The functions of exosomes targeting astrocytes and astrocyte-derived exosomes targeting other cell types
Hongye Xu, He Li, Ping Zhang, Yuan Gao, Hongyu Ma, Tianxiang Gao, Hanchen Liu, Weilong Hua, Lei Zhang,Xiaoxi Zhang,*, Pengfei Yang,*, Jianmin Liu
Abstract Astrocytes are the most abundant glial cells in the central nervous system; they participate in crucial biological processes, maintain brain structure, and regulate nervous system function.Exosomes are cell-derived extracellular vesicles containing various bioactive molecules including proteins, peptides,nucleotides, and lipids secreted from their cellular sources.Increasing evidence shows that exosomes participate in a communication network in the nervous system, in which astrocyte-derived exosomes play important roles.In this review, we have summarized the effects of exosomes targeting astrocytes and the astrocyte-derived exosomes targeting other cell types in the central nervous system.We also discuss the potential research directions of the exosome-based communication network in the nervous system.The exosome-based intercellular communication focused on astrocytes is of great significance to the biological and/or pathological processes in different conditions in the brain.New strategies may be developed for the diagnosis and treatment of neurological disorders by focusing on astrocytes as the central cells and utilizing exosomes as communication mediators.
Key Words: astrocytes; communication; exosomes; neurological disorders; targeting mechanism
Introduction
Astrocytes are the most widely distributed glial cells in the central nervous system (CNS) (Sofroniew and Vinters, 2010).Because of their unique structure and functions, astrocytes are the critical regulatory and supportive cells in the CNS (Rothstein et al., 1994, 1996; Abbott et al., 2006; Ransom and Ransom, 2012; Vasile et al., 2017).Studies have shown that astrocytes can be characterized into a variety of subsets, manifesting specific functions in different conditions or diseases (Freeman, 2010; Zhou et al., 2019; Sofroniew, 2020; Yue and Hoi, 2023).Under physiological conditions, astrocytes are involved in the maintenance of the normal function of the nervous system by participating in the formation of the blood-brain barrier, supporting the normal functions of neurons, and regulating the balance of ions both inside and outside the cell (Sontheimer, 1994; Sofroniew and Vinters, 2010).However, under certain pathological conditions, astrocytes will participate in neuroinflammation and aggravate nervous system damage (Burda and Sofroniew, 2014; Pekny and Pekna, 2014; Lu et al., 2017; Salman et al., 2017a, b).
The exosome is a subcategory of extracellular vesicles that has attracted the most interest from researchers among all other extracellular vesicles.It is worth noting that almost all eukaryotic cells can generate exosomes(Kowal et al., 2014).Current evidence suggests that exosomes are extensively involved in the regulation of various biological processes and intercellular communication by transporting biological components, including nucleic acids, proteins, and lipids (Mathivanan et al., 2010; Colombo et al., 2014).In recent years, the function of exosomes in the CNS has attracted the increasing attention of investigators.Studies have gradually revealed the functions of exosomes from neurons, microglia, astrocytes, oligodendrocytes, and neural stem cells (NSCs).Being one of the most important supportive cells in the CNS, astrocytes also secrete exosomes with a variety of components under different conditions (Gharbi et al., 2020; Upadhya et al., 2020; Zhao et al.,2021; Oyarce et al., 2022).Astrocytes also receive biological cargo carried by the exosomes from other cells and accordingly change their functions and phenotypes (Oushy et al., 2018; Men et al., 2019; Gharbi et al., 2020; Willis et al., 2020; Ogaki et al., 2021).Thus, astrocytes work as relay stations during exosome-based intercellular communication in the CNS (Figure 1).Herein, we have reviewed the exosome-based intercellular communication focused on astrocytes with the aim to evaluate its value for medical use in the future.
Search Strategy
PubMed database was used for literature retrieval and the following search terms were used: (Astrocyte*[tw] OR “Astrocytes” [Mesh]) AND(Exosome*[tw] OR “Exosomes” [Mesh]).The results were further screened bytitle and abstract, which presented the intercellular communication between astrocytes and other cells based on exosomes, from 2006 to 2023.
The Effects of Upstream Cell-Derived Exosomes on Astrocytes
The effects of stem cell-derived exosomes on astrocytes
Mesenchymal stem cells (MSCs) are a sort of multipotential stem cells that can differentiate into hepatic cells, adipocytes, muscular cells, cardiac cells,and even neural cells (Crapnell et al., 2013).It is derived from bone marrow,muscles, umbilical cord blood, and many other tissues (Teixeira et al., 2013;Lai et al., 2015).MSCs manifest neuroprotective effects by direct or indirect intercellular communication through gap junctions or the secretion of biological factors (Caplan and Dennis, 2006; Teixeira et al., 2013; Moravej et al., 2017).MicroRNAs (miRNAs) are single-stranded, short ncRNA molecules that are involved in various biological processes, including cell proliferation,differentiation, and apoptosis (Nahand et al., 2020).Exosome miRNAs have great research outlook in clinical therapy and biomarkers, they have been found to be associated with multiple diseases.A growing number of studies have attempted to alleviate or treat diseases through exosomes.This indicates that miRNAs in exosomes have great significance in preventing and controlling diseases in clinical research (Li et al., 2023).In 2012, Xin et al.described the exosome-based intercellular communication between MSCs and brain parenchymal cells for the first time.MSC-derived exosomes (MSCExos) transferred miR-133b to astrocytes and neurons, enhancing the growth of nerve synapses after an ischemic attack.In 2017, the same group reported that MSC-Exos carrying miR-133b significantly increased the number of exosomes released from astrocytes under oxygen-glucose deprivation (OGD)conditions (Xin et al., 2017).Interestingly, the exosomes from those astrocytes that received MSC-Exos manifested better effects in promoting the growth of rat neuronal synapses (Xin et al., 2017).
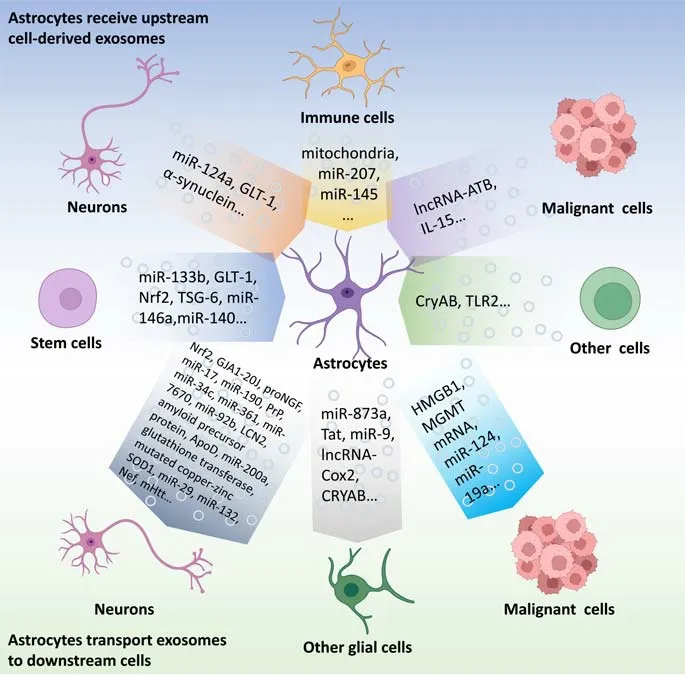
Figure 1 |Intercellular communication based on exosomes: focus on astrocytes.The exosome-based intercellular communication focused on astrocytes.The exosomes from stem cells, neurons, immune cells, malignant cells, olfactory sheath cells, Schwann cells, and endothelial cells can affect the functions of astrocytes.Conversely, AS-Exos can influence the functions of other cells including neurons, microglia, oligodendroglia,malignant cells, and other cell types.Created with BioRender.com.ApoD: Apolipoprotein D; Cox2: cyclooxygenase-2; CryAB: α-B-crystalline; GJA1-20J: gap junction alpha1-20J; GLT-1: glutamate transporter-1; HMGB1: high mobility group box 1; LCN2:lipocalin-2; lncRNA: long non-coding RNA; lncRNA-ATB: long non-coding RNA activated by transforming growth factor-β; MGMT: O6-alkylguanine DNA alkyltransferase; mHtt:mutated huntingtin protein; proNGF: pro-form of neurotrophic factor; PrP: prion protein;SOD1: superoxide dismutase 1; Tat: transactivating regulatory protein; TLR2: Toll-like receptor 2; TSG-6: tumor necrosis factor-stimulated gene-6.
Eventually, the exosome-based communication between MSCs and astrocytes caught the increasing attention of investigators.The effects of MSC-Exos on astrocytes have been studied in specific diseases and conditions.It was reported that intravenous injection of MSC-Exos improved the diabetesinduced cognitive impairment of mice by repairing damaged neurons and astrocytes (Nakano et al., 2016).In the rat model of traumatic brain injury (TBI), intravenous administration of MSC-Exos upregulated the level of glutamate transporter-1 (GLT-1) in astrocytes and downregulated the activation of p38 mitogen-activated protein kinase pathway, reducing glutamate-mediated excitotoxicity in astrocytes (Zhuang et al., 2022).Another study indicated that MSC-Exos inhibited the activation of the inflammatory nuclear factor erythroid 2-related factor 2/nuclear factor kappa-B (NF-κB) pathway, reducing inflammation-induced astrocyte activation and protecting the function of nerves after spinal cord injury (SCI) (Wang et al., 2018).Furthermore, exosomes derived from bone MSCs (BMSCs)stimulated by tumor necrosis factor-α could release tumor necrosis factorstimulated gene-6, inhibiting the NF-κB signaling pathway in activated astrocytes, thereby improving the function of the blood-brain barrier (Tang et al., 2021).According to a study in 2018, in diabetic rat models induced by streptozotocin, an enriched environment stimulated the upregulation of miR-146a in the exosomes secreted by endogenous BMSCs, which exerted anti-inflammatory effects on damaged astrocytes and prevented diabetes-induced cognitive impairment (Kubota et al., 2018).Furthermore, the antagonists of prostaglandin E2 receptor-4 increased exosome release from MSCs by inhibiting the signal pathway of prostaglandin E2/prostaglandin E2 receptor-4 in MSCs.The MSC-Exos administered to mice with hippocampal damage inhibited the proliferation of reactive astrocytes, reducing the widespread inflammatory reaction, ameliorating the infiltration of microglia into the damaged hippocampus, and increasing blood-brain barrier integrity (Chen et al., 2019a).Besides, interleukin (IL)-1β-treated MSC-Exos acting on the signal pathway of Nrf-2 showed a similar neuroprotective effect (Liu et al.,2021).Another study indicated that umbilical cord MSC-derived exosomes ameliorated the activation of microglia and astrocytes, improving the neurological outcomes of rats after traumatic brain injury (TBI) (Cui et al.,2022).Recent studies used exosomes-loaded electroconductive hydrogels as novel therapeutic strategies.Fan et al.(2022) used electroconductive hydrogels loaded with BMSC-Exos (EH-BMSC-Exos) to treat SCI.They found that EH-BMSC-Exos induced the polarization of microglia into the M2 phenotype by activating the NF-κB pathway and promoted the differentiation of NSCs into neurons and oligodendrocytes rather than astrocytes.Besides,EH-BMSC-Exos also activated the phosphatase and tensin homolog/phosphoinositide3-kinase/protein kinase B/mammalian target of rapamycin pathway, thereby increasing axon outgrowth (Fan et al., 2022).These mechanisms endowed EH-BMSC-Exos with the ability to inhibit inflammation and enhance neural regeneration after SCI.
Besides MSC-Exos, exosomes derived from NSCs were also found to adjust the function of astrocytes.NSCs are multipotential cells with the ability to differentiate into neurons, astrocytes, and oligodendrocytes.It was previously believed that NSCs were exclusively present in embryonic or fetal mammals.A study in 2019 proved that NSCs also present in specific regions of the adult mammals’ brain, which facilitated the study of the NSCs (Tuazon et al.,2019).It was reported that exosomes derived from fetal mouse NSCs under ethanol stimulation carried elevated levels of miR-140-3p, which promoted the maturation of astrocytes (Tseng et al., 2019).Another study showed that exosomes derived from NSCs exerted neuroprotective effects in a mouse model of middle cerebral artery occlusion by preserving the function of astrocytes (Sun et al., 2019).
The effects of neuron-derived exosomes on astrocytes
Neurons are the basic functional unit in the nervous system.They initiate nervous impulses and transmit them through synapses and synaptic vesicles from one neuron to the other.Besides inter-neuron communication, neurons also communicate frequently with other neural cells including astrocytes.Communication between neurons and astrocytes is essential for the CNS to maintain its normal physiological functions, in which exosome-based intercellular communication forms an indispensable part (Theodosis et al.,2008; Allen and Lyons, 2018).GLT-1 is one of the major transporters in the brain, accounting for 1% of the total brain protein (Lehre and Danbolt, 1998).Increased levels of GLT-1 can reduce glutamate-mediated neurotoxicity under pathological conditions (McBean and Roberts, 1984; Rimmele and Rosenberg,2016).A study showed that neurons secreted exosomes containing miR-124a,increasing the levels of GLT-1 protein in the targeted astrocytes in the rat model of amyotrophic lateral sclerosis (ALS) (Morel et al., 2013).Afterward,miR-124-3p indirectly increased GLT-1 levels by inhibiting the inhibitory effect of miR-132 and miR-218 on GLT-1 expression (Men et al., 2019).Meanwhile,another study also reported that neuron-derived exosomes containing miR-124-3p inhibited astrocyte activation by targeting myosin heavy chain 9,which acted as carriers for the delivery of biologically functional miR-124-3p into recipient microglia, thereby promoting neurological recovery following SCI in rat models (Jiang et al., 2020).Therefore, the neuroprotective effects of miR-124-3p in neuron-derived exosomes on astrocytes are diverse and deserve further investigation.Furthermore, exosomal miR-181c-3p derived from cortical neurons exerted protective effects on neuroinflammation in astrocytes via downregulation of CXCL1 in an ischemic brain injury rat model(Song et al., 2019).Methamphetamine, a highly addictive psychostimulant drug, is commonly abused worldwide (Carvalho et al., 2012).A recent study showed that neurons treated with methamphetamine secreted exosomes containing α-synuclein, which induced inflammatory responses in the astrocytes that absorbed these exosomes (Meng et al., 2020).To sum up,the impact of neuron-derived exosomes on the targeted astrocytes may be protective or injurious to the CNS under different conditions, which deserves further investigation.
The effects of immune cells-derived exosomes on astrocytes
Exosomes derived from immune cells under different stimulation can also exert various influences on astrocytes.In the study of Alzheimer’s disease(AD), the exosomes derived from macrophages under silybin pre-treatment inhibited astrocyte activation, additionally reducing inflammatory neuronal damage (Huo et al., 2021).According to another study about cerebral ischemia-reperfusion injury, astrocytes transported exosomes carrying mitochondria to the neurons after injury, and the functional normality and structural integrity of the released mitochondria were critical to neuronal metabolism.Heptapeptide-loaded macrophage-derived exosomes inhibited the mitochondrial-damaging dynamin-related protein-1/fission 1 interaction, reducing mitochondrial damage in astrocytes, which ensured the health of released astrocytic mitochondria to the neurons and alleviated mitochondria-mediated neuronal injury (Liu et al., 2022c).According to a study on psychiatric disorders, miR-207 transported by natural killer cellderived exosomes targeted leucine-rich repeats in the astrocytes, inhibiting the activation of the NF-κB pathway, which mitigated depressive symptoms in stressed mice (Li et al., 2020).
As the innate immune cells of the CNS, microglia-derived exosomes also influence the functions of astrocytes.A recent study showed that microgliaderived exosomes containing miR-145-5p, a negative regulator of astrocyte proliferation, downregulated the activity of SMAD family member 3 and reduced the proliferation of astrocytes, inhibiting the formation of an astrocytic scar after SCI (Ye et al., 2022).
The effects of malignant tumor cells-derived exosomes on astrocytes
The exosomes sourced from malignant cells usually serve as a guide for migration and invasion.Glioma is one of the malignant tumors in the CNS.However, the molecular mechanisms underlying the invasion of glioma cells are still poorly understood.Long noncoding RNA (lncRNA) activated by transforming growth factor-β (lncRNAATB) was initially identified in hepatocellular carcinoma.Recent studies suggested that glioma-derived exosomes containing lncRNA-ATB activated astrocytes by inhibiting miR-204-3p in an argonaute 2-dependent manner.In turn, astrocytes activated by glioma-derived exosomes promoted glioma cell migration and invasion(Bian et al., 2019).In addition, exosomes derived from non-solid tumors also showed similar functions.It was reported that exosomes derived from B cell precursor-acute lymphoblastic leukemia cells transported IL-15 to astrocytes,increasing its vascular endothelial growth factor A generation, which increased the leukemic invasion into the CNS (Kinjyo et al., 2019).
The effects of other cell-derived exosomes on astrocytes
Exosomes from other cells are also known to influence the functions of astrocytes.Exosomes containing miR-126 sourced from endothelial progenitor cells protect astrocytes from hypoxia/reoxygenation- and high glucoseinduced injury (Halurkar et al., 2022).Olfactory sheath cells derived exosomes containing α-B-crystalline protein blocked nuclear NF-κB translocation in astrocytes and inhibited neurotoxicity-related astrocyte transcription (Saglam et al., 2021).Besides, Schwann cell-derived exosomes reduced chondroitin sulfate proteoglycan deposition by increasing Toll-like receptor 2 expression in astrocytes through the NF-κB/PI3K signaling pathway, thereby promoting functional recovery in mice after SCI (Pan et al., 2021).Moreover, exosomes derived from obesity-induced endothelial cells promoted astrocyte activation and aggravated brain damage after cerebral ischemia in rats (Perez-Corredor et al., 2023).
The Effects of Astrocyte-Derived Exosomes on Downstream Target Cells
The effects of astrocyte-derived exosomes on neurons
The effect of neuron-derived exosomes on astrocytes has been summarized above.Similarly, the effects of astrocyte-derived exosomes (AS-Exos) on neurons are also relevant to the biological and pathological processes in the CNS.According to a recent study, astrocyte-derived small extracellular vesicles promote synapse formation via fibulin-2-mediated TGF-β signaling (Patel and Weaver, 2021).Besides, AS-Exos perform different biological functions in different CNS diseases.
The effects of AS-Exos on neurons in trauma-related CNS diseases
Traumatic brain injury has high morbidity and mortality, and nearly 69 million TBI cases are reported worldwide each year (Kaplan et al., 2023).TBIinduced neurological dysfunctions and cognitive deficits significantly affect the prognosis of patients (Pavlovic et al., 2019).In rat and mouse models of TBI, AS-Exos decreased the production of reactive oxygen species and neuronal apoptosis by activating the antioxidative nuclear factor erythroid 2-related factor 2 pathway, thereby playing a neuroprotective role (Zhang et al., 2021).Moreover, astrocytes transported exosomes carrying Gap junction alpha-1 (GJA1)-20k, which was derived from the GJA1 mRNA that initiates downstream rather than the first triplet during translation,producing a noncanonical GJA1 protein with an N-terminal full truncation,repairing and protecting the injured neurons in TBI.These GJA1-20k proteins reduced connix43 phosphorylation, restored mitochondrial function, and downregulated the apoptosis rate, thereby promoting neuronal recovery(Chen et al., 2020b).However, AS-Exos is not always neuroprotective in the case of trauma.In a mouse model of SCI, increased expression of proNGF, a pro-form of neurotrophic factor, in exosomes secreted by reactive astrocytes triggered neuronal apoptosis and exacerbated SCI (Cheng et al., 2020).
The effects of AS-Exos on neurons in hypoxic-ischemic cerebral injuries
Currently, researchers have also made a series of advances in studying the effects of AS-Exos on neurons in hypoxic-ischemic cerebral injuries.Another study showed that AS-Exos exerted protective effects on OGD-treated cells by carrying miR-17-5p, which further inhibited the proapoptotic B-cell lymphoma-2 interacting protein 2 expression (Du et al., 2021).Furthermore,miR-190b in AS-Exos inhibited OGD-induced neuronal apoptosis in mice by targeting autophagy-related gene 7 to regulate autophagy (Pei et al.,2020).Prion protein is a glycosylphosphatidylinositol-anchored cell surface glycoprotein that functions as a sensor of oxidative stress (Onodera et al.,2014) and plays an important role in the cellular defense against damage caused by hypoxia, ischemia, excitotoxicity, and hypoglycemia (McLennan et al., 2004; Roucou et al., 2004; Spudich et al., 2005; Onodera et al.,2014).According to these studies, prion protein and neuroglobin in ASExos mediated beneficial cellular responses, improving neuronal survival under hypoxia and ischemic conditions (Guitart et al., 2016; Venturini et al.,2019).Several miRNAs in AS-Exos have protective effects against cerebral ischemia and reperfusion injury.miR-34c in AS-Exos targeted Toll-like receptor 7 and down-regulated the NF-κB/mitogen-activated protein kinase axis.Besides, miR-361 in AS-Exos targeted S-nitrosylation of cathepsin B and down-regulated the adenosine 5′-monophosphate-activated protein kinase/mammalian target of rapamycin pathway (Wu et al., 2020).
In the exploration of the treatment strategies for hypoxic-ischemic cerebral injuries, researchers found that circSHOC2 in ischemic-preconditioned AS-Exos inhibited neuronal apoptosis and improved neuronal damage by regulating autophagy and acting on the miR-7670-3p/sirtuin 1 axis (Chen et al., 2020a).Besides, ischemic-preconditioned AS-Exos were absorbed by neurons and attenuated OGD-induced neuronal death and apoptosis, which was mediated by miR-92b-3p in these exosomes (Chen et al., 2020a).Exosomes secreted by ischemic astrocytes treated with semaphorin 3A inhibitors further promote neuronal axon elongation (Hira et al., 2018).However, exosomes derived from reactive astrocytes induced neuronal death by carrying injurious lipocalin-2(Liu et al., 2022b).
The effects of AS-Exos on neurons in chronic neurodegenerative diseases
Alzheimer’s disease is a devastating neurodegenerative disease associated with the build-up of protein aggregates within and surrounding neurons in the hippocampus and cortex (Goedert and Spillantini, 2006).Amyloid-β (Aβ)peptides generated from the amyloid precursor protein play critical roles in the development of AD.According to recent studies, exosomes derived from cholesterol-accumulated astrocytes played important roles in trafficking amyloid precursor protein/Aβ peptides and influenced neuronal viability in the affected regions of the AD brain (Wu et al., 2021).In another interesting study, AS-Exos increased 5-fold in number when astrocytes were stimulated by ultrasonic energy in cellular experiments, and these exosomes reduced the toxicity of Aβ to neurons, exerting neuroprotective effects on AD mice(Deng et al., 2021).Apolipoprotein D is one of the few genes consistently over-expressed in the aging brain of all vertebrate species tested so far.However, apolipoprotein D is reported to ameliorate membrane injuries,facilitating cell survival under oxidative stress (Loerch et al., 2008).The study in 2018 showed that AS-Exo protected neuronal function by transporting apolipoprotein D to neurons (Pascua-Maestro et al., 2018).Furthermore, it can affect neurodegenerative diseases by regulating the balance of metabolic homeostasis and ubiquitin-dependent protein metabolism (Xie et al., 2022).A study on Parkinson’s disease found that AS-Exos delivered miR-200a-3p to neurons, exerting neuroprotective effects by down-regulating the expression of mitogen-activated protein kinase 4 (Shakespear et al., 2020).AS-Exos also released glutathione transferase M2-2, which prevented dopaminergic neurons from amino pigment neurotoxicity (Segura-Aguilar et al., 2022).
While the vast majority of clinical amyotrophic lateral sclerosis (ALS) cases are sporadic, mutations in the human copper-zinc superoxide dismutase 1(SOD1) cause about 20% of all inherited cases of ALS (Renton et al., 2014).AS-Exos transferred mutated copper-zinc SOD1 to spinal cord neurons, and then selectively induced the death of motor neurons (Basso et al., 2013;Silverman et al., 2019).Recent studies showed that IL-6 levels in AS-Exos are elevated in patients with ALS and positively correlated with the rate of disease progression (Chen et al., 2019b).Furthermore, in patients with AD and TBI, the levels of complement in AS-Exos were elevated, which potentially damaged neurons (Goetzl et al., 2018, 2020).Huntington’s disease is a fatal,autosomal-dominant, inherited neurodegenerative disorder that is caused by an expanded polyglutamine repeat in the N-terminal region of the huntingtin gene (Ross and Tabrizi, 2011).According to a study in 2017, the mutated huntingtin protein (mHtt) reduced the expression of αB-crystal protein and the number of exosomes secreted by astrocyte cells, thereby contributing to non-cell-autonomous neurotoxicity in Huntington’s disease (Hong et al.,2017).
The effects of AS-Exos on neurons in infectious diseases in the CNS
Hu et al.(2012) found that AS-Exos also participated in the pathological processes of human immunodeficiency virus (HIV)-related neurological diseases.Thein vitroexperiment indicated that the expression of miR-29 in AS-Exos was increased under the stimulation of morphine and HIV transactivating regulatory protein (Tat), resulting in the decreased expression of PDGF-B in the neuronal SH-5Y5Y cells, which impaired neuronal viability (Hu et al., 2012).Another study showed that HIV Tat induced the expression of miR-132 in AS-Exos which impaired the functions of neurons by shortening their synapses (Rahimian and He, 2016).Furthermore, the human immunodeficiency virus type 1 (HIV-1) accessory protein, Nef, in AS-Exo was also involved in CNS damage (Pu?ar Dominku? et al., 2017).
The effects of AS-Exos on other glial cells
Astrocytes, oligodendrocytes, and microglia are the major glial cell types in the mammalian CNS (Though microglia are myeloid cells in the CNS, they are discussed in this part together with other glial cells).These glial cells actively modulate neural activity and brain functions, and they also play essential roles in the development and progression of diverse neurological diseases.Their activation and malfunctions occur in a distinct spatial and temporal pattern,and frequent molecular conversations always exist between these cells during these activation processes (Jha et al., 2019).The role of microglia in TBI is a double-edged sword based on their different polarizations (Loane and Kumar,2016; Paolicelli et al., 2022).Therefore, promoting microglia phenotypic transformation in specific circumstances may be a possible therapeutic strategy for TBI.A recent study showed that activated AS-Exos enriched with miR-873a-5p ameliorated cerebral injury and improved neurological function after TBI by promoting microglial polarization into another phenotype via inhibiting extracellular regulated protein kinases and NF-κB p65 phosphorylation (Long et al., 2020).Another study indicated that astrocytes stimulated by HIV Tat upregulated the expression and release of miR-9 in the exosomes.These miR-9 enriched AS-Exos were taken up by microglia,increasing the migration of microglia by downregulating the expression of the target of miR-9, PTEN, a critical suppressor of cell motility (Yang et al.,2018).The study by Hu et al.(2018) showed that morphine-exposed ASExos were internalized by microglia, activating Toll-like receptor 7, followed by upregulation of lncRNA-cyclooxygenase-2 expression, ultimately leading to impaired microglial phagocytosis.In the mouse model of experimental autoimmune encephalomyelitis induced by myelin oligodendrocyte glycoprotein and pertussis toxin, extracellular α-B-crystalline protein in ASExos suppressed inflammation via inhibiting microglial activation.Therefore,augmentation of α-B-crystalline protein-mediated interaction between astrocytes and microglia may be a potential approach for the therapeutic intervention in multiple sclerosis (Guo et al., 2019).Oligodendrocytes in the CNS construct myelin, a multilayered lipid membrane structure that wraps axons to enhance the speed of action potential propagation and provide axons with metabolic trophic support (Moore et al., 2020).During development,and throughout adulthood, myelinating oligodendrocytes are generated through the terminal differentiation of oligodendrocyte progenitor cells(OPCs).OPCs are CNS-resident stem cells that originate in the brain and spinal cord ventricular zones before proliferating and migrating to populate the CNS (Richardson et al., 2006).Appropriate regulation of OPCs differentiation is critical for the proper development and function of the CNS.According to a recent study, AS-Exos improved the differentiation and maturation of OPCs and facilitated OPCs migration.However, AS-Exos under severe hypoxia significantly inhibited the proliferation of OPCs.Therefore, AS-Exos under specific conditions may be hold therapeutic potential for myelin regeneration and repair in white matter injury or other demyelinating-related diseases (Xu et al., 2021).
The effects of AS-Exos on tumors in the CNS
Glioblastoma is one of the most aggressive malignant tumors in the CNS,requiring further investigation for prognostic biomarkers and methods of early diagnosis.SAM and SH3 domain-containing 1 is a member of the SLY family of scaffold proteins widely expressed in normal brain tissues and astrocytes, manifesting tumor suppressive functions (Yang et al., 2015).It was reported that high mobility group box 1 in AS-Exos increased the expression of SAM and SH3 domain-containing 1 when acting on glioma C6 cells (Ma et al., 2019).Diffusive infiltration of tumor cells throughout the brain is a core characteristic of high-grade glioma, and it is responsible for treatment failure even if maximal surgical resection is performed (Cloughesy et al.,2014; Sanai and Berger, 2018).Although temozolomide has been shown to prolong survival, the treatment response is usually limited by the progression of drug resistance (Hombach-Klonisch et al., 2018).A study showed that O6-alkylguanine DNA alkyltransferase mRNA levels were significantly higher in reactive AS-Exos than in non-reactive astrocytes, and Exos derived from normal human astrocytes reversed temozolomide sensitivity in glioma cellsin vivo(Yu et al., 2018).In addition, AS-Exos ameliorated the brain metastasis of lung adenocarcinoma by transporting miR-142-3p, targeting oncogenic transient receptor potential ankyrin-1 (Berrout et al., 2017).
Summary and Outlook
In this review, we have summarized the exosome-based intercellular communication focused on astrocytes.The exosomes from stem cells,neurons, immune cells, malignant cells, olfactory sheath cells, Schwann cells, and endothelial cells can affect the functions of astrocytes (Table 1).Conversely, the AS-Exos can influence the functions of other cells such as neurons, microglia, oligodendroglia, malignant cells, and other cell types(Table 2).
Exosome-based intercellular communication focused on astrocytes are a valuable study target in the experimental research of the CNS because of its characteristics (Zhou et al., 2019).As the major cell type in the CNS, astrocytes are considered an essential source and crucial receptors of exosomes, which deserves further confirmation.Additionally, although astrocytes are not the major cell type accounting for the essential function, signal initiation,and transduction in the CNS, they play critical roles in supportive functions,namely filling gaps between neurons, maintaining homeostasis, participating in the formation of the blood-brain barrier, and repairing injured brain tissue..Therefore, the large amount and critical functions of astrocytes make them meaningful targets and sources of exosomes.
The study on exosome-based intercellular communication has formed its unique research paradigm, such as investigating the source cells, contents,target cells and the functions of exosomes.There is no doubt that the components of exosomes are the most important aspect being focused on by researchers.The components of exosomes depend on their generators and the conditions imposed on their generators.For example, with respect to astrocytes, under OGD or inflammatory conditions, their exosomes may exert protective effects on neurons (Chen et al., 2020a; Long et al., 2020).Whereas, under Aβ and HIV infection conditions, AS-Exos may exacerbate neuronal damage (Hu et al., 2012; Wu et al., 2021).On the other hand, it was found that exosomes work as a network rather than a line from one cell to the other cells.Astrocytes receive exosomes from microglia, neurons, stem cells,and even malignant cells, and the exosomes from astrocytes also influence the multiple cell types in the CNS.Hence, we should put astrocytes into the whole network of exosome-based intercellular communication under specific conditions and comprehensively consider the effects of these exosomes from different sources on astrocytes and the different biological effects of AS-Exos.In recent years, several new techniques have emerged to investigate the cellular sources of exosomes in different tissues.The simplest way to grasp the cellular sources of exosomes is to test the levels of the markers of different cells in the tissue-derived exosomes (Brenna et al., 2020).The method by Liu et al.(2022a) can better manifest the percentage of tissuederived exosomes in every single cell.This method overlapped the “cluster maker” of each cell cluster identified in the single-cell RNA sequencing and the exosomes protein list obtained from the protein mass spectrometer.Then, the proportion of each protein in the exosome protein list and the transcriptional level of the gene encoding this protein in every single cell were matched by algorithms to identify the cellular sources of tissue-derived exosomes.The method by Wu et al.(2019) provided researchers with the prospect to analyze the surface proteins on individual exosomes.Theyreported a new method called proximity-dependent barcoding assay.Briefly,they attributed a barcode to each exosome, and the surface proteins of each exosome were also signified.This method can better analyze the cellular sources of tissue-derived exosomes.With these new methods, the role of AS-Exos can be separately investigated under the background of exosomebased intercellular communication in the brain.Furthermore, it may also be possible to use more advanced imaging platforms to detect exosome release in real time and high-throughput screening and computer-aided drug design platforms to design engineered exosomes to target astrocytes (Wevers et al.,2018; Aldewachi et al., 2021; Salman et al., 2021).Exosomes belong to extracellular vesicles; however, as it is sometimes difficult to define exosomes and extracellular vesicles, it is possible that some of the studies may have missed certain related results.
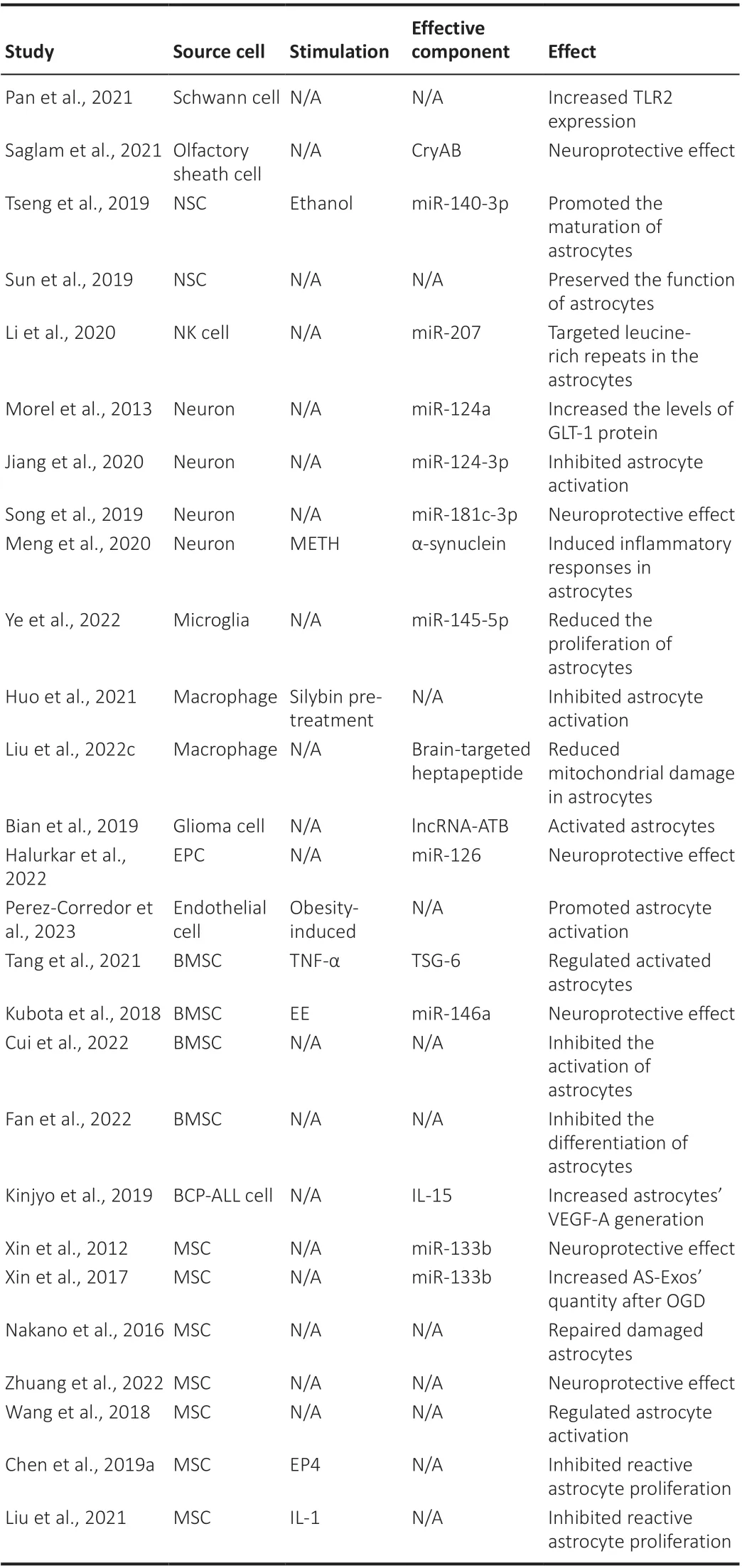
Table 1 |The effects of upstream cell-derived exosomes on astrocytes
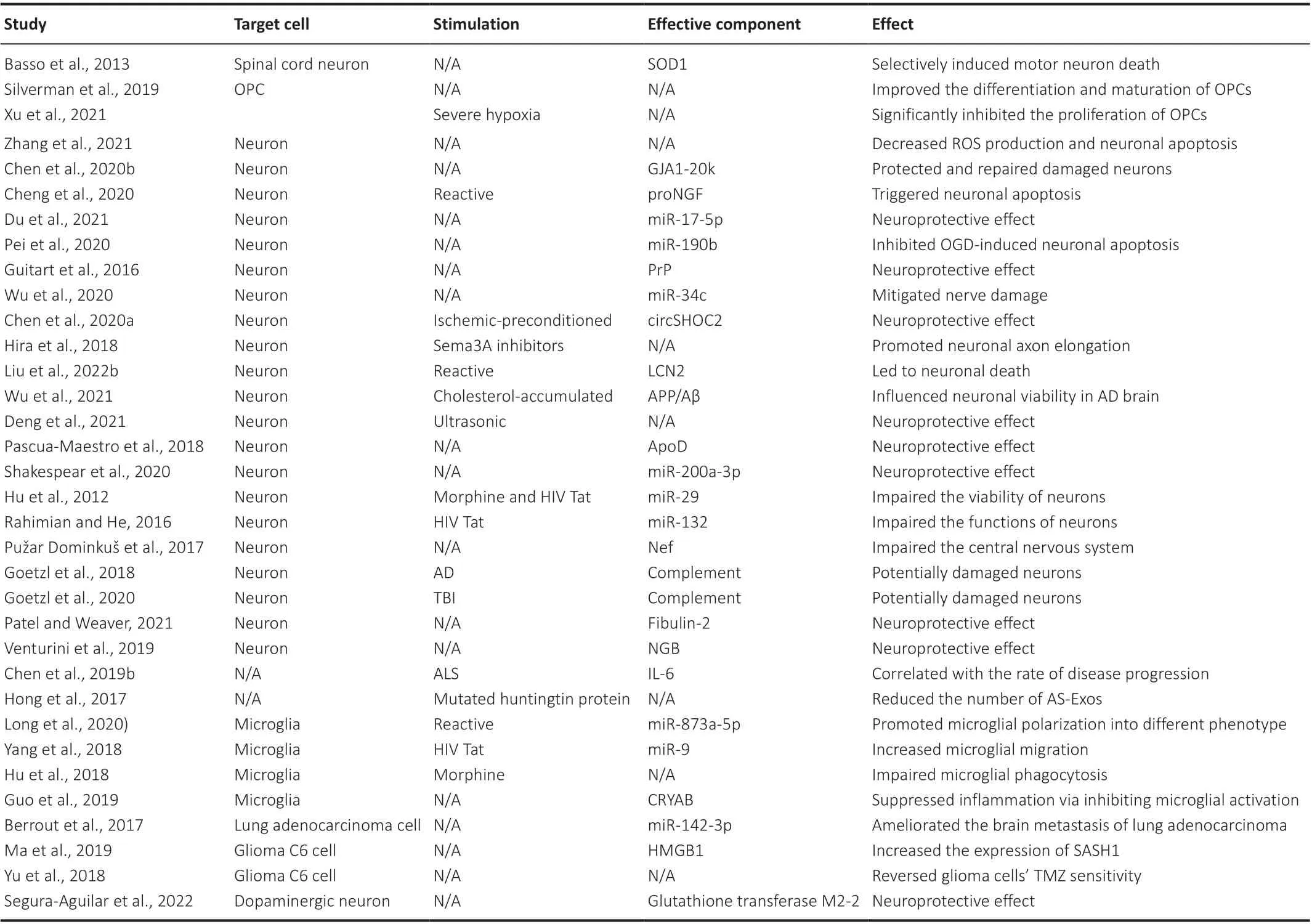
Table 2 |The effects of AS-Exos on downstream target cells
In summary, the exosome-based intercellular communication focused on astrocytes is of great significance to the biological or pathological processes in different brain conditions.Novel technologies can provide us with new perspectives to better understand the role of astrocytes in this network.
Author contributions:Conceptualization and manuscript revision: XZ, TG, PY;literature collection and analysis: YG, HM, HL, WH, LZ, JL; manuscript draft:HX, HL, PZ.All authors approved the final version of the manuscript.
Conflicts of interest:The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability statement:The data are available from the corresponding author on reasonable request.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Paula Gabriela Franco, Universidad de Buenos Aires,Argentina; Carsten Theiss, Ruhr-Universitat Bochum, Germany.
Additional file:Open peer review report 1.
 中國(guó)神經(jīng)再生研究(英文版)2024年9期
中國(guó)神經(jīng)再生研究(英文版)2024年9期
- 中國(guó)神經(jīng)再生研究(英文版)的其它文章
- Does MgSO4 protect the preterm brain? Dissecting its role in the pathophysiology of hypoxic ischemic encephalopathy
- Exosomes derived from microglia overexpressing miR-124-3p alleviate neuronal endoplasmic reticulum stress damage after repetitive mild traumatic brain injury
- On implications of somatostatin in diabetic retinopathy
- Rebuilding insight into the pathophysiology of Alzheimer’s disease through new blood-brain barrier models
- Post-transcriptional mechanisms controlling neurogenesis and direct neuronal reprogramming
- Hypothalamic circuits and aging: keeping the circadian clock updated
