Surface oxidation study of molten Mg-Al alloys by oxide/metal/oxide sandwich method
Mohammad Mahdi Jalilvand,Hassan Saghafia,Mehdi Divandari,Mehdi Akbarifar
School of Materials Science and Engineering,Iran University of Science and Technology,Narmak,Tehran 1684611314,Iran
Abstract The dynamic oxidation of molten Mg-Al alloy was investigated via the oxide/metal/oxide(OMO)sandwich method.The characteristics of sandwiches were explored using optical microscopy,scanning electron microscopy,X-ray energy dispersive spectroscopy,and X-ray diffraction analyses.The results showed the formation of porous oxide film with varying thicknesses from 0.43 to 16.7 mm.Both the measurements and calculations confirme the literature finding that the oxidation product consists mainly of MgO and MgAl2O4 compounds.The increase in thickness and amount of folds formed on the oxide film implies the significan effect of aluminum in reducing the oxidation resistance of magnesium.
Keywords: OMO sandwich;Dynamic oxidation;Surface film Oxide morphology;Thermodynamics;Mg-Al alloys.
1.Introduction
Scattering in the mechanical performance of casting components is considered as a possible disadvantage of the casting technique.Recent studies have shown that the presence of oxide film related defects is the main reason for scattering in the mechanical properties of the casting parts [1,2].The surface of the molten magnesium is rapidly oxidized.During the pouring step,due to the turbulence of the melt fl w,the oxidized magnesium may enter to the bulk of the metal and create a dry surface/dry surface contact between two oxidized molten Mg which is called double oxide layer or "bifilm" At the outside of the bifilm the molten metal wets the oxides.It is suggested that the double layer oxide films can serve as suitable sites for nucleation and growth of the gas bubbles and also shrinkage pores [3].Due to the high affinit of the molten magnesium and aluminum for reacting with oxygen,investigating the characteristics of the double-layer oxide film is of great importance in the case of such metals.[1-3].
The melt fl w turbulence occurring in the casting processes breaks the surface oxide and exposes the bare surface of the molten metal to the atmosphere,repeatedly [4].Therefore,due to the fact that the melt is in motion during the pouring step,the oxide film are consistently subjected to mechanical stresses.Oxide film would be deformed or torn apart under such a dynamic situation [5].So,dynamically formed oxide film possess a different morphology in comparison to the film formed in static conditions [6-10].
Most of the alloying elements added to the magnesium,lower its liquidus temperature,reduce its oxidation resistance,and also its ignition temperature because of the tendency to cause local melting and magnesium evaporation [11,12].However,depending on the type and protection capability of the oxide fil formed on the magnesium and its alloys,the effect of the alloying elements can be either in favor of oxidation or against it [13,14].Adding some elements has led to the development of the Mg alloys with high ignition temperature or non-flammabl Mg alloys [15].It was reported that adding Be,Ca,and also a mixture of them can readily enhance the oxidation and ignition resistance of the Mg alloys[16-19].
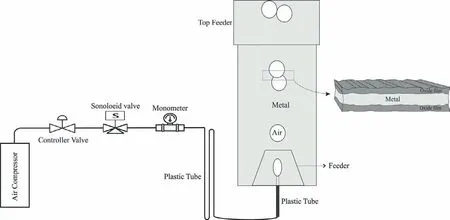
Fig.1.Schematics of the bubble generation system and production of the oxide-metal-oxide sandwich.
Aluminum is widely used as a major alloying element in the industrial series of magnesium alloys such as AZ and AM alloying groups [20].Magnesium alloying with aluminum in an atmosphere containing oxygen reduces the ignition resistance;this is due to the decrease in the liquidus temperature[21].The negative effect of aluminum on the oxidation resistance of the magnesium alloys is attributed to the lower liquidus temperature and the eutectic microstructure,which results in the selective oxidation and evaporation of magnesium [22].
To provide the conditions of the dynamic oxidation during the pouring step,Divandari and Campbell have proposed a technique based on releasing artificia bubbles into the molten metal [23].Colliding and entrapment of the released bubbles in the cast part creates a layer between two adjacent bubbles which has been called the oxide/metal/oxide(OMO)sandwich[23].Considering the limited amount of oxygen presented in the bubbles,the oxide film formed around them are considered as short-time or young oxide film [24].The sandwiches provide unique information about the dynamically formed oxide film on the surface of molten metals.The morphology,thickness,and composition of such oxide film are some of the information which can be acquired using the OMO sandwich method [25].
The aim of this study is to investigate the dynamic oxidation of the molten Mg-Al alloys.For this purpose,the aforementioned method was used to investigate the dynamic oxidation of pure Mg,Mg-3%Al,and Mg-%6Al(weight percent).The characteristics of the oxide film including composition,thickness,and morphology on the samples were studied.
2.Experimental details
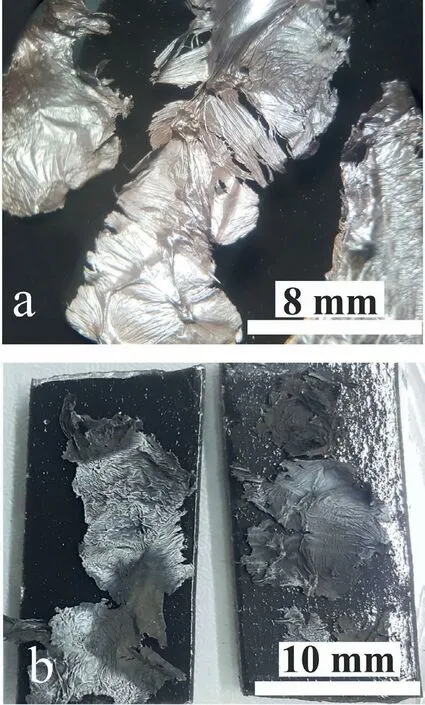
Fig.2.Macroscopic appearance of the OMO samples,(a) pure Mg,(b) Mg-3Al.
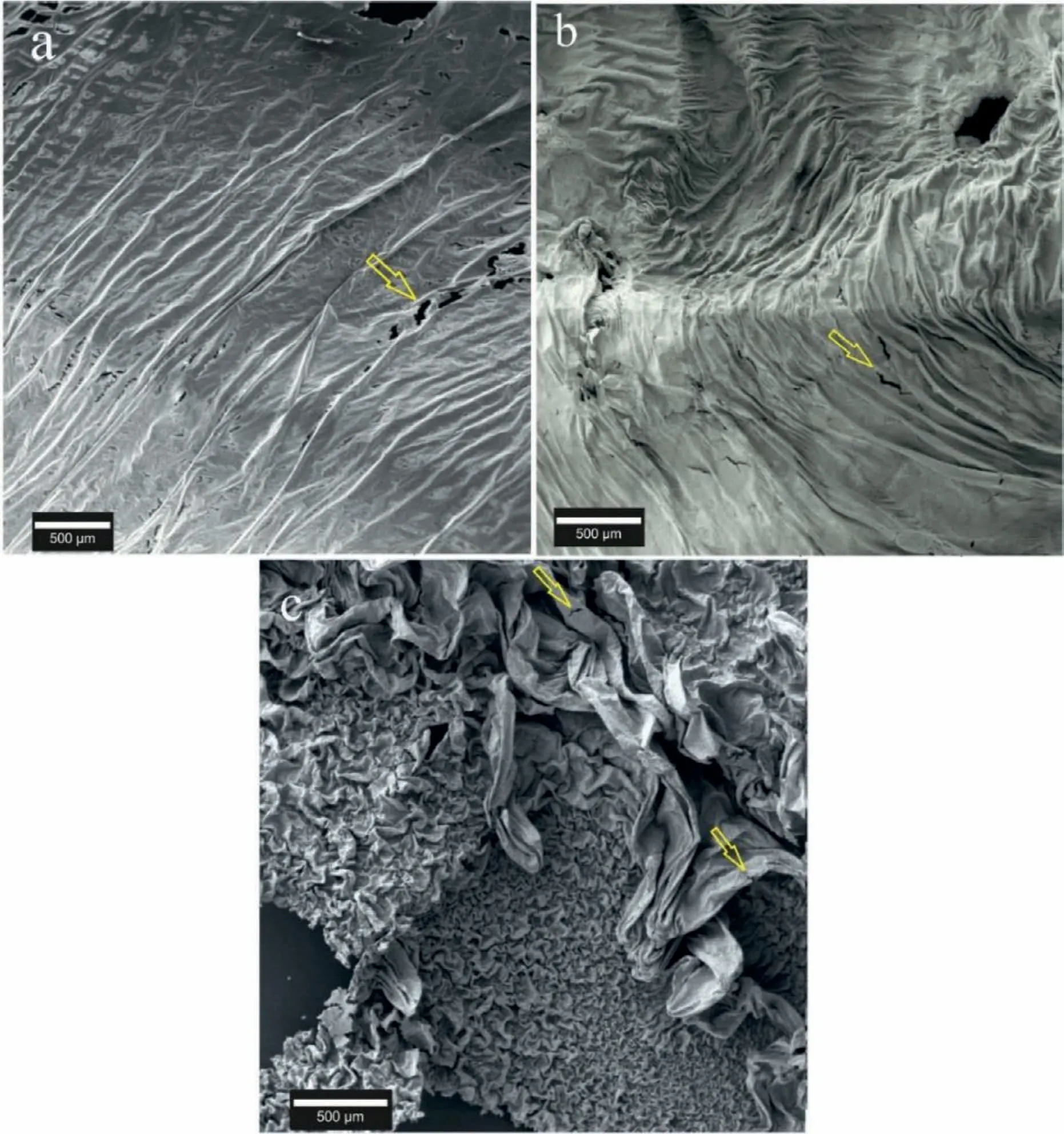
Fig.3.OMO sandwiches of (a) pure Mg,(b) Mg-3Al,and (c) Mg-6Al samples.The difference in the morphology is notable.
As shown in Fig.1,special equipment including a control valve,solenoid valve,manometer,and electrical interrupter,were used to produce and release artificia bubbles of specifi size and shape into the melt.Eventually,the air bubble is blown into the mold at a pressure of 0.2 atm through a quartz tube with an inner and outer diameter of 1 mm and 3 mm,respectively.The air bubbles were blown to the mold during the pouring step in specifie intervals (two bubbles per second).
The casting model was a thin plate of 15 mm thickness.A top feeder was designed to postpone the solidificatio process in order to increase the time/chance of bubbles entrapment in the casting.Also,a trapezium-shape section was designed at the bottom of the plate to prevent the solidificatio of the melt surrounding the quartz tube.
A bottom pour gating system was performed to minimize the turbulence of the melt during the pouring stage (Fig.1).Also,by performing this system the entrance velocity of the melt into the mold is decreased.More information about the method used in this study has been presented in another research[26].The mold material consisted of silica sand,4%wt.sodium silicate as the binder and 1% dry sulfur powder.
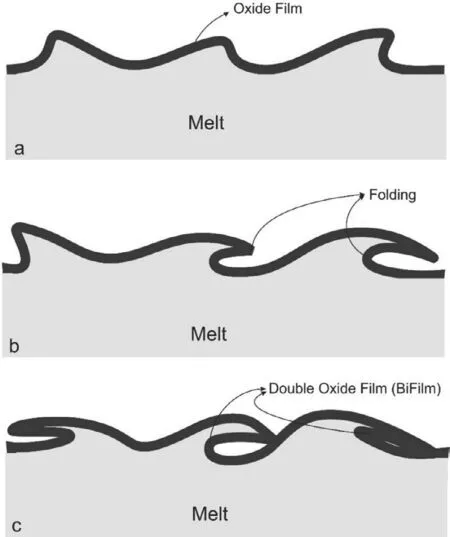
Fig.4.Formation and entrapment of double oxide fil defects during the pouring stage of the casting process as a result of surface turbulence.
Two ingots of commercially pure magnesium and aluminum were used as the raw material.Melting was done in an electrical-resistance furnace.To protect the samples against the risk of flaming MagRexR○coverall flu was employed in the melting process.Regarding the fina composition of the alloys,the alloying process was carried out using proper calculations.According to the industrial importance of the Mg-Al alloying system as well as to investigate the effect of the increasing trend of aluminum content in this system,the fina composition of the alloys was defined Table 1,presents the result for the quantitative analysis of the cast samples obtained by the spark optical emission spectrometer.Accordingly,samples were labeled as Mg-3Al and Mg-6Al for Mg-2.62Al and Mg-5.35Al alloys,respectively.The pouring temperature was 50 °C above the melting temperature of each sample.
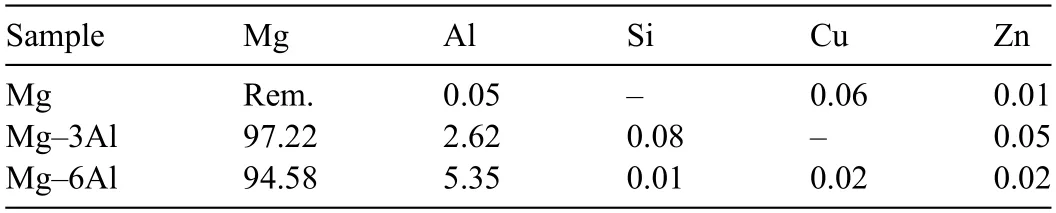
Table 1 Chemical composition of the alloys studied in this work.

Table 2 The linear coefficien of thermal expansion (CTE) of different materials[30,31].
After successfully performing the method,the samples were cooled and carefully cut.The OMO sandwiches were extracted from the samples (Fig.1) employed in this study.Samples were then analyzed by X-ray diffraction (XRD) and scanning electron microscopy (SEM) equipped with energy dispersive spectroscopy (EDS).
3.Results and discussion
3.1.Macroscopic feature
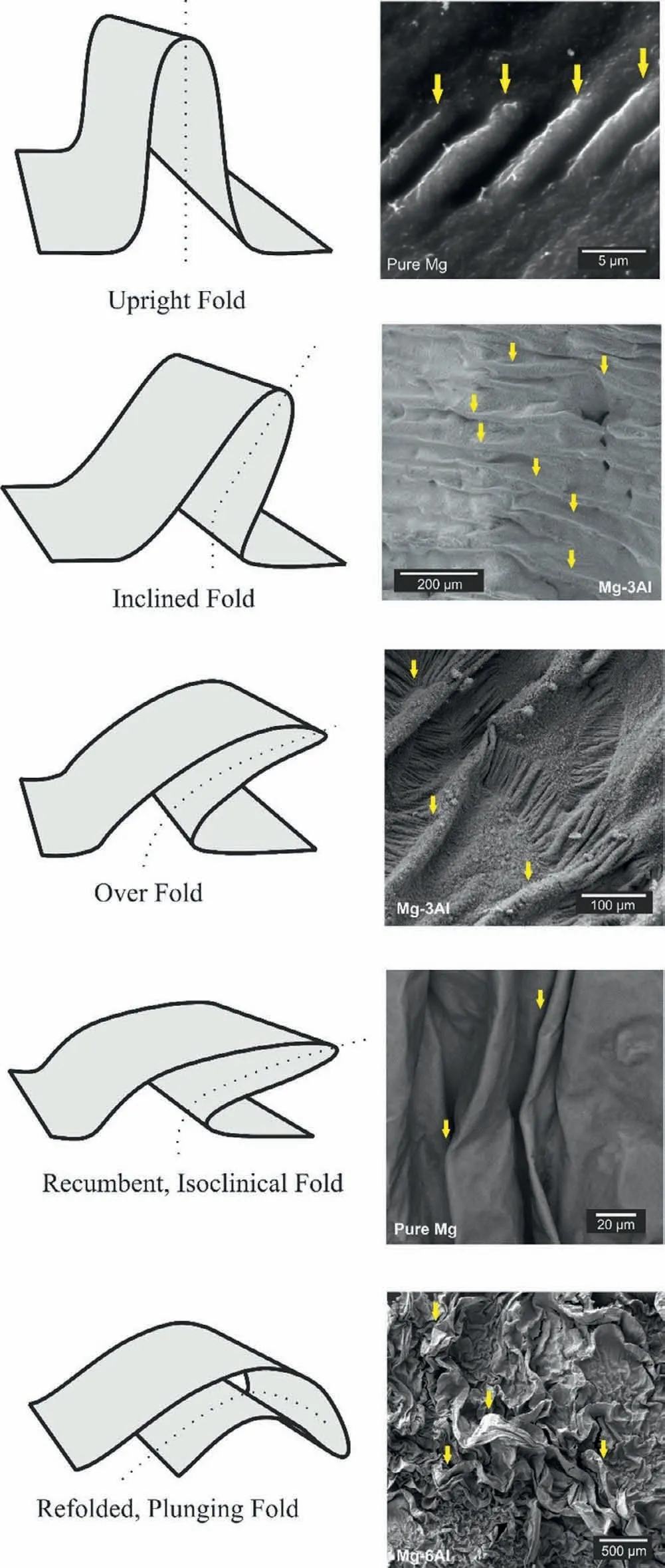
Fig.5.Classification of the folds formed on the OMO sandwiches.
The surface roughness is an important factor in light reflectio from a material.The light reflecte from a smoother surface at angles equal to the primary emission angle,results in a shiny appearance.Considering Fig.2,which shows the images taken from the OMO sandwiches,the appearance of the pure Mg sandwich sample is shiny (Fig.2a).Adding Al to the melt resulted in a darker appearance of the sandwich surface (Fig.2b).Supposedly,aluminum additions have increased the surface roughness of the oxides films The scattering of the light beams due to surface roughness affects the sample appearance.The change in the appearance of the MgO oxide fil from shiny to dark has been attributed to the surface roughness and evaporation of magnesium [27].

Fig.6.SEM image taken from (a) pure Mg,(b) Mg-6Al OMO sample showing the two-phase structure.Oxide/Oxide regions have a brighter appearance comparing to Oxide/Metal/Oxide regions.Schematics from top and cross-section views from the two-phase structure of the (c) pure Mg,and (d) Mg-6Al OMO sandwiches.
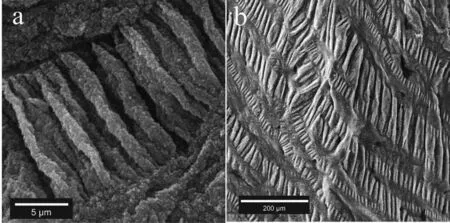
Fig.7.SEM images of the Mg-3Al sandwich showing the stich line morphology at different magnifications

Fig.8.The schematics drawing showing the evolution of stich lines on the oxide fil of the Mg-3Al sample.
3.2.Morphological investigations
Fig.3 shows the SEM images of the pure Mg,Mg-3Al,and Mg-6Al OMO sandwiches.As shown in this figure the surfaces of these samples include many folds.Also,the locations of cracks and ruptures have been marked by yellow arrows.The dynamic conditions during the formation of these oxide film have caused such a highly folded morphology.The fluctuation and upward movement of the bubbles in the melt can be considered as one of the sources for the creation of the dynamic condition.Regarding the SEM images of Mg-3Al and Mg-6Al samples,the number of folds and also their size is higher than that of pure magnesium sample.Probably,the addition of Al to the Mg melt has weakened the oxide fil rigidity to be easily folded.The strength of the oxide fil seems to play an important role in the formation of these folds [28].
Two different types of folds,which are distinguished by their size,are visible on the OMO sandwich of the Mg-6Al sample (Fig.3c).Mechanical stresses and also the growth mechanism are probably the reasons behind the formation of larger folds in this sample.The mechanical stresses are the results of the high-velocity upward movement of bubbles in the melt and pressure difference between two adjacent bubbles [5].The magnitude of these stresses is such that apart from the folding and deformation of the oxide films deforms the metal entrapped between the oxide layers.Thus,it is expected that the larger folds form on the sandwich surface as a result of the mechanical stresses.The mechanical forces could increase the surface area of the bubble,and temporarily increase the surface area of the oxide film On returning to its spherical shape,the surface area of the fil would be too large to fi the bubble.So,as it is in the solid state,deformation would be unavoidable.
It has been reported that the addition of 1 at%.of Al has accelerated the oxidation of pure magnesium [29].Adding 10 at% of Al to Mg doubles the oxidation rate constant of magnesium at 400 °C.Therefore,it can be concluded that decreasing the oxidation resistance of the alloying samples increases the size of folds.
As described in the introduction section,melt turbulence during the pouring and fillin stage might lead to the formation and entrapment of double oxide fil defects.Folding of the oxide fil is the main factor for the formation of these types of defects.This concept is schematically shown in Fig.4.As it could be seen from Fig.4 there is a dry surface/dry surface contact between the oxidized metal.The double-layer oxide films are considered as a crack in the fi nal casting part and decrease the mechanical performances of the parts.They also can serve as potential sites for nucleation and growth of the shrinkage pores and gas bubbles [3].
Fig.5 schematically shows the different types of folds which have formed on the OMO samples.The oxide fil in the pure Mg sandwich is mainly composed of upright and inclined folds.On the other hand,in the Mg-3Al sample in addition to the inclined folds,the surface oxide has experienced the over folding of the layer in some regions.In thecase of the Mg-6Al OMO sample,the isoclinical and plunging folds are formed.By decreasing the oxidation resistance of the melt formed oxide fil would be thicker.Also,there is possibly a chance for detachment of oxide fil from the substrate for thicker oxide film The mentioned factor has influence the morphology of the folds formed on the oxide films These changes in the oxide film can be attributed to the effect of Al additions on the oxide films
Fig.6 (a and b) reveals two different regions in the SEM images of OMO sandwiches.This two-phase microstructure is schematically illustrated from both top and cross-section views in Fig.6 (c and d).During the formation of OMO sandwiches,there is always a certain amount of molten metal entrapped between two oxide layers.As the temperature decreases,the solidificatio process begins.Consequently,solidphase nucleates and starts to grow.Further growth and decrease in the temperature would be accompanied by volume reductions.This leads the two oxide layers to come in contact with each other in some areas [7-10].Other researchers have observed similar two-phase structure in OMO sandwich samples of different alloys such as aluminum and zinc alloys[6-8].This morphology can be considered as an indicator of double layer oxide film in which two dry surfaces of oxides are in contact with each other.
Fig.7 demonstrates SEM images of the Mg-3Al sandwich showing a stich-like morphology of folds that have also been observed in other samples.As shown in Fig.7(a and b) many stich lines formed on the OMO sandwich of the Mg-3Al sample.The concept of forming such morphology is schematically shown in Fig.8.Exerting different types of stresses in the oxide film which has a weak strength against tensile stresses,results in a tear in some areas.The tearing of the oxide fil lets the molten metal and oxygen atoms to react with each other.As a result of that,the new oxide fil starts to form rapidly on the old oxide fil which is already in solid-state.The new oxide fil grows,in a special manner,and heals the old oxide film It then folds and wrinkles due to some reasons such as the difference in the linear thermal expansion coefficien between the oxide film and base metal.
Table 2 presents the linear expansion coefficien values for magnesium,aluminum,as well as the oxides of these two metals at room temperature.According to the values in Table 2,it can be claimed that the amount of volumetric shrinkage of the molten alloys is greater than that of oxide films Thus,during cooling and solidificatio of the molten metal,the oxide formed on the surface cannot be adapted to the metal beneath,which has gone through greater shrinkage than the oxide.As a result,the oxide fil would be folded or wrinkled to compensate for the change [25].The folds created by this mechanism are in microscopic scale.Although,the mechanical forces can form macroscopic folds.
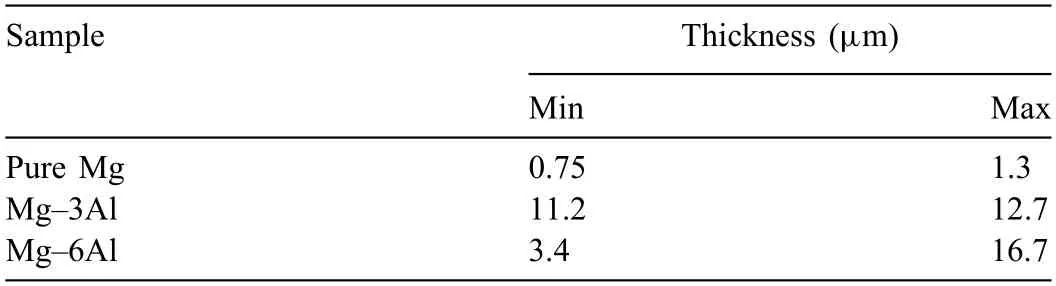
Table 3 The results of the oxide fil thickness estimations for different samples.
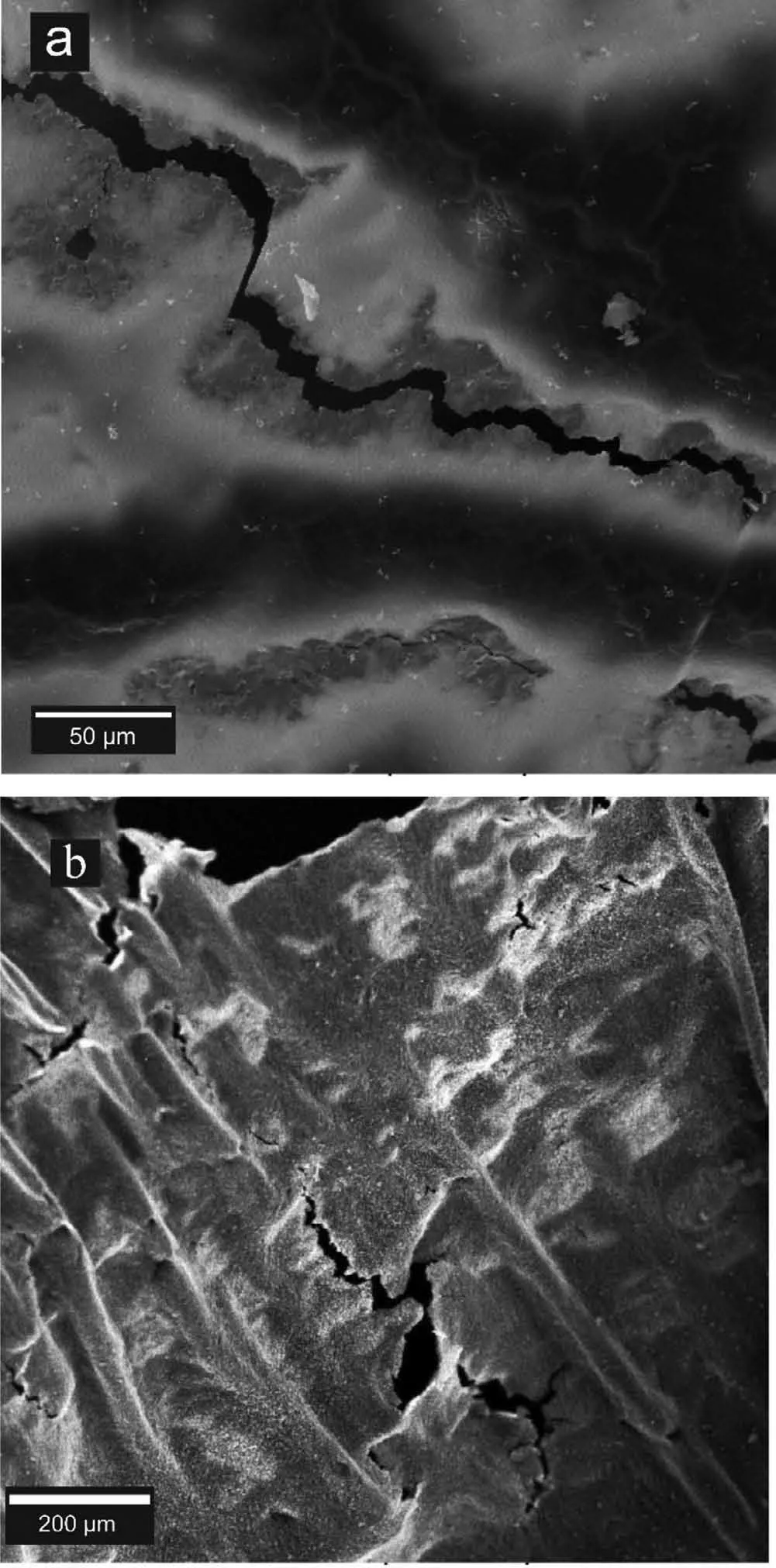
Fig.9.SEM image of crack and ruptures found on (a) pure Mg and (b)Mg-3Al OMO samples.

Fig.10.(a,c and d) SEM images taken from the Mg-3Al sample showing a porous microstructure with corresponding EDS results taken from the point indicated in image (a).Note that this morphology has been found in other samples.
Returning to Fig.3 the cracks and ruptures found in the oxide samples of the Mg-Al system are shown by yellow arrows.Closer views of a crack found on the OMO samples is presented in Fig.9.The shrinkage forces resulted from the solidificatio of the metal confine between the two oxide layers,cause tensile stresses.Since the tensile strength of the oxide fil is lower than that of the base metals,the oxide fil would crack and tear in areas where the metal is not entrapped(and/or sucked out to adjacent directions).This is also the case for the areas in which the quantity of the entrapped metal is low.Mechanical stresses,which can be greater than shrinkage stresses,especially during sample extraction,are other causes of cracking in the oxide film It should be noted that the cracks caused by shrinkage stresses are rarely found in Mg-6Al samples.This can be attributed to the effect of Al additions on increasing Mg strength through the solid solution or increasing the oxide fil strength [20,28].
The cracking and rupturing of the oxide film covering the melt expose the underlying metal to the air so that the oxidation progresses.The internal stresses cause the crack formation on the oxide film Thus,an oxide fil with higher mechanical properties is likely to have a better resistance against the oxidation [32].
SEM images at larger magnification of the oxide film are shown in Fig.10.These images indicate a porous structure composed of a large number of oxide spheres.The diameter of these oxide spheres is in nanometers scales.The presence of the pores in this type of microstructure provides suitable channels for the inward movement of oxygen gas (molecular oxygen).Hence,the oxidation process can continue until the complete consumption of the oxygen in reach [33,34].These fin particles are the result of growth from vapor deposition.Mg is known to evaporate from oxide film and the vapor reacts with oxygen above the surface of the fil
Since morphology is one of the key factors in determining the capability of oxide fil protection [35];dynamically formed oxide film in the Mg-Al system can be assumed to be non-protective.The magnesium oxide formed at room temperature is predominantly amorphous and dense with uniform morphology.As the temperature increases,the amorphous fil begins to crystallize and become porous [14].As a result of that,the oxide fil loses its protection capability and the continuous oxidation of the underlying metal occurs[14,36].Accordingly,the low resistance of the Mg-Al alloys against oxidation can be attributed to the porous microstructure of their oxide films
It seems that the oxide fil may grow by either growth of granular oxide particles or with layer thickening mechanisms.Initially,an amorphous layer of oxide forms on the substrate.Next,the granules of the crystalline MgO nucleates on this layer.As the oxidation process goes on,these granular oxides,which are in the nanometric scale,form a crystalline oxide layer on the former layer.The thickening of the oxide fil happens through the growth of the crystalline layer.Fig.11 schematically illustrates the granular growth of the Mg-based alloy samples.
3.3.Thickness estimation
Approximate thickness of the dynamically formed oxide film in the molten state can be obtained by determining the width of folds created on the surface of an oxide fil [5,7,8].Since these folds consist of two layers of oxide,the estimated width should be divided by two to have the oxide fil thickness.It should be noted that the possibility of melt entrapment between two layers of a fold reduces the accuracy of this method.
Table 3 gives the estimated values of thickness for the dynamically formed oxide film in this study.From these values,one can see that the addition of aluminum will increase the thickness range of oxide film from a mean value of 1.025 μm for pure Mg to 11.95 μm for Mg-3Al sandwich samples.The highest thickness for dynamic oxide film was 16.7 μm for the Mg-6Al sandwich sample.
As it can be seen from the results,the dynamic oxide film formed on the magnesium alloys are thicker than those of the aluminum alloys which has been reported to be approximately 50 nm[9,10].Compared to the dense and protective fil formed on the aluminum alloys in the molten state,the presence of such thick and rough film reveals the request for paying more attention to the oxidation of magnesium alloys.
3.4.Phase identificatio
The EDS results obtained from points marked in the SEM images of the pure Mg,Mg-3Al,and Mg-6Al sandwiches are presented in Fig.12.Regarding Fig.12(a) which illustrates the dual-phase microstructure of the pure Mg sandwich,the difference between the intensity of the peaks corresponding to O indicates that the bright regions are formed by the overlapping of the oxide layers on each other.The existence of oxygen in the oxide layer is evident in the EDS results obtained from alloying samples.X-ray diffraction tests were also used to identify the compounds present in the sandwiches.Fig.13 shows the XRD patterns of the samples.Identifying the peaks,it can be seen that most of them are related toα-magnesium.MgO oxide was detected in all sandwiches.However,it is observed that the peaks corresponded to MgO in the pure magnesium sandwich are less intense.This may be due to the low oxide content and thickness of the oxide fil in the pure Mg sandwich.Adding aluminum to magnesium increases the relative intensity of the MgO peaks.

Fig.12.Results of EDS analyses obtained from specifie points in (a) Pure Mg,(b) Mg-3Al,and (c) Mg-6Al OMO sandwiches.
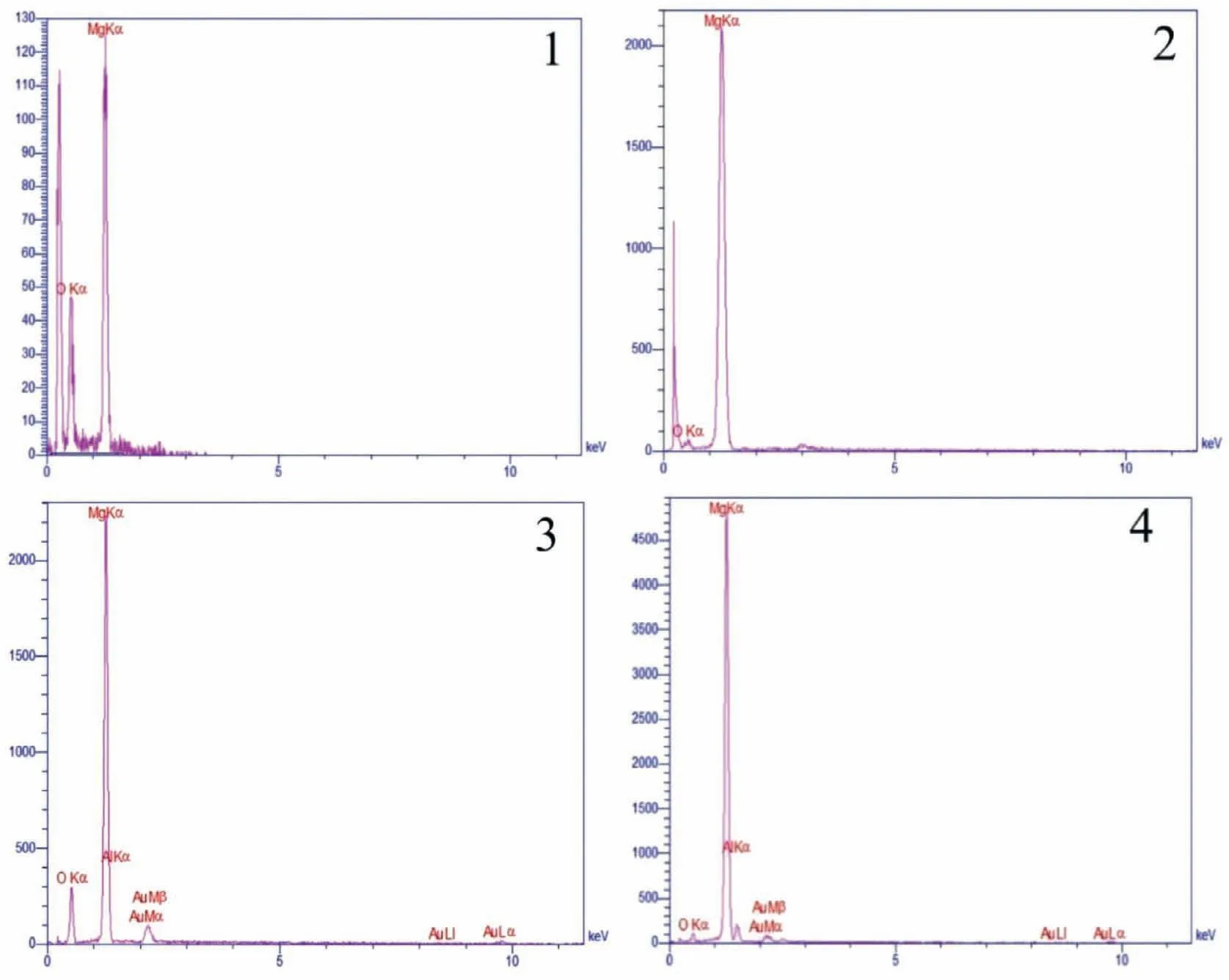
Fig.12.Continued

Fig.13.XRD patterns of the OMO sandwiches.
Three oxide compounds,including MgO,Al2O3,and spinel MgAl2O4,have the chance to form during oxidation of the Mg-Al alloy systems.[37,38].The Gibbs energy equations for the formation of these three compounds,in the presence of oxygen,are presented in Table 4.TheΔG°of the compounds is calculated at 973 K[39,40].Reaction 1 is more thermodynamically favorable regarding the lowerΔG of formation.MgO oxide,which is already considered as the reactant for reactions No.5 and 3,forms through reaction 1.Therefore,from the thermodynamics,this compound is the most stable oxide in Mg-Al alloy melts within the range of this study.Despite the results of the XRD analyses (Fig.13),which have only detected the presence of the MgO compounds,formation of the MgAl2O4spinel is also suggestedaccording to the thermodynamic calculations.It should be noted that the fraction of a phase in the composition of oxide fil plays an important role in detecting of this compound by XRD.Supposedly,the MgAl2O4compound was not detected by XRD due to its low amount.
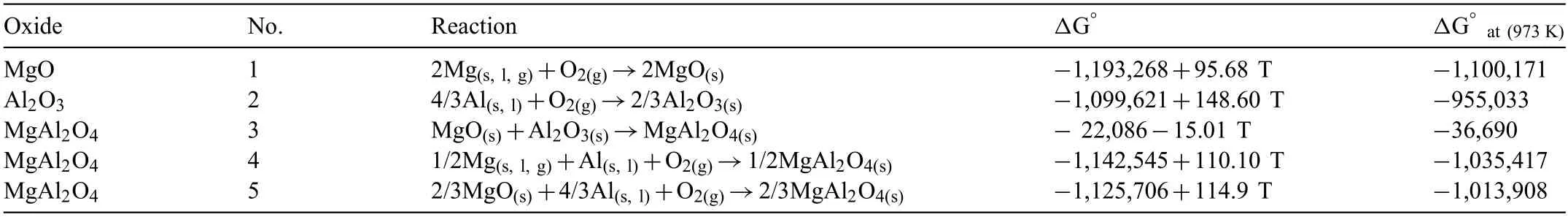
Table 4 The Gibbs free energy for formation of different oxide compounds at 700 °C [39,40].
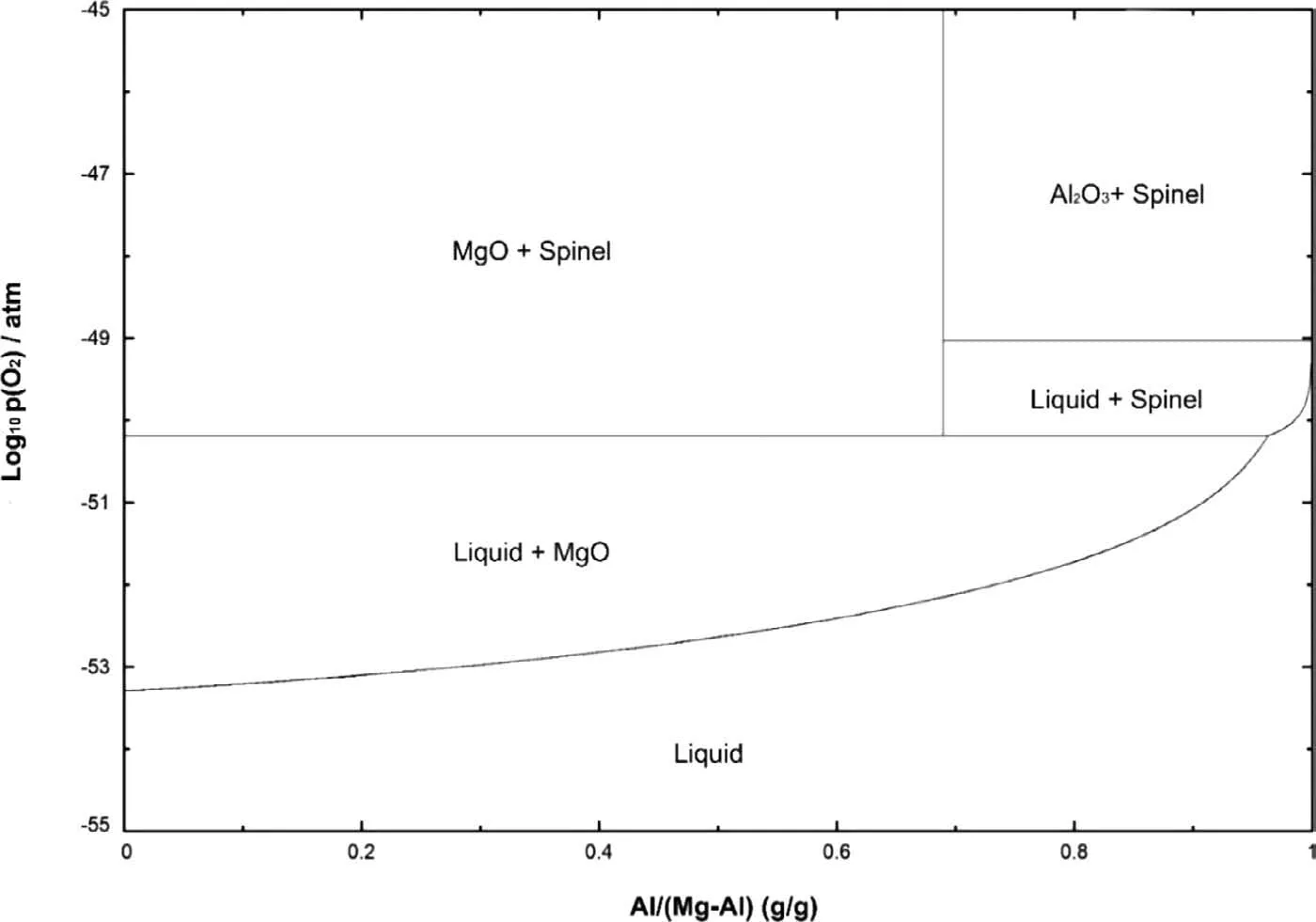
Fig.14.Thermodynamic phase stability formed in the Mg-Al-O2 system at 700 °C [41].
Fig.14 shows phase diagram of the Mg-Al-O2system obtained from FactSage 7.3.Based on this figure increasing the oxygen pressure,MgO is the primary oxide in the Mg-Al system at 700 °C within the range of Al content in this study.Above the oxygen pressure of 10?50atm (PO2≥10?50atm),the MgO and MgAl2O4are both stable oxides at 700 °C.It worth to mention that the diagram is plotted in equilibrium condition whereas the growth of the oxide film occurs in highly non-equilibrium conditions [42].
Akbarifar et al.has suggested that the high surface activity of Mg atoms is an obstacle in the way of Al atoms reacting with oxygen,In the case of Al-Mg alloys [43].It should be noted that in addition to thermodynamics,oxidation kinetics has a major impact on the composition of an oxide fil [43].Considering Fig.10,the porous structure of the MgO oxide fil provides suitable channels for inward diffusion of the oxygen ions to the metal surface.
Fig.15 shows the SEM images taken from the surface of the Mg-3Al sandwich.As shown in this figur two different morphology,granular and cubic,are visible in the images.Wang et al.have recently reported that the morphology of MgO depends on the physical state of the metallic substrate.The MgO which has formed from the liquid state is octahedral while the cubic ones are formed from Mg vapor [44].
Shih et al.[38] have reported the formation of MgAl2O4phases on the solution-treated AZ80 alloy sample heated in air at 427 °C for one hour,using the ESCA analysis(Electron Spectroscopy for Chemical Analysis).Moreover,Feliu et al.have suggested the formation of this compound for Mg-based alloys containing 3.1wt.% to 6.2wt.% Al [45].
By consumption of Mg atoms in the substrate for forming the MgO compound,the concentration of Al on the metal surface increases.This is thermodynamically favorable for the formation of spinel.The mobility of atoms is facilitated due to the fact that the alloy is in the liquid state above 700 °C.Regarding the thermodynamic assessments which were discussed in this section,the MgAl2O4spinel can form by reactions 4 and 5.Pouros morphology of the oxide film formed on Mg-Al alloy samples provides a chance for the reaction which leads to the formation of spinel.The spinel formed by this mechanism is more likely to be located within the MgO oxide fil [38].On the other hand,the metastable MgO has the chance to react with the Al and O2to form MgAl2O4via reaction 5.This spinel tends to be formed at the oxide/substrate interface [46].
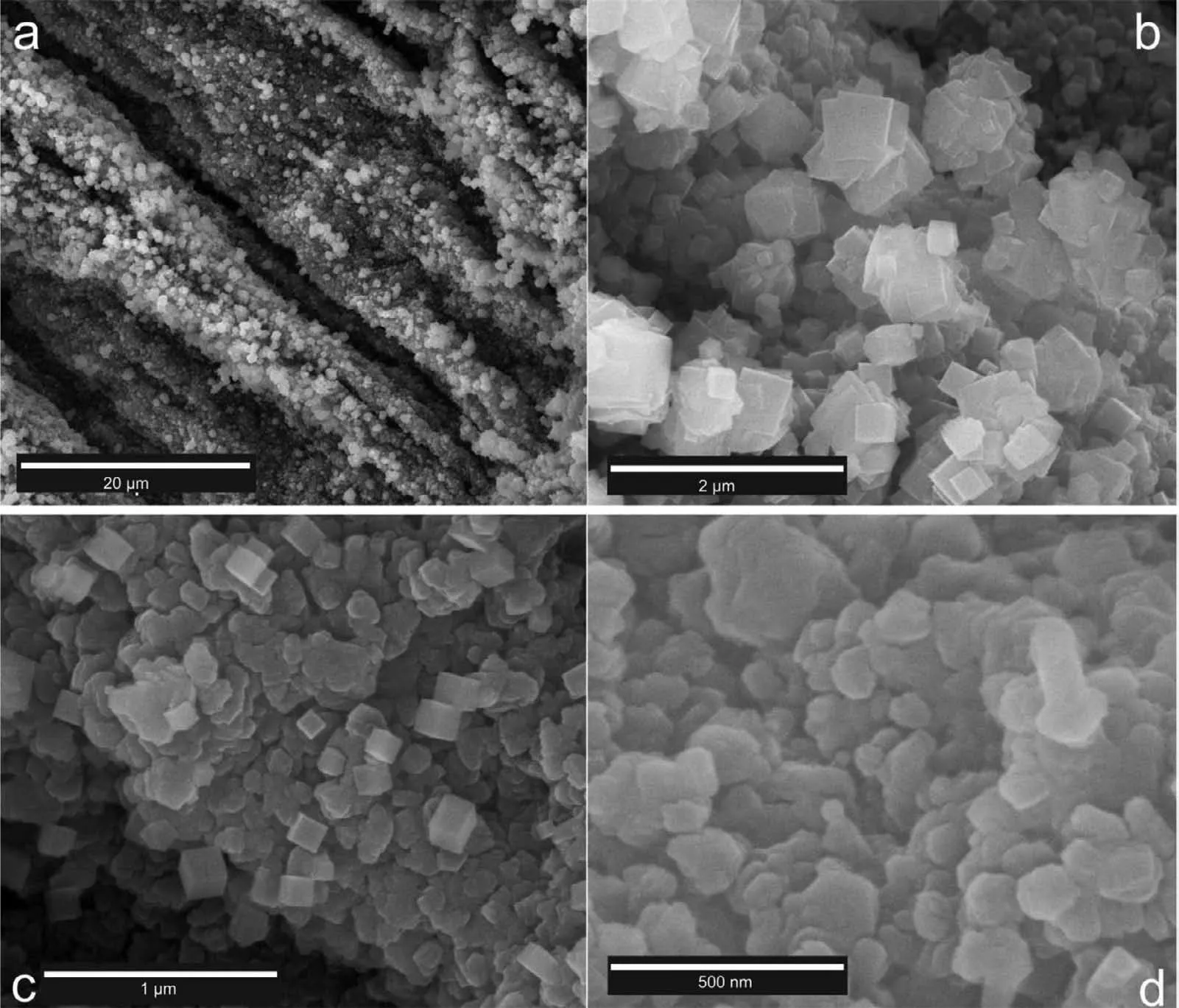
Fig.15.SEM images of the Mg-3Al sandwich at different magnifications (a).5000 X,(b).50,000 X,(c).100,000 X,and (d).200,000 X.
To sum up,based on the analyses and also thermodynamic calculations,it can be concluded that the short time dynamic oxidation of the Mg-Al alloys in this study has resulted in the formation of crystalline MgO and probably MgAl2O4spinel oxides.
4.Conclusion
The dynamic oxidation of the molten Mg-X(%wt)Al alloys was studied via the so-called oxide/metal/oxide (OMO)method.Based on the investigations,the followings can be concluded:
1.The morphology of the oxide film consisted of different types of folds.Due to the low tensile strength of the oxide films these film have been torn and cracked in some areas.
2.At the nanometric scale,all the oxide film formed in this system were porous.These pores are created by the gap between oxide crystals and provide suitable channels for the transfer of oxygen.
3.The addition of aluminum significantl changes the morphology of the oxide films As the aluminum content increases,the oxide film become more wrinkled.
4.The thickness of the oxide fil was measured according the technique in which half of the width of the folds formed on the oxide fil is considered as its thickness.The thickness was increased by adding aluminum to magnesium.
Declaration of Competing Interest
The authors declare that there is no conflic of interest.
Acknowledgment
We would like to express our sincere gratitude to Dr.Mohammad Divandari (ETH Zurich) for his scientifi comments and grammatical review.Thanks go to the Iran University of Science and Technology and the Porous and Cellular Materials Laboratory staff of this university for their cooperation and assistance.
 Journal of Magnesium and Alloys2022年6期
Journal of Magnesium and Alloys2022年6期
- Journal of Magnesium and Alloys的其它文章
- EDITORIAL BOARD
- Aims and Scope
- Production and characterisation of new bioresorbable radiopaque Mg-Zn-Y alloy to improve X-ray visibility of polymeric scaffolds
- Quantitative study on the tension-compression yield asymmetry of a Mg-3Al-1Zn alloy with bimodal texture components
- Microstructure analyses and phase-fiel simulation of partially divorced eutectic solidificatio in hypoeutectic Mg-Al Alloys
- Promoting wetting of Mg on the SiC surfaces by addition of Al,Zn and Zr elements:A study via first-principl calculations
