Oil recovery tests with ionic liquids: A review and evaluation of 1-decyl-3-methylimidazolium triflate
Al Somoz , Alerto Arce , An Soto ,*
a CRETUS, Department of Chemical Engineering, Universidade de Santiago de Compostela, E-15782, Santiago de Compostela, Spain
b School of Engineering, Universidade de A Coru~na, E-15403, Ferrol, Spain
Keywords:Enhanced oil recovery (EOR)Ionic liquids Core flooding Interfacial tension 1-Decyl-3-methylimidazolium triflate
ABSTRACT The main advantages of the use of ionic liquids in enhanced oil recovery are their tunability and stability in harsh environmental conditions. In this work, a comprehensive review of ionic liquids proposed to improve current chemical oil recovery methods has been presented, focusing on core flooding experiments. With an almost infinite number of possible ionic liquids, the amount of experiments carried out up to now has been very limited. However, results are promising, with additional recovery after secondary flooding of up to 32% of the original oil in place. Most formulations with ionic liquids have been proposed for sandstone reservoirs, the number of studies with carbonate cores being very scarce. The possibilities of a new room temperature surface active ionic liquid,1-decyl-3-methylimidazolium triflate,for this application were analyzed. It was shown that it is able to drastically reduce the water/oil interfacial tension. An optimized formulation was proposed for carbonate reservoirs. After secondary flooding with brine, an additional recovery of 10.5% of original oil in place was achieved at room conditions.A combination of the proposed method followed by a polymer flooding step with polyacrylamide led to a lesser but still significant recovery, reducing the costs associated to the ionic liquid.
1. Introduction
Demand for energy is increasing worldwide,and petroleum will be needed not only as a source of energy, but also of materials for many years. Most of the current world oil production comes from mature fields due to the declining number of new oil field discoveries.Therefore,increasing oil recovery from these aging resources is the primary concern for oil companies and authorities globally(Pal et al., 2018; Saxena et al., 2019). After primary and secondary recovery methods, a reservoir still contains two thirds of the original oil in place (OOIP). This is because the efficiency of these methods is limited by capillary forces that retain the residual crude oil in pore structures. Moreover, at the reservoir scale, there are certain areas in which the fluid injected during secondary recovery does not penetrate adequately due to the low permeability of the rocks or because of preferential pathways (Shah and Schechter,1977). Enhanced oil recovery (EOR) techniques are being developed to overcome these problems. The main EOR techniques include thermal recovery, miscible gas injection and chemical methods (Sheng, 2011).
Among chemical EOR methods,surfactant injection has received in the last decades a great deal of attention because of its ability to reduce water/oil interfacial tension (IFT) to ultra-low values.Toxicity, high cost, and degradation at harsh conditions of temperature and salinity are the main issues that companies face during surfactant flooding process (Bin Dahbag et al., 2019). Surfactant retention also plays a significant role in chemical EOR application because it drastically affects the economics of the process (Ahmadi and Shadizadeh, 2015). In practice, it is very common to implement a tertiary surfactant flooding in combination with other chemicals in so-called alkali-surfactant-polymer(ASP) flooding. Alkalis are deployed to improve the interfacial activity of surfactants through in situ saponification by reaction of the alkali with the naphthenic acids (saponifiable components) of crude oil.Moreover,alkalis are cheap,so their application improves project economics. In addition, the employment of polymers aims to increase displacing fluid viscosity and thus to increase sweep efficiency (Sheng, 2011; Tackie-Otoo et al., 2020).
Surface Active Ionic Liquids(SAILs)have been shown to improve the features of conventional surfactants (Bera and Belhaj, 2016).Ionic liquids (ILs) are salts with melting or glass transition temperatures below 373.15 K. In many cases these temperatures are low enough that SAILs are liquids at room conditions, thus facilitating their manipulation. Other properties useful for their application in EOR are their negligible vapor pressure and their high thermal stability and solvating capacity. Moreover, in comparison with traditional surfactants SAILs have been found to have advantages regarding stability in harsh environments (high salinity and temperature) (Benzagouta et al., 2013; Hezave et al., 2013). However,their most interesting attribute is likely tunability.Cations and anions can be selected to make task-specific compounds, so they can be designed with respect to the characteristics of each individual reservoir (Pillai et al., 2018; Nandwani et al., 2019a).
In this paper,a comprehensive review of SAILs proposed for EOR is presented focusing on core flooding experiments. Moreover, a new IL, namely 1-decyl-3-methylimidazolium triflate is proposed for chemical EOR.Selection criteria were based on stability in brine solution and IFT reduction performance. Phase behavior of SAIL/brine/oil systems is studied by means of pipette tests. An optimal formulation is defined through the minimization of water/oil IFT by changing salinity, and surfactant and alkali concentrations.Adsorption tests and core flooding experiments in sandstone and carbonate reservoirs are carried out to analyze the capability of the SAIL to extract oil. Furthermore, the performance of alkalisurfactant (AS) and ASP flooding is compared.
2. Review
In the last decade,the number of papers proposing the use of ILs for EOR has increased exponentially.However,the number of core flooding tests carried out up to now is very limited, although this technique is considered the most suitable to determine and evaluate the effect of injecting fluids to enhance oil recovery in crude oil reservoirs. In this current section, the most important work on oil recovery with SAILs are presented. Test conditions may be either ambient temperature and low confining pressure or high temperature and pressure. The core material often comes from an oil reservoir but some tests use sand packs prepared manually with uniform sand grains. In addition, rocks are classified as sandstone or carbonate reservoir rocks.
2.1. Tests using real reservoir rocks
Hezave et al. (2013) tested the SAIL [C12mim]Cl and found out that an increase in the concentration of the surfactant led to a significant decrease in water/oil IFT even at high salinity conditions.The authors also carried out core flooding tests and obtained extractions of about 50%of the OOIP in secondary water flooding,and an additional 8%of OOIP in tertiary flooding with a 4000 ppm SAIL solution in water.Higher recovery was observed using a formation brine-surfactant solution at the same SAIL concentration,obtaining a recovery of 13%of OOIP.It should be noted that the authors report high adsorption values (16 mg of surfactant per gram of rock).Rocks used for the analysis had a porosity between 14 and 23%and a permeability around 8-16 mD. However, no further information was offered about the type of rock.
Bin Dahbag et al. (2015) suggested the use of a tetra-alkyl ammonium sulfate (Ammoeng 102) after screening nine ILs. Solubility in brine solutions, surface activity and solution stability at high temperatures were the selection criteria. Optimization of Ammoeng 102 formulation was done based on IFT reduction. Core flooding experiments were conducted on Berea sandstone samples.The injection of 20 wt% brine solution (83% NaCl and 17% CaCl2)allowed an extraction of about 43% of the OOIP. An additional oil recovery (AOR) of about 5% of the OOIP was achieved in tertiary flooding with the injection of 500 ppm of Ammoeng 102 diluted in the same brine solution.However,Ammoeng 102 was proven to be more effective injected in secondary mode(as a slug followed with brine or as a continuous surfactant injection)than in tertiary mode.Achieved recoveries ranged from 63% to 70% of OOIP. However, a high tendency for the surfactant to be adsorbed on rock surface at reservoir conditions was noticed,which increased at higher salinity conditions.
Deep Eutectic Solvents(DESs)are mixtures generally composed of two components that form an eutectic system with a melting point lower than that of each individual component.This is due to the formation of strong hydrogen bonds between the two components, one being a hydrogen bond donor and the other a hydrogen bond acceptor.DESs are seen as an interesting alternative to ILs in some applications. Mohsenzadeh et al. (2015) used two DESs, Choline Chloride:Glycerol (1:2) and Choline Chloride:Urea(1:2), for oil recovery. Surprisingly, formulations were defined using 50% volume concentration of DES on formation brine, far too high for real applications. Core flooding experiments were carried out in Berea Sandstone core plugs at three different temperatures using formation brine. The results revealed that the injection of Choline Chloride:Glycerol in tertiary recovery mode resulted in AOR of 14.0% at 318.15 K, 22.3% at 333.15 K and 29.8% at 353.15 K based on the OOIP. The injection of the Choline Chloride:Urea DES produced an AOR of 23.2%, 30.8% and 26.3% at 318.15 K, 333.15 K and 353.15 K, respectively. Heavy oil recovery increased by increasing the temperature.IFT between crude oil and formulation with DES ranged from 31 mN/m to 40 mN/m,and little information is given about the possible mechanism of this recovery. Moreover,according to the authors, the emulsions used were not stable.
Gou et al. (2015) used water-soluble complexes containing a hydrophobically associating co-polymer and a series of imidazolium-based ionic liquids including [Cnmim]Br (n = 6, 8,10,12,14 and 16).The polymer,denoted as PAAD,was prepared with acrylamide, acrylic acid and N,N-diallyl-2-dodecylbenzenesulfonamide. The incorporation of [Cnmim]Br(n = 10,12,14 and 16) in PAAD led to a white thick gel, while the addition of [C6mim]Br around critical micelle concentration resulted in a large decrease in the viscosity of the solution.The authors concluded that PAAD/[C8mim]Br complex was the most effective chemical. Temperature and PAAD, [C8mim]Br and NaCl concentration in the formulation were optimized via IFT measurements.They performed core flooding tests in Berea sandstones at 343.15 K using brine at a concentration of 20 g/L of NaCl.They tested the optimized combination of copolymer/IL and compared it with the use of the individual chemicals. They found out that the best results were obtained with a combination of 3 g/L of PAAD and 5 g/L of[C8mim]Br extracting 21.25% of the OOIP.
Jia et al.(2017)examined the use of a blend of a cationic SAIL:Ndodecyl-N-methylpyrrolidinium bromide (L12) with a traditional anionic surfactant:sodium dodecyl sulfate(SDS)for chemical EOR.Cores from sandstone reservoirs were used in flooding experiments at 338.15 K L12/SDS ratio and process temperature were optimized via IFT studies. At the optimal L12/SDS molar ratio of 1:2.5, the blend effectively reduced the IFT of water/oil to an ultralow value(~4×10-3mN/m)using a low surfactant concentration(500 mg/L).An extraction of 25%of OOIP was achieved by adding the surfactant solution mixed with 2000 mg/L of partially hydrolyzed polyacrylamide (HPAM) in tertiary mode.
Manshad et al. (2017) screened four ionic liquids, namely:[C12mim]Cl, [C18mim]Cl, [C8Py]Cl and [C18Py]Cl. [C18mim]Cl was found to be the best IL based on IFT reduction and wettability alteration towards water-wet.Core flooding with the selected IL at a low concentration of 170 ppm revealed a 13% increase in oil recovery compared to flooding with brine. These tests were performed using carbonate core samples. Wettability measurements proved that the effect of the wettability is not particularly significant, the increase in oil production being mainly due to the IFT reduction.
Nabipour et al. (2017) tested two ionic liquids [C12mim]Cl and[C18mim]Cl alongside other conventional surfactants. Emulsification and compatibility tests were performed to study the tolerance of surfactants to harsh conditions of salinity. The IL-based surfactants were compatible with formation brine but [C18mim]Cl was discarded due to its poor dispersion in seawater with a high concentration of divalent ions. [C12mim]Cl was selected due to its capacity to shift the wettability of reservoir rock towards more waterwet whilst reducing crude oil/seawater IFT. Core flooding experiments were carried out using a surfactant solution of 1000 ppm in seawater.The authors showed the IL to be more effective than other conventional surfactants.They carried out the tests in a native core;however, they do not specify if it was made from sandstone or carbonate material. To verify the contribution of wettability alteration to oil recovery, tests adding a soaking step of 21 days(designed to change the wettability of the rock towards water-wet)were performed. With the soaking step, the tertiary oil recovery efficiency was increased from 8% up to 12.7% of the OOIP. The effectiveness of soaking to enhance the wettability alteration mechanism is highlighted in the paper.
Rodríguez-Palmeiro et al. (2017) found that a formulation consisting of 4000 ppm of [P44414]Cl in a solution of 4 wt% NaCl and 5000 ppm NaOH was able to reduce the IFT between water and crude oil from 19.2 mN/m to 0.1 mN/m. They carried out coreflooding experiments at room temperature using Berea Sandstones, and compared extraction using three different processes.Firstly,as a tertiary oil recovery method,the optimized formulation was injected. Secondly, the brine + SAIL solution was injected before the optimized formulation. Finally, brine + NaOH was injected before the formulation. The authors tested adsorption in terms of resistance factor and residual resistance factor and found that there was no significant retention of the SAIL.An AOR of about 8.1%of the OOIP was reached when the optimized formulation was used after secondary recovery with brine (4 wt% NaCl). It was concluded that direct injection of the optimized formulation gave practically the same result as injection of the formulation after the brine + NaOH solution. The injection of SAIL before the optimized formulation led to the worst result.
Liu et al. (2018) studied the ability of 1-butyl-3-methylimidazolium dioctylsulfosuccinate (BMOT) to reduce the IFT between crude oil and water, obtaining an ultra-low value on the order of 10-3mN/m. This was selected as the most promising surfactant by comparison with the traditional anionic surfactant,sodium dioctylsulfosuccinate(AOT)and the anionic SAIL 1-butyl-3-methylimidazolium dodecyl sulfate (BMDS). The effect of BMOT concentration, salts and temperature on IFT was studied. An artificial core from a sandstone reservoir was used in the core flooding tests.The extractions were carried out at 335.15K.12.5%of the OOIP was obtained using BMOT(1500 mg/L)which clearly improved on results with AOT or BMDS.
Zabihi et al.(2019)studied two different families of surfactants including conventional and IL-based surfactants.From the family of ILs, they tested several imidazolium and pyridinium chlorides varying the length of alkyl chain.To that aim brine/oil formation IFT was measured using a spinning drop tensiometer.The conventional surfactant sodium dodecylbenzenesulfonate(SDBS)was capable of reducing the IFT value even at high salinity, but surfactant concentrations higher than 1600 ppm led to precipitation problems.[C12mim]Cl and [C18mim]Cl showed the best capability for IFT reduction and precipitation problems were not encountered.Further emulsification and Amott wettability tests revealed a greater capability of SDBS for emulsification while the ILs showed higher capability to alter the wettability of the rock surfaces toward mixed wet.Finally,the core flooding tests performed revealed that[C12mim]Cl and [C18mim][Cl] were able to enhance the tertiary oil recovery up to 13% and 16.5%, respectively, while the SDBS increased recovery up to 26%. The difference in performance was justified in terms of the involved mechanisms.While the dominant mechanism in the recovery with ILs was the wettability alteration,the main mechanism for SDBS was the IFT reduction.
Details regarding oil, brine, rock type, and temperature conditions for all the works considered in this section are shown in Table 1.
2.2. Tests using unconsolidated sand packs
Pereira et al. (2014) proposed the use of a non-surfactant IL[C2mim][OTS], as an ionic additive to improve oil recovery. The results showed that using a 2 wt% solution of [C2mim][OTS] in brine, 20.56% of the OOIP could be recovered in tertiary flooding,almost double the extraction with brine solution alone.A vertically oriented acrylic column uniformly packed with dry sand was used as the core. This increase in oil recovery is believed to have been caused by the aromatic character of the IL that contributes to the increase of the interactions with aromatic oil fractions.This may be an interesting oil recovery mechanism associated with aromatic ILs.
Tunnish et al. (2016) also proposed the use of an IL without a surfactant character: [C2mim][Ac]. For core flooding experiments,unconsolidated sand packs made from Ottawa sandstones were used at 290.15 K with formation brines of 0.1 and 1 wt% of NaCl(also containing divalent ions).The best result was obtained when a brine solution (0.1 wt% NaCl) containing 3000 ppm [C2mim][Ac]was injected. A tertiary extraction of 16.68% OOIP was achieved,even though the IFT between the optimized formulation and crude oil was quite high (7.31 mN/m). They also tested the use of formation brine but results showed a better oil recovery for lower salinity ratios in the absence of divalent ions.The authors also point out that the main mechanisms of improvement in oil recovery were wettability alteration and increase of viscous forces. The IL proved to be more effective than traditional surfactants such as SDS.
Sakthivel et al. (2016) also tested SDS and a variety of alkyl ammonium ILs, at several temperatures and salinities, to enhance the recovery of low waxy crude oil.[OHPrNH3][CF3COO]was noted as the most promising IL, even though its water/oil IFT was 9.38 mN/m. Core flooding experiments were carried out using a horizontal sandpack column, and surfactant flooding with SDS or IL.A second stage of polymer flooding with an aqueous solution of polyacrylamide (PAM) was also tested. The IL + polymer flood showed significant increase in oil recovery as compared to the rest of the flooding schemes.17-23% of the OOIP was recovered using IL + polymer flood system under low salinity conditions, which further improved to 28% under high saline conditions. It was observed that the ILs possessing longer alkyl chain lengths increased oil recovery more than those with shorter alkyl chain lengths.
Sakthivel et al. (2017) investigated the use of lactam and imidazolium based ionic liquids (ILs) for EOR. SDS was used for comparative purposes. With a similar procedure to the previous paper,it was found that among all the ILs studied,[CP][C6H13COO]led to the best performance. Better results were found for high salinity conditions. It should be noted that the measured IFT between SDS or IL and crude oil were always higher than 8 mN/m.When flooding was carried out with aqueous solution of IL and polymer,the AOR was quantified in the range of 22-25%,whereas in the case of high saline conditions it was enhanced up to 31%.

Table 1 Core flooding works on Ionics liquids for EOR application using real reservoir rocks.
Nandwani et al. (2017) tested three long-chain 1-alkyl-3-methylimidazolium bromides ([C12mim]Br, [C14mim]Br and[C16mim]Br) in terms of aggregation in water and dynamic IFT under high salinity conditions.The most promising was found to be the one with longest chain: [C16mim]Br, providing an IFT value of 3.9 mN/m.The core flooding experiments were performed at room temperature using a vertically placed acrylic sand packed column.3 wt% NaCl and 0.3 wt% of surfactant concentration were selected for aqueous formulations after optimization via IFT measurements.Greater extraction was obtained with the IL than with the traditional cationic surfactant cetyl trimethyl ammonium bromide(CTAB) at the same composition. It was shown that, unlike other conventional surfactants, [C16mim]Br interfacial activity remains even at high temperature conditions.
Nandwani et al. (2018), in line with previous work, performed flooding experiments using a horizontal sand pack holder. Pure[C16mim]Br and a mixed system containing this IL and the nonionic surfactant Tergitol 15-S-9, at a molar ratio 4:1, were tested.To that aim, pipette tests were carried out to determine optimal formulations.The mixed surfactant system showed a middle phase microemulsion, related to the drastic reduction of IFT to very low values (2x10-2mN/m). Surfactant flooding, with the IL at the optimal salinity,led to an oil recovery of 3.7%of the OOIP,whereas for the blend the percentage of oil recovered was 9.79%of the OOIP.The optimal salinities found for crude oil and kerosene were 30.1 and 32 wt% respectively. Thus, the proposed formulation is a potential candidate to be used in oil reservoirs with high salinity formation brine.
Pillai et al. (2018) studied three tetrafluoroborate ILs ([C8mim][BF4],[C10mim][BF4],and[C12mim][BF4])to reduce IFT and change wettability of reservoirs. Results demonstrated that the ILs remained stable at high temperature and saline conditions, and were able to significantly reduce IFT. They also carried out emulsification tests and static adsorption studies, leading to the conclusion that [C12mim][BF4] was the most promising SAIL for EOR applications. This IL provided a IFT value of 2.1 mN/m. Thus,sand pack flooding experiments were conducted,with this IL,using crude oil.An organic alkali,triethanolamine(TEA),and the polymer HPAM, were used as chemical additives to improve oil extraction.Initially, the HPAM polymer slug was injected after conventional water flooding which resulted in an AOR of 15.7%. In a second experiment, a slug of [C12mim][BF4] and polymer was injected achieving 28.17% of AOR after chase water flooding. Finally, the extraction was improved by the injection of a chemical slug containing [C12mim][BF4], polymer and alkali. AOR of 32.28% was obtained.
Nandwani et al. (2019a) proposed a chemical formulation containing the SAIL[P66616]Br and a nonionic surfactant(Tergitol 15-S-9). Formulation was optimized via pipette tests, achieving a Winsor type III microemulsion.It was observed that after the water flooding process the individual nonionic surfactant recovered only 7.28% additional oil, whereas the optimized chemical formulation,containing both SAIL and non-ionic surfactant in an equimolar ratio, recovered 16.68% additional oil at the same salinity. They simulated a carbonate reservoir by tightly packing calcite powder.Experiments were carried out at room temperature with 9.25 wt%brine.
Tunish et al. (2019) carried out chemical flooding experiments using alkali and alkali + IL (AIL) mixtures in unconsolidated sand packs. They used Na2CO3as alkali and four different imidazoliumbased ILs: [C2mim][Ac], [C2mim]Cl, [C4mim][NTf2] and [C12mim]Cl, only the latter being a surfactant. Best results were obtained with[C2mim][Tf2N](13.47%OOIP)followed by[C2mim][Ac](11.47%OOIP) and [C2mim]Cl (10.40% OOIP). Surprisingly, extraction using[C12mim]Cl led to the worst result even though it was the IL that led to the lowest IFT. The increase of IL concentration (0.7 wt%Na2CO3+ 3000 ppm [C2mim][Tf2N]) and slug size led to further improvement in tertiary recovery reaching 21.12% of OOIP. The authors found that a viscous effect was not predominant and point to changes in surface wettability and IFT reduction as dominant mechanisms.
It should be mentioned that all these tests were carried out without confining pressure,so the results could be affected by the existence of side flows. Details regarding oil, brine, rock type, and temperature conditions for all the works considered in this section are shown in Table 2.
2.3. Discussion
Despite the huge potential of ILs to improve current EOR methods, the number of flooding experiments carried out up to now is still very limited. Most studies are conducted using unconsolidated sand packs. Porosity and permeability of this kind of systems is much higher than that of real reservoirs. This leads to lower capillary pressures and a consequent easier crude oil extraction.ILs have mostly been proposed as surfactants but also no surface active ILs have been suggested as chemicals in EOR. In the case of SAILs,they drastically reduce the IFT water/oil even in harsh conditions,but ILs can also increase viscosity,change wettability,or in some cases promote solubilization of oil through aromatic interactions. Most of ILs proposed for the application are aprotic,however the use of protic ILs(Sakthivel et al.2016,2017)also led to interesting results.
Optimal formulations are mostly defined using IFT as the target property.In very few cases the formulation was obtained by phase equilibria studies. SAILs tested in core-flooding experiments corresponded to a Winsor type I behavior. Blends with traditional surfactants have been proposed to obtain Winsor Type III systems.However, no noticeable increase in the recovery is detected with this behavior.
After secondary recovery (with water or brine), additional oil recoveries of up to 32%of OOIP have been shown in the literature,using ILs or combinations of these salts with other surfactants or polymers. Nonetheless, many of these studies are carried out on unconsolidated sand packs. Thus, the recovery in oil reservoirs could be drastically lower. Regarding experiments with real reservoir rocks,oil recoveries with pure ILs have achieved up to 16.5%of additional recovery, and using combinations with other chemicals values up to 25% have been shown. Surprisingly, up to now only[C18mim]Cl has been proposed for carbonate rocks. Most of the studies propose the use of SAILs in sand pack rocks where a high adsorption would be expected due to the cationic nature of the proposed ILs. The number of adsorption studies found in the literature is very scarce. Oil recovery obtained with DES derived from Choline Chloride is notable.However,the quantity of the chemical used make the proposed formulation unrealistic for real applications. More studies with these kinds of chemicals are required to analyze the possibilities of these chemicals for EOR.
The practically unlimited number of ILs,and in particular SAILs,drastically multiplies the possibilities of surfactant EOR.These salts can be tailor-made to solve the main problems associated with this oil recovery method, such as stability of the surfactant at high temperature and salinity conditions, whilst keeping adsorption at very low values.However,a significant number of papers published to date are incomplete or inconclusive. Both experts on ILs and surfactant EOR must combine their knowledge and resources in order to find a suitable formulation,and carry out adequate tests to prove its efficiency.Once laboratory results are solid and promising,field-scale simulations will be required as a necessary step before carrying out the first pilot tests in the field.
3. EOR using 1-decyl-3-methylimidazolium triflate
3.1. Materials and methods
3.1.1. Materials
In the present work, the room temperature IL 1-decyl-3-methylimidazolium triflate, [C10mim][OTf], was purchased fromIolitec(purity>99 wt%).Prior to its characterization,it was purified under high vacuum(<1 Pa)while stirred magnetically and heated at ca. 373-383 K for elimination of water and potential volatile impurities. Brine solutions were prepared by weight dissolving sodium chloride purchased from Panreac (purity >99 wt%) in distilled water.Sodium hydroxide in the shape of anhydrous pellets(purity >99 wt%)and n-octane(purity >99 wt%)were supplied by Sigma-Aldrich.Potassium iodide(purity>99 wt%),used as a tracer for dynamic adsorption tests,was also supplied by Sigma-Aldrich.
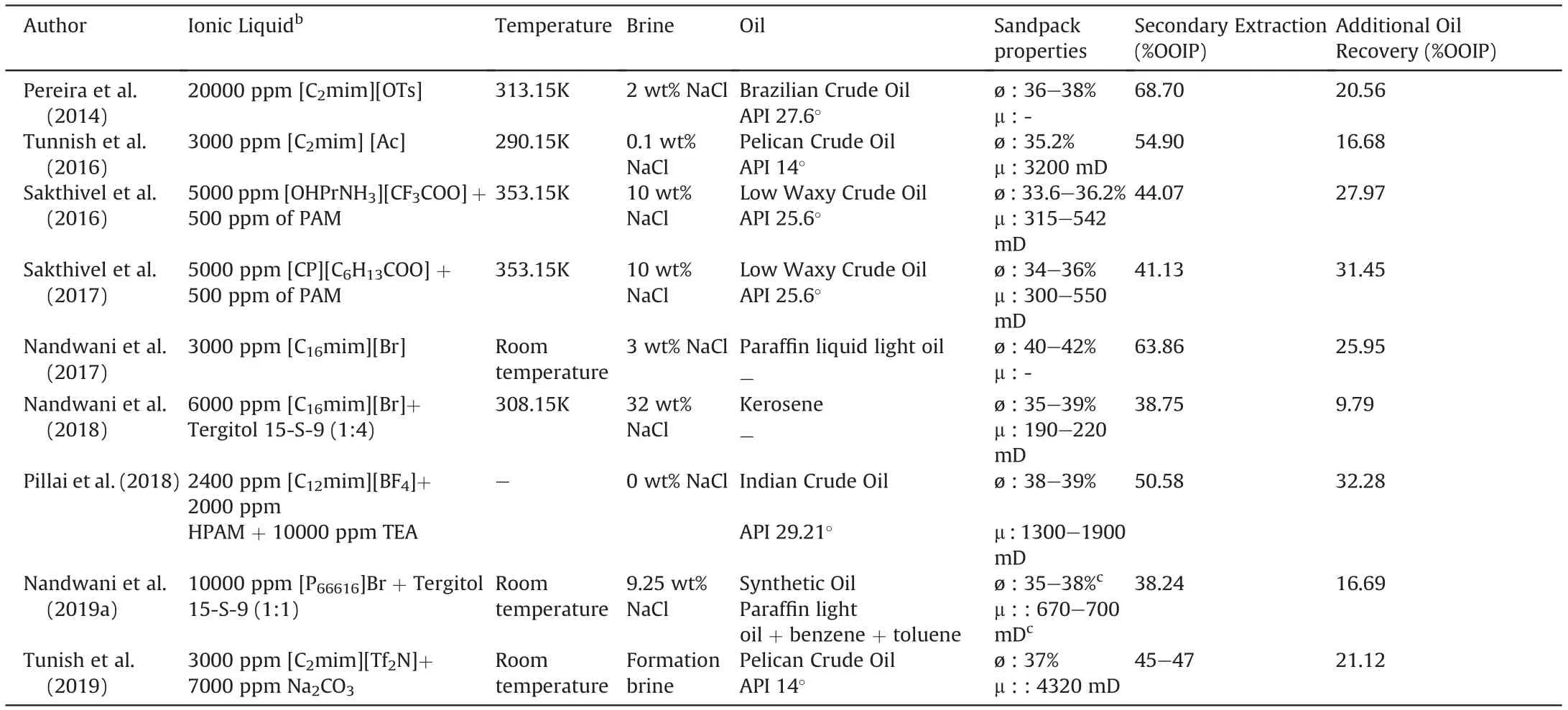
Table 2 Core flooding works on Ionics liquids for EOR application using unconsolidated sand packs.
Partially hydrolyzed polyacrylamide (HPAM) with a molecular weight of 5-8 million Daltons and a hydrolysis degree of 30%(Flopam 3230S), and polyacrylamide (PAM) without hydrolysis(Flopam FA 920 SH) with the same molecular weight, were kindly provided by SNF Floerger.
Crude oil was kindly supplied by Repsol (refinery of A Corun~a,Spain). Its main properties are summarized in Table 3 and ASTM D86 test presented in Table S2 of SI.
Sandstone cores were purchased from Vinci Technologies and used for dynamic adsorption tests.Their mineralogy was analyzed by X-Ray diffraction.Results are shown in Table 4.Low permeability carbonate cores made from limestone and dolomite were used for dynamic adsorption and core flooding tests. These cores were purchased from Kocurek Industries and their mineralogy was also analyzed by X-Ray diffraction. Results are shown in Table 5.
3.1.2. Methods
3.1.2.1. Ionic liquid characterization. [C10mim][OTf] water content was measured using Karl-Fischer titration in a Metrohm 899 coulometer.Density was determined in an Anton Paar DMA 5000M density meter.An Anton Paar LOVIS 2000 ME microviscometer was used to measure IL viscosity. Surface tension in aqueous solutions was measured using the Wilhelmy plate method in a Krüss K11 tensiometer in order to determine the critical micelle concentration(cmc). Experiments were performed at 298.15 K and repeated at least twice to ensure repeatability.
3.1.2.2. Pipette tests. Salinity tests were performed following the encased-glass-pipette method(Puerto et al.,2012).In this test,1 cm3of brine with SAIL and 1 cm3of oil were added into individual pipettes sealed at the tip.Thereafter,pipettes were top sealed,mixed in a rotatory mixer at room temperature for 24 h and then put into a test tube filled with silicon oil. Finally, the glass encased pipettes were left to equilibrate in a dry block heater OVAN model BD200-RE at the target temperature until phase volume remained constant. N-octane was chosen as the oil because it is known that optimal salinities of various surfactants using this oil are not greatly different from crude oil (Puerto et al., 2012). Surfactant concentration in the pipette was 2 wt%overall.A salinity range from zero to 15 wt% NaCl was studied at 298.15, 323.15 and 348.15 K.
3.1.2.3. Dynamic interfacial tension. Dynamic IFT between crude oil and surfactant solution were measured using a Krüss SITE100 spinning drop tensiometer. All measurements were carried out at 298.15 K,using circulating oil from a Julabo EH-5 thermostatic bath

Table 3 Crude oil properties(provided by supplier).

Table 4 Berea Sandstone mineralogy.

Table 5 Carbonate core mineralogy.
to keep the temperature constant, and atmospheric pressure. The aqueous solution (dense phase) was used to fill the capillary tube ensuring that there were no air bubbles.A drop of 4 μL of crude oil(light phase)was injected in the middle of the capillary tube,using a Hamilton micro-liter syringe, rotating at slow speed (100 rpm).Rotating velocities between 2000 and 7000 rpm were applied in order to obtain a drop length at least 4 times larger than its diameter. The IFT was calculated according to the Vonnegut equation:

where Δρ is the density difference between dense and light phase,ω is the angular velocity and D is the diameter of the oil drop.All the experiments were performed at least twice to ensure repeatability.
3.1.2.4. Adsorption and core flooding. Adsorption and flooding experiments were conducted in a core-flooding system equipped with a Hassler Core Holder H00-021-0,and two floXlab BTSP 500-5 piston pumps equipped with an accurate pressure sensor for brine or surfactant solution and oil. The Core Holder and pumps were purchased from Vinci Technologies (Fig. S1 in Supplementary Information).Experiments were carried out at room temperature and a constant injection rate of 2 mL/min. To prevent hydraulic side flow,a confining pressure was maintained at a value at least 35 bar higher than the flooding pressure using an Enerpac P142 manual hydraulic pump.
Both sandstone and carbonate cores, as described in materials section, were used in experiments. The reservoir plugs were 3.51 cm in diameter and 7.62 cm length.To characterize the rocks,the core was initially vacuumed for 24 h and then saturated with brine (2 wt% NaCl). After 24 h, pore volume (PV) was determined using the dry and wet weights of the core. Absolute permeability(kw) of the brine was calculated by flooding the core with brine solution at different injection rates. The pressure difference between inlet and outlet(ΔP)was recorded for each injection velocity.Injection flow rate versus pressure drop yields a straight line. The slope of the straight line was used to calculate the absolute permeability of brine using Darcy’s law.
Dynamic adsorption tests were carried out to evaluate the retention of the IL in the rocks.Around 60 mL of tracer or IL solution in brine were continuously injected at a rate of 2 mL/min.Effluent fractions were collected for analysis every 4 mL. IL and tracer concentrations were determined by UV absorption using an HP Presario SR1000 UV/Vis spectrophotometer.
In oil recovery tests,after brine saturation the core was flooded
with crude oil by increasing flow rate from 2 mL/min to 10 mL/min until there was no further water coming from the effluent. OOIP was then calculated as the volume of oil retained in the core.Initial oil saturation(Soi)and initial water saturation(Swi)were calculated using Eqs. (2) and (3) respectively:

The core was allowed to rest for 4-5 days.After this aging step,the plug was ready to initiate the core flood.First,a water flood was done injecting 3-4 PV of brine(2 wt%NaCl)at a constant flow rate of 2 mL/min until oil was no longer produced and the differential pressure remained stable. Oil recovered after water flood (ORWF)was determined and residual oil saturation (Sor) was calculated as follows:

The core was then subjected to tertiary flooding.For the case of AS flooding, the core was flooded with 3-4 PV of the optimized formulation at a constant flow rate of 2 mL/min. For the ASP flooding,one pore volume(1 PV)of the optimized formulation was flooded through the core at a constant rate of 2 mL/min followed by the injection of 3 PV of solution of polymer in brine increasing its flow rate from 1 mL/min to 2 mL/min to maintain a differential pressure of at least 20 bar.In addition,a third core flooding test was carried out implementing polymer flooding after secondary recovery.In this test 4 PV of polymer solution in brine were injected at 2 mL/min to determine the ability of the polymer to extract oil.Oil fractions collected correspond to the oil recovered after tertiary flooding(ORTF).Thus,additional oil recovery(AOR)was calculated as a function of the OOIP:

3.2. Results and discussion
3.2.1. Ionic liquid characterization
The main characteristics of [C10mim][OTf] are listed in Table 6.Results of cmc measurements can be seen in Fig. 1. A value of 8.03 mmol/kg was obtained, which corresponds to 3890.84 ppm,with a surface tension at this concentration of 28.86 mN/m.
3.2.2. Pipette tests
Microemulsion systems can be classify as Winsor I,Winsor II or Winsor III (Winsor, 1948). In a Winsor I system there is an equilibrium between an oil-in-water microemulsion and an upper excess oil phase,whereas in the Winsor II system the equilibrium is between a water-in-oil microemulsion and a lower excess water phase.The Winsor III system has a middle microemulsion phase in equilibrium with an upper excess oil phase and a lower excess water phase.
Phase behavior of [C10mim][OTf] was studied with n-octane by carrying out pipette tests (Fig. S2 of Supplementary Information).Winsor type I systems were found for salinities from 0 to 15 wt%NaCl at 298.15,323.15 and 348.15 K.It is known that Winsor type III microemulsions lead to ultra-low interfacial tensions (10-3mN/m)but they usually experience high adsorption and bad solubility in brine (Spildo et al., 2014). Moreover, surfactant loss through partitioning into the crude oil can be responsible for 30%of surfactant losses.As an advantage,in the case of very hydrophilic surfactants(Winsor type I systems),the surfactant loss is near zero(Salari et al.,2011).

Table 6[C10mim][OTf] water content and physical properties at 298.15 K and atmospheric pressure.
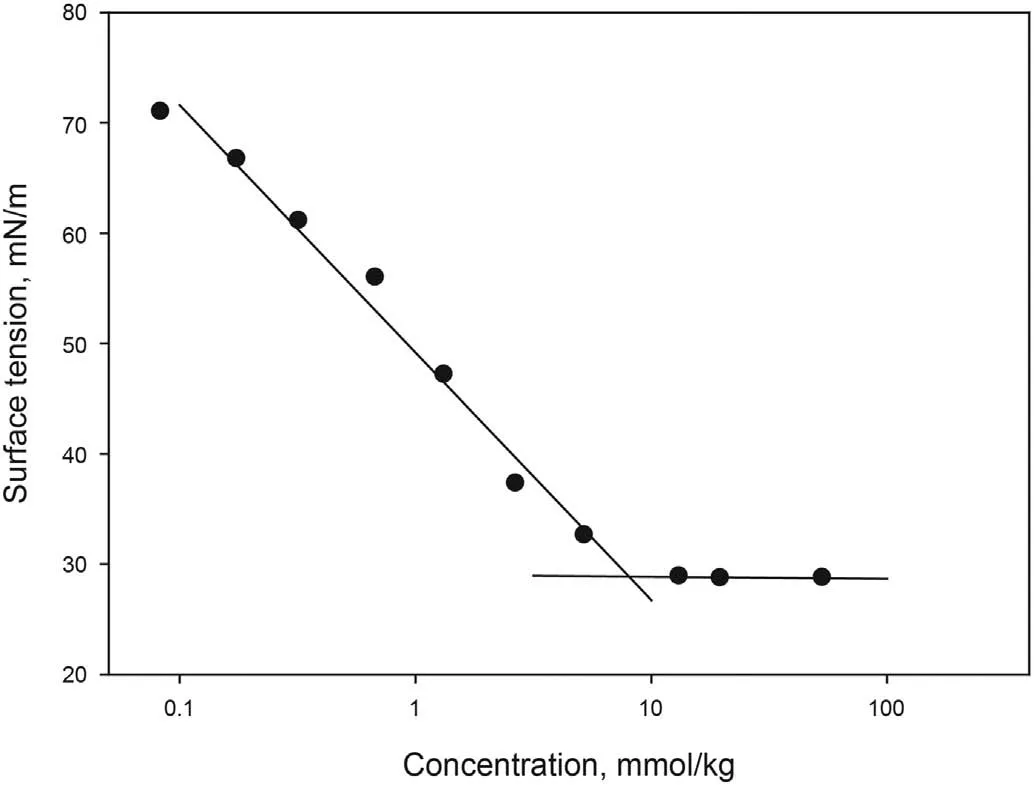
Fig.1. Surface tension of aqueous solutions of [C10mim][OTf] at 298.15 K.
The knowledge of phase behavior is the first and fundamental step to select an adequate surfactant for EOR but required,decisive tests are core flooding experiments using reservoir cores (Sheng,2011). As the selected IL showed Winsor Type I behavior in all the range of salt concentrations,an optimization of the composition of the formulation was carried out by means of dynamic IFT measurements prior to the core flooding tests.
3.2.3. Dynamic interfacial tension
Four different concentrations of[C10mim][OTf]were prepared in distilled water: 2000 ppm, 3000 ppm, 4000 ppm and 5000 ppm,and IFT with crude oil measured at 298.15 K and atmospheric pressure.As shown in Fig.2,the dynamic IFT between crude oil and surfactant solution decreases when increasing SAIL concentration.Equilibrium values of 6.70 mN/m, 2.42 mN/m, 0.36 mN/m and 0.41 mN/m were obtained for 2000 ppm,3000 ppm,4000 ppm and 5000 ppm respectively.Concentrations of 4000 and 5000 ppm led to similar results,therefore a value of 4000 ppm was selected.This result is in accordance with the value obtained for cmc in section
3.2.1.
The salinity of the aqueous phase has a strong influence on the interfacial tension of crude oil/aqueous systems. For this reason,various experiments were carried out varying sodium chloride concentration from 0 wt% to 4 wt% in the aqueous phase while maintaining a constant concentration of [C10mim][OTf] at 4000 ppm.As seen in Fig.3,an increase in NaCl concentration from 0.1 wt%to 1 wt%led to an increase in the IFT,providing equilibrium values of 0.57 mN/m and 0.93 mN/m respectively. NaCl concentration in the range of 2 wt%-4 wt%provided similar IFT equilibrium values(1.1 mN/m-1.3 mN/m).As formation water contains salts,a value of 2 wt% NaCl was chosen to avoid high values of the IFT.Furthermore, according to Fig. 3, even if the salinity increased,interfacial tension would not be greatly affected.
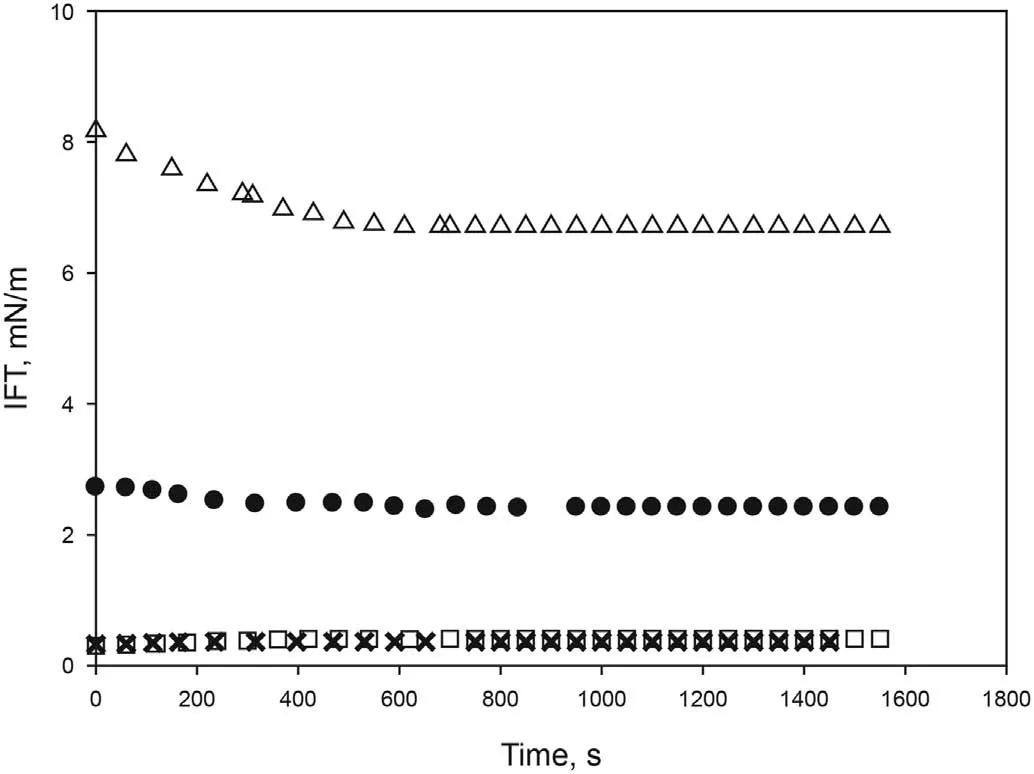
Fig. 2. Effect of [C10mim][OTf] concentration on dynamic interfacial tension between water and crude oil at 298.15 K and atmospheric pressure.

Fig. 3. Effect of NaCl concentration on the dynamic interfacial tension between aqueous solution of [C10mim][OTf] (4000 ppm) and crude oil at 298.15 K and atmospheric pressure.×0 wt%,▽0.1 wt%,○0.2 wt%,?1 wt%,□2 wt%,?3 wt%,●4 wt%.
Alkalis improve the interfacial activity,generating surfactants in situ reacting with the naphthenic acids of crude oil, so they can improve the economy of the process since they reduce the amount of surfactant needed to obtain low IFT(Tackie-Otoo et al.,2020).For this reason,three different concentrations of an alkali(NaOH)were prepared using a solution of [C10mim][OTf] (4000 ppm) in brine(2 wt% NaCl) and varying concentration of NaOH from 0% to 1.5%.Fig.4 shows a significant decrease of dynamic IFT from 1.20 mN/m in the absence of NaOH to 0.56 mN/m for 1 wt% NaOH. Higher concentrations of the alkali did not further reduce the IFT, thus a value of 1 wt% NaOH was selected. The optimized formulation is clear and stable with time.
3.2.4. Adsorption
Adsorption of surfactants in porous media is a fundamental issue in EOR. Loss of surfactants owing to their interactions with reservoir rock and fluid affects the effectiveness of the chemical solution injected, and may make the process economically unfeasible (Bera et al., 2013). It is known that surfactant adsorption depends on surfactant, solid surface and aqueous phase, the electrostatic attraction between charged surface of the rock and charged head group of surfactant likely being the most important factor. (Curbelo et al., 2007; Salari et al., 2011; Ma et al., 2013;Hanamertani et al., 2018).
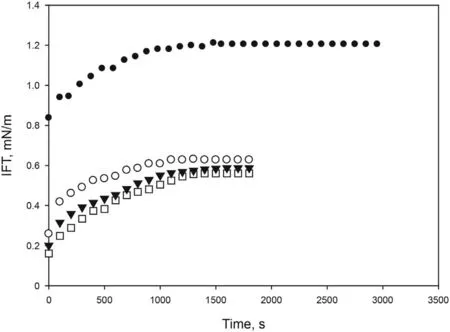
Fig. 4. Effect NaOH concentration on the dynamic interfacial tension between brine solution (2 wt% NaCl) of [C10mim][OTf] (4000 ppm) and crude oil at 298.15 K and atmospheric pressure. ●0 wt%, ○0.5 wt%, □1 wt%,▼1.5 wt%.
The adsorption of the optimized formulation was studied in Berea sandstone. After 4 PV of injected optimized formulation(4000 ppm IL,1 wt% NaOH, 2 wt% NaCl), no ionic liquid concentration was detected in the effluent, so the adsorption was very high.This is not surprising.As mentioned above,the adsorption of ionic surfactants is strongly influenced by the composition of the rock that makes the core surface negatively or positively charged.Sandstone cores used in this study contain 4% muscovite and 4%albite (Table 4) which are silicate-like minerals, and the proposed SAIL is a cationic surfactant.So adsorption takes place mainly due to the presence of negatively charged components of sand particles,such as silica, which are negative at neutral pH or in water (Bera et al., 2013).
The adsorption of the optimized formulation in carbonate cores was also studied. Fig. 5 shows the ratio between surfactant concentration of the effluent and injected formulation as a function of injected PV. The SAIL and the tracer potassium iodide were compared. As shown, both tracer and [C10mim][OTf] are detected for the first time in the effluent at approximately 0.5 PV, but the tracer takes longer to reach initial concentration. Therefore,adsorption of the SAIL in carbonate rock can be assumed to be low enough to consider the formulation suitable for the application.For carbonate material, the presence of calcite and other minerals containing Mg2+makes the core surface positively charged(Tagavifar et al., 2017). Thus, the strong repulsion between positively charged rock surface and positively charged surfactant, prevents adsorption from occurring.
This low adsorption is in agreement with previously findings showing that imidazolium-based ILs lead to less adsorption in carbonate rocks than pyrrolidinium or pyridinium or even other conventional cationic surfactants. (Nandwani et al., 2019b).
3.2.5. Core flooding
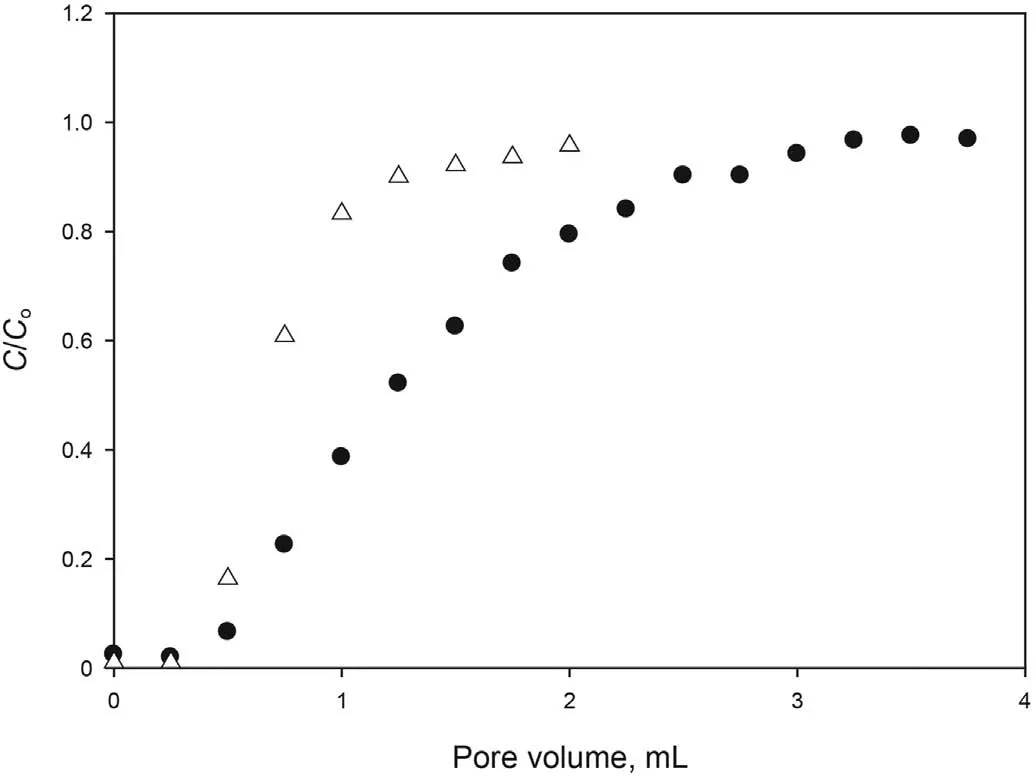
Fig.5. Dynamic adsorption of the optimized formulation at 25°C in Carbonate rock.●Tracer Δ Optimized formulation.
Three core flooding runs were performed in fresh carbonate cores as reported in Table 7.Test 1 was started with secondary brine flooding until reaching the residual oil saturation(Sor)followed by tertiary flooding of 4 PV of optimized formulation containing 4000 ppm of [C10mim][OTf] and 1 wt% NaOH in brine solution.Direct injection of the optimized formulation was preferred to a two-step injection with initially the alkali and then the surfactant because it has been shown that both procedures lead to the same oil extraction (Rodríguez-Palmeiro et al.,2017).
With the aim of saving surfactant,a Test 2 combining surfactant and polymer flooding was carried out.A first attempt using HPAM was performed. However, an uninterrupted increase in pressure was experienced and no additional oil was obtained in the effluent.This is due to the high adsorption of the polymer caused by the electrostatic attraction of the negatively charged HPAM and positively charged carbonate surface. So, the use of this polymer was discarded and the non-ionic polymer (PAM) was selected. Fig. 6 shows viscosity of aqueous solutions of PAM. The mechanism of enhanced recovery involved in polymer flooding is based on decreasing the mobility difference between displacing and displaced fluids.Thus the displacing phase should have lower mobility than the displaced phase. As mobility is inversely proportional to viscosity (Raffa et al., 2016), a concentration of 1000 ppm of PAM was chosen in order to obtain a viscosity of 2.23 mPa s, twice the viscosity of the crude oil.In Test 2,brine was flooded in secondary mode until reaching residual oil saturation,and for the EOR step a slug of 1 PV of optimized formulation was injected into the core,followed by continuous injection of 3 PV of aqueous solution of PAM at the same salinity. To support the idea that combined method is better in terms of oil recovery,a new flooding test(Test 3)was carried out.In this essay,after conventional water flooding,4 PV of PAM at the same concentration (1000 ppm) in brine were continuously injected.
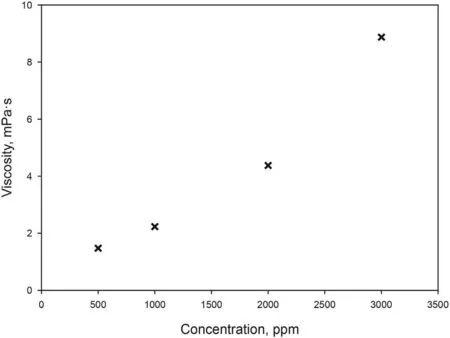
Fig 6. Effect of PAM concentration on viscosity.
No stable emulsion was found in the effluent in any of the flooding experiments. The results obtained (Table 7) show that a significant EOR can be achieved using the SAIL [C10mim][OTf].The results indicate an additional oil recovery of 10.48%and 7.98%of the OOIP for AS and ASP flooding, respectively. In the case of polymer flooding, a very low additional oil recovery was achieved (1.1% of the OOIP).This result indicates that the increase in the viscosity of the injected formulation has little effect on crude oil extraction,the most effective mechanism being the reduction of IFT with the surfactant. This justifies the better results obtained in terms of oil recovery with AS in comparison with ASP methods. However, in terms of potential economic savings, the costs associated to the latter would be significantly lower, making the method more promising.
4. Conclusions
The recent awareness of the possibilities of ILs has led to developed or under development technologies being reviewed

Table 7 Summary of core flooding experiments.
considering the contributions that these salts can provide. This is the case of surfactant EOR. The main advantages highlighted regarding the use of SAILs in EOR are their tunability and stability in harsh environments (high salinity and/or temperature). However,the number of flooding experiments carried out up to now is still very limited and the conclusions obtained must be considered with caution. Despite this, from the literature review and work presented here some tentative conclusions may be established.
· The early results are promising and research on this topic should be encouraged.
· There is a need to uniformize, as far as possible, the experimental methodology used in core flooding experiments to facilitate comparison among ILs.
· There is a lack of adsorption studies that should always be considered prior to flooding experiments.
· Up to now, most ILs have been proposed for sandstone reservoirs.The number of studies with carbonate cores is very scarce.
· The SAIL[C10mim][OTf]is able to drastically reduce the water/oil IFT. The value of this property increases with NaCl and reduces with NaOH concentration.
· A formulation consisting of 4000 ppm of [C10mim][OTf] and 1 wt% NaOH in brine solution (2 wt% NaCl) was proposed for EOR. A high adsorption was detected in sandstone rocks which discounts its use in these kinds of rocks.In the case of carbonate cores, after secondary recovery with brine, an additional 10.5%of OOIP recovery was achieved at room conditions.
· 8% of the OOIP was extracted using just one PV of surfactant followed by an aqueous solution of PAM. Even though a lower recovery was achieved, the low cost of polymer in comparison with the IL could make the process competitive.
· The main mechanism associated with the improvement in the oil recovery for the proposed formulation is IFT reduction.Increasing of viscous forces has a lesser effect.
Acknowledgements
The authors acknowledge the Ministry of Science and Innovation and State Research Agency for financial support throughout project PGC2018-097342-B-I00, including European Regional Development Fund. We would also like to thank SNF Floerger for supplying polymer samples and Repsol (A Coru~na) for providing the crude oil used for the experiments.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.petsci.2021.10.025.
- Petroleum Science的其它文章
- Primary logistics planning of oil products under the imbalance of supply and demand
- Advances in enhanced oil recovery technologies for low permeability reservoirs
- A novel method of quantitative evaluation and comprehensive classification of low permeability-tight oil reservoirs: A case study of Jidong Oilfield, China
- Measurement of pore diffusion factor of porous solid materials
- Catalytic oxidation of pentanethiol on basic nitrogen doped carbon hollow spheres derived from waste tires
- Temperature effect on the dynamic adsorption of anionic surfactants and alkalis to silica surfaces

