Molluscicidal effect of green synthesized silver nanoparticles using Azadirachta indica on Biomphalaria alexandrina snails and Schistosoma mansoni cercariae
Salwa S. Younis, Iman F. Abou-El-Naga?, Khaled H. Radwan
1Medical Parasitology Department, Faculty of Medicine, Alexandria University, Alexandria, Egypt
2Agriculture Genetic Engineering Research Institute, Cairo, Egypt
ABSTRACT Objective: To assess the molluscicidal effect of the eco-friendly green synthesized neem silver nanoparticles (neem-Ag NPs) against Biomphalaria alexandrina, the snail intermediate host for Schistosoma mansoni, and their cercaricidal potential.Methods: Methanol extracts from neem fruits were used for green synthesis of neem-Ag NPs. The neem-Ag NPs were characterized using UV-visible absorption spectra, dynamic laser light scattering technique, and transmission electron microscopy. The potential molluscicidal effect against adult and juvenile Biomphalaria alexandrina and the effect of the sub-lethal concentration on hatching of snail eggs and Schistosoma mansoni cercariae were evaluated. Results: The surface plasmon resonance of neem-Ag NPs showed a sharp absorption peak at λmax = 518 nm together with multiple peaks. The hydrodynamic diameter was (77.15±34.53) nm, the polydispersity index (0.338±0.000) and the zeta-potential –14.07 mV. Moreover, transmission electron microscopy showed that the average size of the nanoparticles was (27±2) nm. Agglomeration was evident and a light-colored capping layer could be seen coating the nanoparticles. Juvenile snails (LC50: 0.83 ppm) were more susceptible to neem-Ag NPs than adults (LC50: 1.07 ppm). In addition, neem-Ag NPs and neem at LC50 concentrations inhibited the egg-hatching of snails and showed cercaricidal activity in a timedependent manner. Conclusions: Neem-Ag NPs have lethal activities against Biomphalaria alexandrina snails and their eggs, as well as Schistosoma mansoni cercariae. Hence, neem-Ag NPs could be a potential agent to control schistosomiasis.
KEYWORDS: Biomphalaria alexandrina; Neem; Schistosoma mansoni; Azadirachta indica; Silver nanoparticles; Molluscicide
1. Introduction
Schistosomiasis is a debilitating highly prevalent neglected infectious disease in many tropical and subtropical countries. It is the second most important parasitic disease after malaria in terms of social, economic, and public health impact[1]. Snails of genus Biomphalaria are the intermediate host of Schistosoma mansoni (S. mansoni) and Biomphalaria alexandrina (B. alexandrina) (Ehrenberg 1813) is the snail host in Egypt[2]. The climate conditions suitable for the snails contributed to the high endemicity of schistosomiasis[3]. Chemotherapy using praziquantel has been the most adopted means to control the disease. However, the excessive use of the drug raises concerns about the emergence of resistant parasites[4]. Other strategies for controlling schistosomiasis include the use of molluscicidal agents against the intermediate hosts, along with health education and good sanitation[5]. World Health Organization[6] recommends the use of niclosamide, a synthetic molluscicide, as a strategy to combat the disease. The combination of niclosamide and mass chemotherapy is considered one of the best ways to interrupt the transmission cycle of Schistosoma. However, the high production value of niclosamide and its toxicity to non-target organisms are major setbacks for its general adoption[7]. Because chemical molluscicides have adverse environmental effects, some plants that are inexpensive, effective for snail control, and safe for aquatic organisms could be a suitable alternative to control snails[8].
Azadirachta indica A. Juss is a tree commonly known as neem and has been used in traditional medicine, especially in India, for thousands of years. Now, it is widely distributed in Africa, Asia, and many tropical parts of the world[9]. Many promising phytochemicals such as azadirachtin, nimbolide, gedunin, and azadirone can be extracted from different parts of neem tree. Neem has a wide range of effects including anticancer, antimicrobial, antipyretic, antiinflammatory, antidiabetic, and pesticidal activities. Therefore, neem tree is known as a “village pharmacy” and is considered by the United Nations as “The tree of the 21st century”[9]. In addition to these various activities, neem has also shown molluscicidal effects against many snails including Biomphalaria[10]. Neem extracts have no or very low acute toxicity to aquatic organisms while subacute and sub-chronic toxicity can be eliminated by using lower concentrations[11].
The application of nanotechnology in biomedicine has attracted increasing interest with the main goal of efficient delivery of bioactive ingredients and thus dose reduction. It has been widely applied against various parasites[12], and most notably against Schistosoma[13,14]. Silver nanoparticles (Ag NPs) are increasingly used in medical applications and have been used to control freshwater snails including Biomphalaria snails[15,16]. Ag NPs are also effective against cercariae, the infective stage of Schistosoma parasite[15-17]. These nanoparticles have been synthesized by different physical, chemical, and biological methods. However, biological methods have drawn significant attention over other methods, especially their green synthesis using various plant extracts. This can be attributed to their eco-friendly nature, economic value, high availability, and stability offered by plants to synthesize nanoparticles[18].
Neem plant extracts have been used as bio-reducing agents to convert silver nitrate to Ag NPs and as stabilizers for the nanoparticles. Capping and stabilizing effects of neem enhance the activities of Ag NPs in wound healing and treatment of diabetes and increase the antibacterial effect[19,20]. The availability, bio-reducing, and stabilizing effects of neem, and the proven molluscicidal activity of Ag NPs, together with the environmental safety of neem, could make neem-Ag NPs a desirable molluscicidal compound. The application of green synthesized nanoparticles to control snails is a novel and under-explored approach[21]. The present work aimed to evaluate the molluscicidal potential of neem-Ag NPs on the adult, juvenile, and egg stages of B. alexandrina snails, as well as their activity against the cercarial stage of the parasite. Moreover, the effect of neem extracted from the ripe fruits was also assessed.
2. Materials and methods
2.1. Preparation of neem extract
Ripe fruits were collected from Alexandria governorate in January (the rainy season in Egypt) from neem trees; Azadirachta indica, after identification by specialists at the Botany Department, Faculty of Agriculture, Alexandria University. The co-ordinates of the collection site are between 30° 53’ 35.99” N, 29° 23’ 09.92” E and 31° 16’ 05.89” N, 30° 09’ 06.25” E. About 210 g of the ripe fruits were rinsed under running tap water, and the clean fruits were dried in the shade and then ground. The fine powder of neem fruits (100 g) was soaked with 1 000 mL of methanol in a 2 000 mL conical flask and kept on an orbital shaker (Stuart, England) at 160 rpm at room temperature for 24 h. The extract was filtered through Whatman No.1 filter paper. Plant residues were re-extracted twice with methanol. The pooled filtrates were concentrated under a vacuum at 40 ℃ to dryness. For experimental application, different concentrations (10 ppm, 15 ppm, 20 ppm, 40 ppm, and 60 ppm) from the dried crude extract were freshly dissolved in dechlorinated tap water.
2.2. Preparation of green synthesized Ag NPs
Neem-Ag NPs were prepared in the presence of neem extract as a reducing agent according to previous reports with minor modifications[20]. Nine mL of neem extract was added to 36 mL of an aqueous solution of 1 mM silver nitrate (AgNO3) (Sigma-Aldrich Chemical Co) with vigorous stirring. The reaction vessel was kept in the dark at 37 ℃ in a water bath for 5 h. The color change of the solution to dark brown indicates the formation of Ag NPs. Finally, the Ag NPs produced were purified by centrifugation at 20 000 rpm for 30 min. The pellets were washed several times with distilled water and resuspended in sterile deionized water.
2.3. Characterization of neem-Ag NPs
Surface plasmon resonance (SPR) for both neem extract and neem-Ag NPs was recorded using UV-visible absorption spectra in the wavelength range 100-1 000 nm. The hydrodynamic particle size distribution and zeta potential of aqueous dispersions of neem-Ag NPs were measured by dynamic laser light scattering technique using Malvern Zeta sizer. Dynamic light scattering (DLS) and electrophoretic mobility for Ag NPs in water were performed to characterize the secondary size and the polydispersity index (PDI) of the formulation as indicators for the homogenous particle size distribution of the prepared nanoparticles. Zeta potential was measured to determine the charge of the prepared nanoparticles after dilution with deionized water[20,22]. The size and shape of neem-Ag NPs were determined by transmission electron microscopy (TEM). Approximately 10 μL from the diluted prepared samples were placed on a 200-mesh carbon membrane-covered copper grid. After 30 s, excess fluid was removed with a piece of filter paper and air-dried. Samples were negatively stained with uranyl acetate.
2.4. Snails, their egg masses, and cercariae of S. mansoni
Adult (8-10 mm), juvenile (3-4 mm), and infected adult B. alexandrina snails were purchased from the Schistosome Biological Supply Centre, Theodor Bilharz Research Institute, Giza, Egypt. Adult, juvenile, and infected snails were cultivated separately in glass aquaria containing dechlorinated tap water at (25±2) ℃ and fed fresh lettuce. Pieces of polyethylene sheets were used to collect the egg masses from the uninfected snails. Cercariae were collected from infected snails after exposure to direct sunlight for approximately 1-2 h[23].
2.5. Molluscicidal bioassay tests for adult and juvenile B. alexandrina snails
Molluscicidal bioassay testing was performed on adult and juvenile B. alexandrina snails according to World Health Organization guidelines[24]. Ten adults and ten juvenile snails were placed in each test container containing 50 mL of varying concentrations of neem (10 ppm, 15 ppm, 20 ppm, 40 ppm, and 60 ppm) and neem-Ag NPs (0.5 ppm, 1 ppm, 1.5 ppm, 2 ppm, and 2.5 ppm) diluted with dechlorinated tap water, for 24 h at (25±2) ℃. The snails were washed in dechlorinated tap water and allowed to recover for a further 24 h. Snails with the same corresponding size were exposed to dechlorinated tap water only as control groups. Ten snails were used for each concentration and the experiment was done in triplicate. At the end of the recovery period, the snails were examined and considered dead if they lack the response to gentle irritation with a blunt object, their shell was discolored or their visceral masses were exposed. Dead snails were counted and the lethal concentrations (LC) of neem and neem-Ag NPs were estimated by probit analysis.
2.6. Effect of neem and neem-Ag NPs on hatching of the egg masses
The effect of neem and neem-Ag NPs on hatching of egg masses was carried out on adult B. alexandrina egg masses using the ~15 h old blastula stage. Ten egg masses (adding up to an average of 100 embryos) were collected from the culture of uninfected snails. Egg masses were placed in two test containers containing the estimated LC50concentration of neem and neem-Ag NPs on the adult snails. After 24 h of exposure, the egg masses were transformed into Petri dishes containing dechlorinated tap water. Three or four additional egg masses in dechlorinated tap water were used as controls. Each experiment was carried out in three containers, under light/dark cycles of 12:12 h at (25±2) ℃. Eggs were observed daily until the negative control embryos were fully hatched[25].
2.7. Cercaricidal potential of neem and neem-Ag NPs
Shedding cercariae from previously infected B. alexandrina snails were collected after exposure to sunlight for 1-2 h. One hundred cercariae were transformed into Petri dishes containing 10 mL of the determined LC50concentration of neem or neem-Ag NPs on the adult snails. Assays were performed in triplicate. The same number of cercariae in another Petri dish containing 10 mL of dechlorinated tap water served as a control. Cercariae were observed under a dissecting microscope at successive intervals of 15, 30, 45, 60, 90, and 120 min to monitor their activity and survival. Immobilized cercariae or those with detached tails were considered dead. Mortality rate was calculated.
2.8. Statistical analysis
Data were analyzed using the statistical program for Social Science (SPSS) version 28. The lethal concentration (LC10, LC25, LC50, and LC90) values of neem and neem-Ag NPs for adult and juvenile snails and the 95% confidence limit (CL) of LC50were calculated using the probit regression graphing. The experiment in each group was done in triplicate, and the median and range (min-max) were determined. The significant differences in mortality of the adult and juvenile snails exposed to different concentrations of neem and neem-Ag NPs in comparison to their control group (snails in dechlorinated tap water) were determined by Kruskal Wallis H test. Post hoc test (Dunn’s multiple comparsion test) was used for comparison between different concentrations of the tested agents. The level of significance was set at P<0.05.
2.9. Ethical statement
All animals in this study were handled in accordance with the ethical guidelines of the Faculty of Medicine, Alexandria University. The experiment was performed under protocol number (0305489) approved on 17th March 2022. This study was in accordance with the ethical animal guidelines and regulations set by the Alexandria University Ethics Committee (00012098) for animal experimentation in accordance with the internationally accepted principles for laboratory animal use and care.
3. Results
3.1. Characterization of neem-Ag NPs
Incubation of neem with 1 mM aqueous solution of AgNO3for 5 h changed the color of the reaction mixture from colorless to light yellow and eventually reddish-brown. Analyses of neem and neem-Ag NPs by the UV-visible absorption spectra showed that neem exhibited two peaks. The silver coating led to a shift of the curve of the coated sample with respect to the neem curve and a decrease in the two neem peaks, which could b attributed to the increase in particle size. The SPR due to Ag resulted in a sharp absorption peak in the visible region of the electromagnetic spectrum at λmax= 518 nm. The multiple peaks were due to core-shell design of the coated nanoparticles (Figure 1A).
DLS data for neem-Ag NPs showed that the hydrodynamic diameter (Dh) was (77.15±34.53) nm (Figure 1B) and the PDI was 0.338±0.000. The higher average particle size compared to TEM data was due to the hydrophilic nature of the prepared particles that tend to aggregate or form self-assembly, as shown in TEM images. The zeta potential for neem-Ag NPs was –14.07 mV (Figure 1C).
The TEM micrograph for the prepared neem-Ag NPs showed that the average size of the nanoparticles was (27±2) nm. Agglomeration was evident and a light-colored capping layer could be seen coating the nanoparticles (Figure 1D).
3.2. Molluscicidal activities of neem and neem-Ag NPs against adult and juvenile B. alexandrina snails
Neem and neem-Ag NPs demonstrated molluscicidal activity against B. alexandrina snails. No mortality was detected in the negative control snails. The mortality of adult and juvenile snails exposed to different concentrations of neem and neem-Ag NPs was significantly higher compared to the negative control (P<0.05). The mortality increased with increasing concentration of neem and neem-Ag NPs (Table 1). Juvenile snails were found to be more sensitive to the toxic effect of neem and neem-Ag NPs than adults (P<0.001). The effect of neem and neem-Ag NPs on adult and juvenile snails was found to be concentration-dependent (Figure 2A and B).
Probit analysis showed that the LC50and LC90concentrations of neem for adult snails were 31.51 and 75.42 ppm, respectively, while for juvenile snails the values were 12.45 and 20.30 ppm, respectively (Figure 3A). The LC50and LC90concentrations of neem-Ag NPs for adult snails were 1.07 and 3.89 ppm, respectively and for juvenile snails were 0.83 and 2.18 ppm, respectively (Figure 3B). Table 2 shows the different LC values of neem and neem-Ag NPs on adult and juvenile snails.
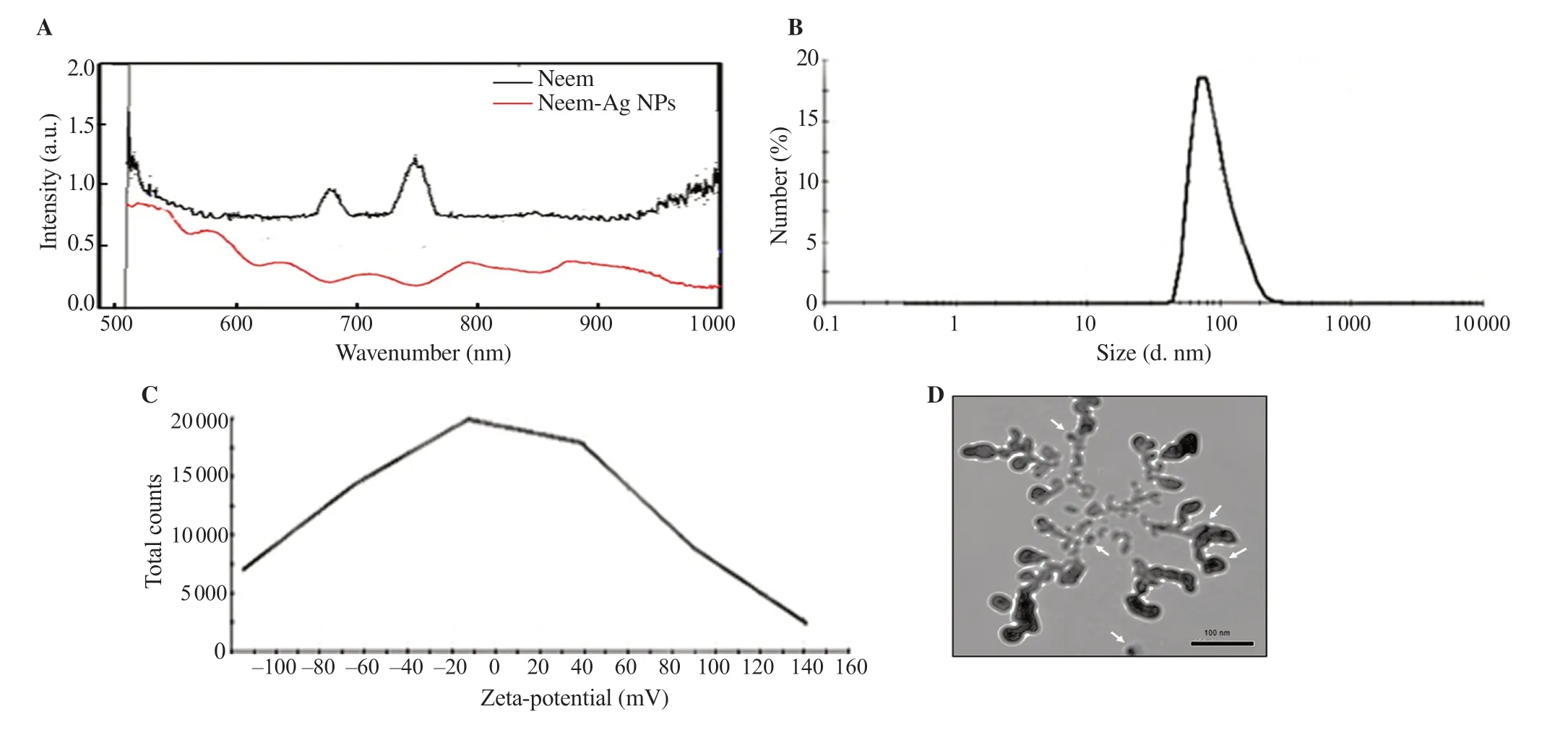
Figure 1. (A) UV-visible absorption spectra of neem-Ag NPs showing a sharp absorption peak at λmax = 518 nm and other multiple peaks. (B) Dynamic light scattering measured by Malvern Zeta sizer shows that the hydrodynamic diameter (Dh) is (77.15±34.53) nm and (C) the zeta-potential for neem-Ag NPs is –14.07 mV. (D) TEM shows that the average size of the nanoparticles is (27±2) nm. Agglomeration is evident and a light-colored capping layer could be seen coating the nanoparticles.
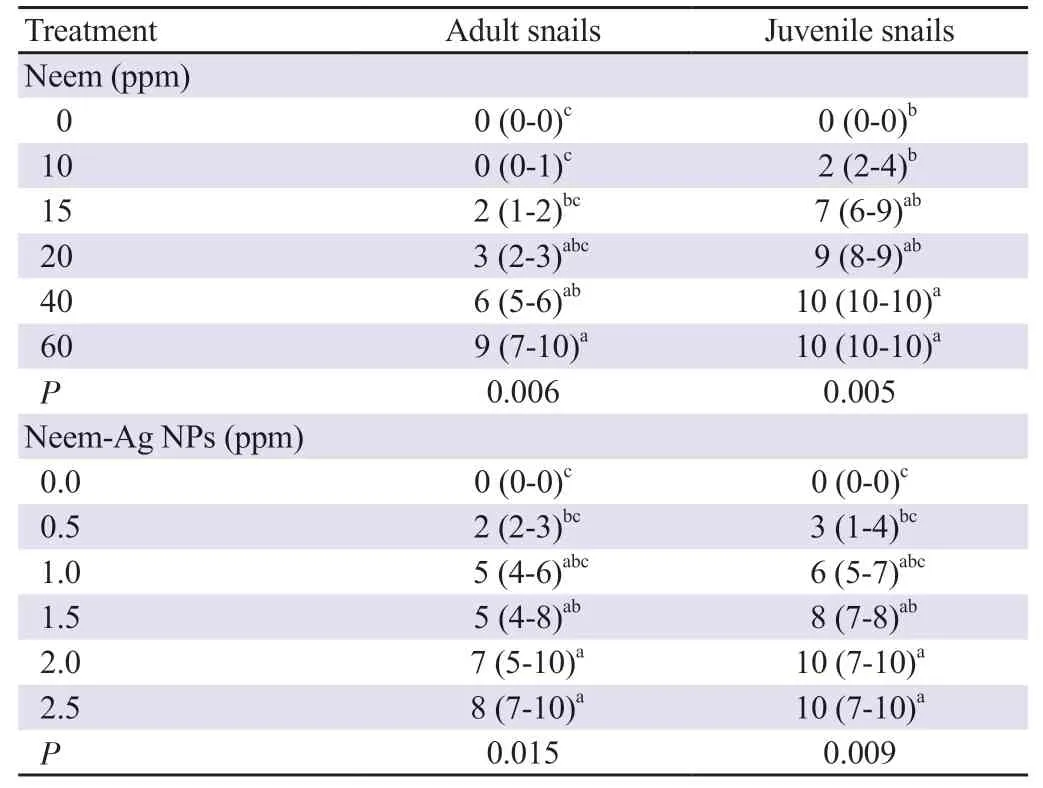
Table 1. Median mortality of adult and juvenile Biomphalaria alexandrina snails using different concentrations of neem and neem-silver nanoparticles (neem-Ag NPs) [%, median (min-max)].
3.3. Effect of neem and neem-Ag NPs on egg hatching
The results showed that exposure to LC50concentration of neem (31.51 ppm) and neem-Ag NPs (1.07 ppm) inhibited hatching of the egg masses by 100%. All negative control egg masses hatched at (16±2) d.
3.4. Effect of neem and neem-Ag NPs on S. mansoni cercariae
The mortality rate of S. mansoni cercariae exposed to LC50concentration of neem and neem-Ag NPs was found to be timedependent as shown in Figure 4A. Cercaricidal activity was first detected after 30 min exposure to neem and neem-Ag NPs, leading to 10% (8%-12%) and 20% (15%-40%) mortality rates, respectively. The mortality rate increased to reach 50% (45%-55%) and 65% (60%-85%) at 45 min and complete death of cercariae was achieved at 60 min. The difference in the mortality rate between neem and neem-Ag NPs at each time point was statistically insignificant. Figure 4B shows that the cercariae treated with dechlorinated tap water had normal rotation and motility accompanied by preservation of the tail. After 90 and 120 min, 0% (0%-5%) and 5% (0%-10%) of cercariae died, respectively. During the exposure to neem and neem-Ag NPs, some cercariae revealed slow rotation around their axes with some contractions, others were dying with dropped head and some were dead with separation of the head and tail. Some examples of alive, dying and dead cercariae are shown in Figure 4B.

Figure 2. Effects of different concentrations of (A) neem and (B) neem-Ag NPs on adult and juvenile Biomphalaria alexandrina snails.
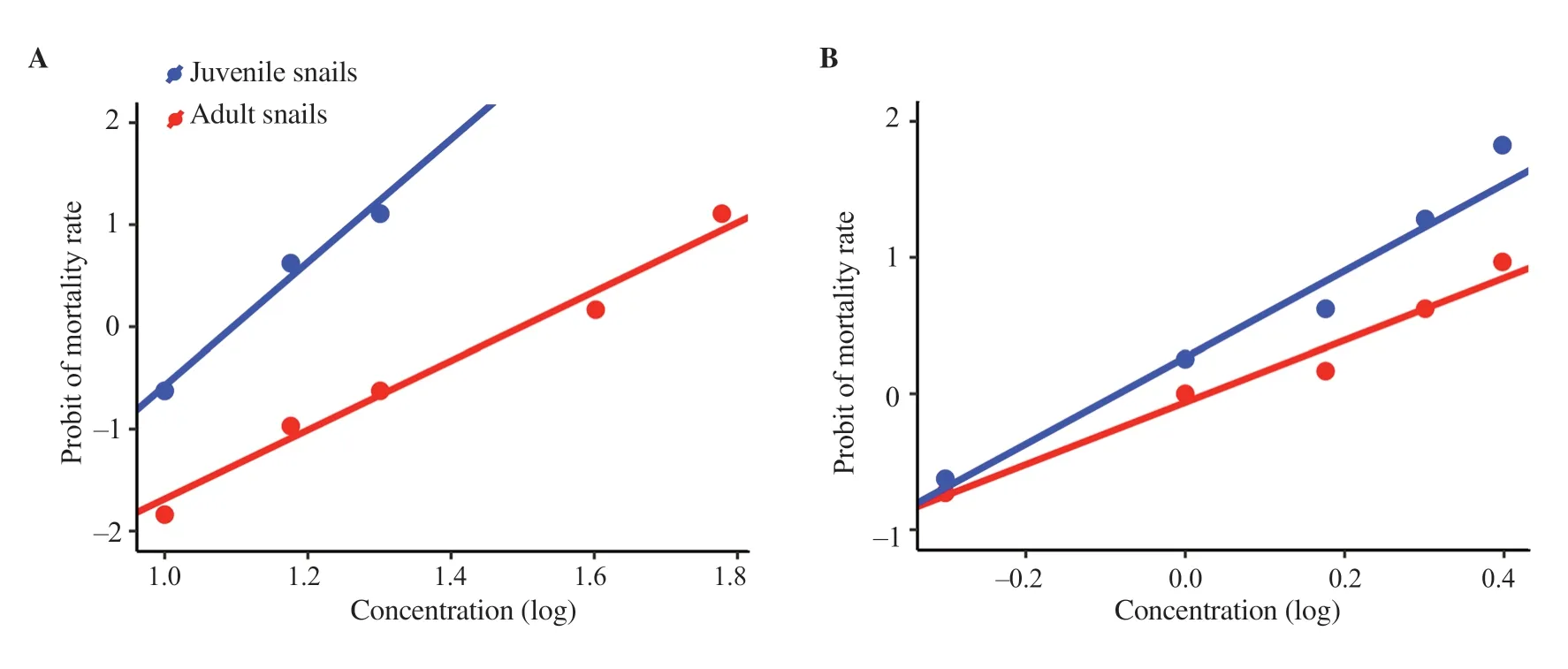
Figure 3. Lethal concentrations of (A) neem and (B) neem-Ag NPs against adult and juvenile Biomphalaria alexandrina snails.

Table 2. Lethal concentrations (LC) of neem and neem-Ag NPs against adult and juvenile Biomphalaria alexandrina snails.
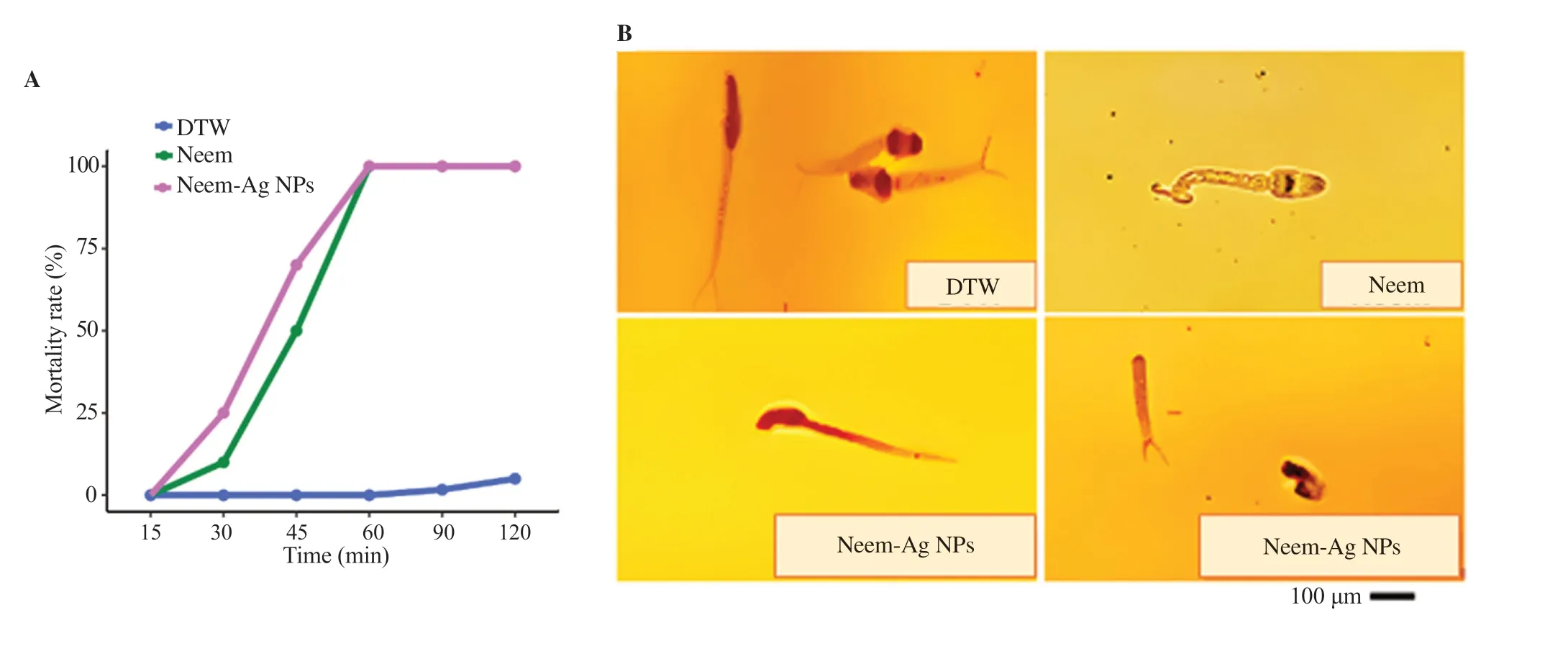
Figure 4. (A) A time-dependent effect of LC50 concentrations of neem and neem-Ag NPs on mortality of cercariae. (B) Cercarial behavior during exposure to different treatments. For the control group (DTW), the image shows live cercariae with preservation of tail. A dying cercaria with tail contractions is shown in the neem exposed group. For the neem-Ag NPs exposed group, one image shows a dying cercaria with tilted head and another image shows a dead cercaria with complete separation of head and tail (×400). DTW: dechlorinated tap water.
4. Discussion
Several physical and chemical methods are adopted for the synthesis of Ag NPs. However, the development of a plant-mediated green synthesis as a simple and eco-friendly biological system would help reduce substances that are harmful to human health and the environment[19]. The rationale for using plants for the synthesis of Ag NPs is that they are highly available, safe to handle, and have variable-reducing metabolites[21]. In the current study, bioreduction of silver nitrate by neem extract led to the production of neem-Ag NPs. The nanoparticulate formula was visually identified by a notable color change that started almost immediately and increased over time until it turned brown. This property is wellknown to Ag NPs[20]. Formation of neem-Ag NPs was confirmed using UV-Visible spectroscopy. A sharp SPR band was detected at λmax= 518 nm. Decreasing the size of the particles increases the excitation of the electrons at the outer surface[26]. Many other peaks were also detected due to the design of the core-shell of the coated nanoparticles. Several studies have shown that neem is a suitable plant source for the green synthesis of Ag NPs due to the high content of natural reducing agents, including flavonoids, terpenoids, and phenolic compounds[22,27].
The hydrodynamic diameter of neem-Ag NPs was found to be larger than the original diameter obtained from TEM results. This inconsistency may be due to weak adhesion of two or more colloidal particles in the solution leading to the larger size measurements detected by the DLS approach[22]. The measured PDI of neem-Ag NPs can facilitate moderate distribution[28], and thus it is recommended to optimize the formula to obtain a narrow range of the dispersion index for better distribution within tissues. The electrostatic repulsions of neem-Ag NPs due to the dissociated acidic groups on their surface resulted in a negatively charged zeta potential value. Biogenic Ag NPs from plant biomass usually have negative zeta potential values[29].
The presence of a capping layer detected by TEM can provide stability to the nanoparticles. Hence, neem has a dual action as a reducing and a stabilizing biomolecule in this biological method implicated in Ag NPs synthesis[22]. The capping controls the size and enhances the activities of the nanoparticles by improving their binding affinity towards the organisms[20]. Moreover, the agglomeration of neem-Ag NPs observed in the current study using TEM was also reported by Lakkim et al.[27] who suggested that it could be due to chemical components in neem that act as stabilizing agents.
The methanol extract of neem showed molluscicidal activity against adult and juvenile B. alexandrina snails. LC90was 75.42 ppm on adult snails, after 24 h of exposure and examination after another 24 h. These concentrations are considered satisfactory as the World Health Organization recommended concentration LC90/48h < 100 ppm for any plant sample to be considered active[30]. However, the lower dose of neem-Ag NPs (3.89 ppm) exerting similar efficacy as the unbound plant extract offered an advantage. Abdel Ghaffar et al.[10] demonstrated more potent activity of methanol than aqueous extract of neem against B. alexandrina. The LC90and LC50were 70 and 40 ppm for the methanol extract and 190 and 100 ppm for the aqueous extract. Neem was also effective against Biomphalaria pfeifferi[31] and other land snails such as Archachatina marginata and Limicolaria aurora[32].
While a line of research has been concerned with the antimicrobial activity of neem-Ag NPs[20,22], no previous reports of the molluscicidal activity have been found. In the current study, the application of neem and neem-Ag NPs significantly increased the mortality of B. alexandrina snails, whereas neem-Ag NPs were more effective. The mortality increased by increasing the concentration of both formulae. The results also showed that the susceptibility of snails to neem and neem-Ag NPs was dependent on the developmental stages of the snails. Juvenile snails were found to be more susceptible to neem and neem-Ag NPs than adult snails. This developmental stage-dependent variation in susceptibility to molluscicide action has been observed in previous studies[33]. The high susceptibility of juvenile snails to molluscicides is important as juvenile snails are more susceptible to infection with S. mansoni[34]. The high resistance to neem and neem-Ag NPs as the snails advance in age could be due to increased tolerance of adverse environmental conditions with age-dependent acquisition of well-developed organs such as mantle and periostracum[33].
The LC50concentrations of both neem and neem-Ag NPs determined against adult snails also demonstrated a lethal effect against S. mansoni cercariae in a time-dependent manner, with a mortality of 100% after 60 min, with significant efficacy of the nanoparticle formula. Both formulae led to some changes in behavior of the cercariae before their death. There was a decrease in motility, some contractions, and tail loss. These changes were found to be not compatible with infectivity[35]. Similar results were obtained after exposure of cercariae to Ag NPs[15,17]. Cheng et al.[17] attributed the cercaricidal effect of Ag NPs to the effect of Ag+ions which could lead to cell death by generating reactive oxygen species that inhibited the respiratory enzymes. Moreover, Ag+ions preferentially bind to cercarial papillae and inhibit fatty acids-induced secretions from cercarial acetabular glands[36], which are key factors for host penetration[37].
Different studies determine the effect of phytochemicals in neem. The fruits of the plant contain various active components including azadirachtin, nimbin, and its derivatives, as well as other limonoids. The methanolic extract of neem seeds present in the fruits contains the highest amount of azadirachtin compared to other parts of the plant, in addition to its high level of phenolic compounds. Azadirachtin is the most important biologically active component, having many antibacterial, antifungal, and antimicrobial properties[38]. Phenolic compounds are responsible for antioxidant activity[39]. The molluscicidal and cercaricidal activities of neem extracted from the fruits of the plant in the present study might be attributed to these active components.
The powerful effect of neem-Ag NPs could be due to their small size that can reach the target tissues, even at low doses in addition to the effect of silver. Common mechanisms of action of AgNPs are the release of silver ions leading to the breakdown of adenosine triphosphate[40], the generation of reactive oxygen species, and cell membrane damage leading to leakage of intracellular contents[41].
Another demonstrated effect of neem and neem-Ag NPs is that they affect the hatching of B. alexandrina egg masses. Application of the LC50concentrations of both formulae to egg masses at the early stage of development led to total failure of egg hatching. Neem also prevented hatching of head and body lice eggs[42]. Some green synthesized nanoparticles and some plant extracts showed toxic effects against early embryonic stages of Biomphalaria glabrata snails, such as curcumin-nisin poly lactic acid nanoparticle[42], dichloromethane from Liagora farinose[43], and potassium usnate from Cladonia substellata[35] that showed low toxicity against early embryos of Biomphalaria glabrata.
Several studies reported the protective role of the egg membrane and the gelatinous egg capsule of embryos against molluscicidal compounds[25]. In the current study, the effect of neem fruit extract on egg masses might be due to its phytochemical constituents. All parts of the neem plant have wide applications; however, neem seeds and their oil attracted the most interest. The oil constitutes up to half the weight of the seeds in the ripe neem fruits[44]. The higher concentration of triterpenoids and limonoids extracted from neem seeds are more active in preventing the hatching of some nematode eggs than the leaf extract[45]. We suggest that the lipophilic feature of neem nimbolide may be a reason for complete inhibition of B. alexandrina egg hatching, as it may facilitate the penetration into snail eggs. Furthermore, the inhibitory effect of neem-Ag NPs on egg hatching could be attributed to an increase in bioavailability that could facilitate delivery of silver-encapsulated neem through the protective gelatinous egg masses. However, Ag NPs and the polyvinylpyrrolidone-NPs have low inhibition against egg hatching of Biomphalaria galabrata snails[16]. The apparent effect of neem and neem-Ag nanoparticles on the early embryonic stages of B. alexandrina could also be due to the intense cell proliferation at this early developmental stage[43]. The change in hydro-mineral contents is a proposed mechanism by which neem can inhibit the embryogenesis of eggs of the crustacean parasite Argulus japonicas[46]. However, further studies are needed to determine the different factors and the phytochemical components that prevent hatching of the snail egg masses. Moreover, the effect of neem Ag-NPs on the developmental stages of the embryos is recommended.
The application of green synthesized nanoparticles in snail control is novel and few studies have referred to the use of this approach against Biomphalaria snails[33]. The natural polymers present in neem plant-mediated synthesis of the Ag NPs through this approach, therefore, produce fewer toxic nanoparticles than using toxic chemical polymers. Green synthesis of nano formula can solve the toxicity problems when applying metal-based nanoparticles[21]. As Biomphalaria snails live with other freshwater organisms in the same microhabitats, it is suspected that excessive use of molluscicides containing Ag NPs could result in additional loading of aquatic ecosystems with silver that could induce some levels of toxicity on other non-target freshwater organisms. Although the green synthesis of nanoparticles decreases the toxicity issues associated with the application of Ag-based nanoformulations, extensive toxicity assessment is recommended and further studies are needed to evaluate the effect of neem-Ag NPs on the surrounding nontarget organisms. After application of a molluscicide, assessment of the snail density, the effect on water quality, and community acceptability of the intervention are important to determine its success for snail control[47].
In conclusion, neem-Ag NPs are synthesized by an eco-friendly green synthesis method. The synthesized nanoparticles are characterized by various techniques. Neem-Ag NPs are active against adult and juvenile B. alexandrina but with different efficacy. The formulation prevents the hatching of snail eggs and has a significant lethal effect on cercariae, the infective stage of the parasite. Therefore, neem-Ag NPs could be a potential solution to control schistosomiasis after studying the effect of neem-Ag NPs on the surrounding non-target organisms. Further studies concerned with safety, stability, and optimization of nanoparticles are recommended to optimize the formula to obtain a narrow range of the dispersion index for better distribution within tissues.
Conflict of interest statement
The authors declare no conflict of interest.
Acknowledgments
We are grateful to Dr. Wagdy Khalil, Department of Cell Biology, National Research Centre, for helping in preparation and characterization of the nanoparticles.
Funding
The authors receive no extramural funding for the study.
Authors’ contributions
IFA designed the study. SSY, IFA and KHR performed the experiment. SSY and IFA collected, analyzed and interpreted the data, wrote and revised the article and prepared the figures. SSY, IFA and KHR approved the final version to be published.
 Asian Pacific Journal of Tropical Biomedicine2023年1期
Asian Pacific Journal of Tropical Biomedicine2023年1期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Information for Authors Asian Pacific Journal of Tropical Biomedcine
- Anti-leishmanial, immunomodulatory and anti-oxidative activity of quercetin against cutaneous leishmaniasis caused by Leishmania major
- L-carvone attenuates myocardial injury and dyslipidemia in rats with isoproterenolinduced cardiac hypertrophy
- Quercetin modulates ovarian autophagy-related molecules and stereological parameters in a rat model of PCOS
- Scopoletin: Anticancer potential and mechanism of action
