellularization
Various techniques have been proposed for tissue decellularization,with the main goal being the removal of intracellular components and nuclear material.This is typically achieved through chemical treatments[7],and in some studies,a combination of physical and chemical methods has been used[83].During the seeding of the biocage,there was rapid migration and colonization of the extracellular matrix within 24 hours,followed by further formation of cluster-like structures that maintained stability for up to 7 days[49].Additionally,an increase in the amount of DNA in the cultured medium was observed throughout the duration of the experiments[83].
To improve the engraftment of the transplant and suppress the immune system,researchers have encapsulated decellularized ovarian tissues within a hydrogel-based capsule.This hydrogel serves as a protective layer and restricts the invasion of immune cells[83,84].Primary ovarian cells isolated from 8-week-old female rats retain their viability and biological activity.These cells were able to reconstruct primordial or primary follicle-like structures within decellularized human ovarian skeletons after transplantation.Immunostaining also demonstrated the presence of cells capable of expressing steroid hormone receptors.Additionally,mesenchymal stem cells seeded on decellularized scaffolds showed a higher proliferation rate than in the two-dimensional conventional culturing system.However,the presence of host cells and lack of neovascularization may indicate that further optimization of the transplant strategy is necessary to improve its effectiveness[21].
Advancements in the decellularization process primarily revolve around optimizing techniques to effectively eliminate DNA and intracellular constituents from ovarian tissue while concurrently augmenting the structural integrity of the remaining extracellular matrix within the ovarian framework[21,85,86].
5.What method is more promising for replacement of sex hormones?
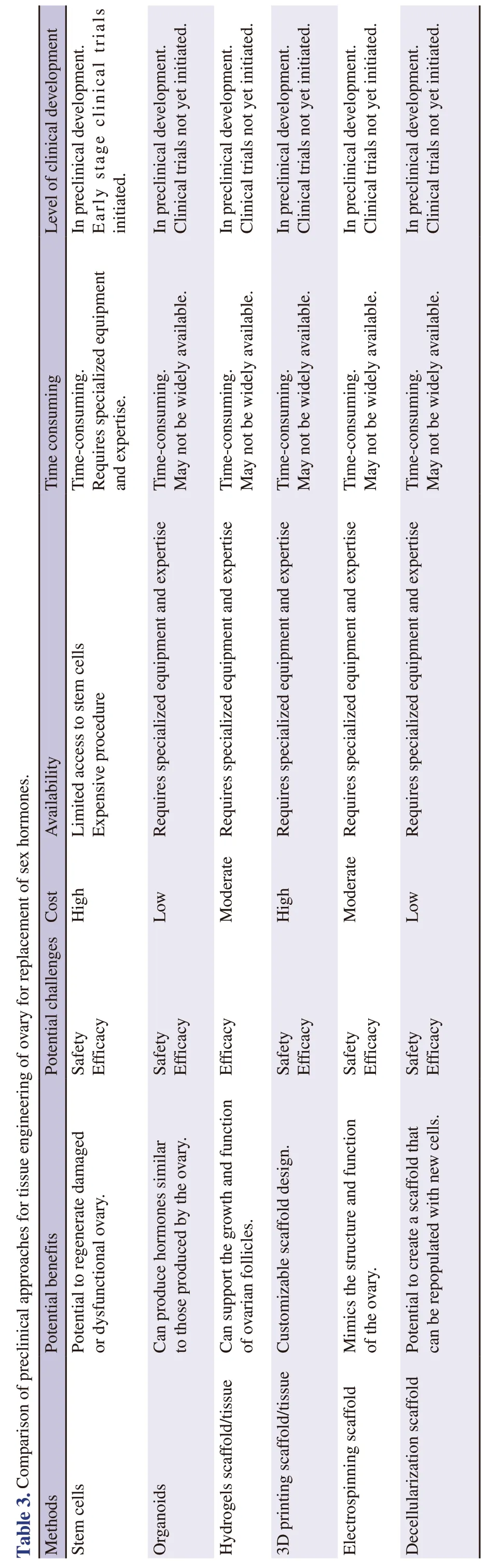
In Table 3,the overall evaluation has been adjusted to reflect the relative advantages and disadvantages of each method as explained above.Based on the information provided,stem cell transplantation and reconstruction of the ovarian scaffold by 3D printing appear to be the most promising methods,followed by ovarian tissue organoids,ovarian follicles in hydrogels,and reconstruction of the ovarian microenvironment by electrospinning.Reconstruction of the ovarian microenvironment by decellularization appears to have the lowest overall score,although it still shows promise and may be developed further with more research.It should be noted that the follicular vascular structure[29] and the ovarian innervation network[28] play an important role in its reproductive activity and are one of the important challenges of ovarian tissue engineering.It is important to keep in mind that these rankings may change as more information becomes available through ongoing research and clinical trials.
6.Conclusions and future prospect
Tissue engineering approaches offer a promising avenue for restoring ovarian function in patients with ovarian insufficiency.While current treatments have limitations and drawbacks,experimental techniques such as stem cell transplantation,ovarian tissue organoids,and various methods of reconstructing the ovarian microenvironment have shown potential for improving outcomes.Although further research and clinical trials are necessary to establish safety and efficacy,these approaches represent an exciting area of investigation for scientists and clinicians.Tissue engineering offers the potential for more personalized,effective,and minimally invasive treatments,ultimately improving the quality of life for women with ovarian insufficiency.With continued development and refinement,tissue engineering approaches may offer a more physiological and sustainable solution for ovarian replacement therapy.
Conflict of interest statement
The authors declare no conflicts of interest to disclose.
Funding
The study receives no extramural funding.
Authors’ contributions
The main frame of the guidelines is written by Dr Aliya Zhylkybekova;the introduction was written by Dr Myltykbay S.Rysmakhanov;Bibliometric sections were written by Nadiar Maratovich Mussin and revised by Asset Askerovich Kaliyev;Tissue engineering section was written by Dr Aliya Zhylkybekova,Dr Mahdi Mahdipour,Dr Nurgul Abdullayevna Abenova,and Gulbakit K.Koshmaganbetova.Editing,framing,referencing and logistics were done by Amin Tamadon.
 Asian Pacific Journal of Reproduction2024年1期
Asian Pacific Journal of Reproduction2024年1期
- Asian Pacific Journal of Reproduction的其它文章
- Promotion of sexual and reproductive health in Pakistan-The role of technology and online awareness
- Adding chitosan nanoparticles of green tea extract in diluent and thawing temperatures ameliorate the post-thawed quality of Boer buck semen
- Pro-fertility effect of Ficus carica fruit extract in streptozotocin-induced male rats
- Subsequent pregnancy outcomes and fertility rates in the case series that underwent bilateral hypogastric artery ligation (BHGAL) due to severe postpartum hemorrhage
- The relationship between DNA fragmentation and the intensity of morphologically abnormal human spermatozoa
- Exploring the relationship between ambient sulfur dioxide and semen quality parameters: A systematic review and meta-analysis
