Rational Design of High-Performance Bilayer Solar Evaporator by Using Waste Polyester-Derived Porous Carbon-Coated Wood
Ning Liu, Liang Hao, Boyi Zhang, Ran Niu, Jiang Gong* , and Tao Tang*
1. Introduction
With ever-growing global population, the freshwater scarcity becomes one of the most pressing problems in human society.Although oceans cover over 70%of Earth’s surface,only 10%of water resources can be accessed by residents.Water treatment techniques such as membrane filtration, distillation, and reverse osmosis have been developed to produce freshwater.[1]However, these techniques suffer from massive energy consumption. Over the past few years,interfacial solar-driven vapor generation, which utilizes clean and renewable sunlight as energy source to generate clean water from seawater,is considered as an appealing approach to alleviate the severe situation of global water scarcity.[2,3]Compared with other desalination technologies,interfacial solar-driven vaporization localizes solar-thermal energy conversion and heats up the air–water interface to simultaneously realize low heat loss and high conversion efficiency.[4-6]Photothermal materials are pivotal elements that act as both solar harvesters and solar-to-thermal converters. So far, a plethora of photothermal materials have been prepared,for example,carbon materials,[7,8]polymeric gels,[9,10]metallic nanomaterials,[11,12]and semiconductors.[13,14]Despite the significant progress, most of these photothermal materials have drawbacks, such as high cost, complicated fabrication process,and difficulty in scalability. More seriously, the surface of photothermal materials is easily deposited by salt precipitated from seawater, which dramatically impairs the water production rate and impedes large-scale practical applications.

Figure 1. Scheme of preparing porous carbon-coated wood (PCW) bilayer solar evaporators for solar steam generation to produce freshwater.
Wood is regarded as one of the most abundant renewable resources on Earth and recently has been widely used for interfacial solar steam generation, owing to its several advantages including low cost, low thermal conductivity, nontoxicity, natural hydrophilicity, self-floating property,and many aligned microchannels.[15-18]Particularly,the large vertical-channel arrays of wood favor for the fast water transportation and enable the long-term desalination of seawater.Unfortunately,natural wood is light in color and does not absorb sunlight well,leading to relatively low efficiency in solar steam generation.Recently,a great deal of effort has been made to improve its solar absorption, which can be classified into three strategies.The first strategy is the surface carbonization of wood by thermal,flame,or laser ablation treatment,while keeping the internal porous structure of wood unchanged.[19-23]However,this strategy could not precisely control the porous structures (e.g.,micropores (<2 nm), mesopores (2–50 nm), and macropores(>50 nm))of the surface carbon layer.Recent work has confirmed that the porosity of carbon is important for water transportation and subsequent vapor formation,[24]but there is still inadequate understanding about the role of porous structures of carbon layer in terms of light absorption,water transportation,and the state of water molecule in the carbon layer. The second strategy is the bulk carbonization to turn wood into carbon foam by annealing at high temperature.[25,26]Compared to wood, carbon foam suffers from drawbacks of low hydrophilicity, poor mechanical property, and low evaporation rate(e.g., <1.65 kg m?2h?1). The third strategy is the surface deposition of wood with high-absorptivity materials, for example, carbon nanotube,[27]graphene,[28,29]porous carbon,[30]metal nanoparticle,[31,32]and semiconductor.[33,34]However, these solar evaporators have respective limitations including high cost of absorptivity materials and complex processing technique. Consequently, simple, cost-efficient,and scalable surface modification methods are highly desired to design wood-based bilayer solar devices with high evaporation rates and wellcontrolled porous carbon layer.
Nowadays,millions of tons of urban and industrial plastic wastes are discarded annually,which has brought about serious waste of resources and many environmental problems, for example, “White Pollution.”[35]From the perspectives of sustainable development and solar energy exploitation,converting waste plastics into carbon-based materials with well-controlled porous structures can kill two birds with one stone, because it not only advances the treatment of plastic wastes, but also offers a cost-efficient approach for large-scale production of carbon-based solar evaporators. During the last two decades, we have been devoted to converting waste plastics into carbon-based materials,[36-41]and recently put forward the controlled carbonization strategy of waste plastics into porous carbons as photothermal materials for interfacial solar vapor generation.[42-45]Unfortunately, the resultant porous carbon shows moderate specific surface areas (e.g.,<700 m?2g?1)and low evaporation rates(e.g.,<1.65 kg m?2h?1).
In this work, we report a novel strategy for the catalytic carbonization of waste polyesters to prepare low-cost porous carbon with welldefined hierarchically porous structures and high specific surface areas(1164 m?2g?1). By dip coating porous carbon onto the surface of wood, a bilayer solar evaporator is facilely fabricated. With the merits of abundant micro-/meso-/macropores and oxygen-containing groups,porous carbon remarkably improves sunlight absorbability, accelerates water transportation and vapor escaping,and reduces water evaporation enthalpy. These combined features above endow the bilayer solar evaporator with high evaporation rate (2.38 kg m?2h?1), good saltresistance, excellent long-term stability, and good scalable capability.
2. Results and Discussion
2.1. Morphology, Microstructure, and Textural Property
The wood-based bilayer solar evaporator was rationally constructed by dip coating porous carbon(PC-x)on the top surface of wood block(Figure 1).PC-x with well-controlled structures was firstly prepared through catalytic carbonization of poly(ethylene terephthalate)(PET)by ZnO at 550 °C.After the ZnO/PET mass ratio (x) rises from 0.5 to 3, more nanopores of 30–200 nm in size are observed in PC-x (Figure 2a?d), meanwhile the apparent packing density of PC-x decreases (Figure S1). Evidently,ZnO acts as a physical template during carbonization, thereby increasing the amount of ZnO leads to the formation of more meso-/macropores in PC-x.TEM and HRTEM images(Figure 2e and f)reveal that PC-1 bears a multitude of micro-/meso-/macropores and discontinuous graphite layers with a layer space of ca.0.35 nm.
Moreover, the textural property of PC-x was studied by using N2adsorption-desorption at 77 K (Figure 2g and Table S1). In a lowpressure range(P/P0< 0.1),PC-0 displays a type-I curve and a moderate N2uptake,meaning the existence of rich micropores.PC-x(x = 0.5,1, 2 and 3) exhibits the combination of type I/IV physisorption isotherms and displays a type-H4 hysteresis loop at P/P0= 0.2 ?0.95 with a sharp N2uptake at P/P0> 0.8, confirming the formation of abundant mesopores and macropores(Figure 2h and i).[46]The micropore size distribution of PC-x (x = 0.5, 1, 2, and 3) calculated by using DFT model is centered on 0.5 nm,smaller than that of PC-0(0.65 nm),implying the positive effect of ZnO on the formation of smaller micropores.To expound the catalytic effect of ZnO during the carbonization of PET,model carbonization experiments were conducted,in which PET or PET/ZnO mixture (the ZnO/PET mass ratio=1) was heated at 350 °C for 30 min. As displayed in Figure S2, the C = O stretching vibration peak at ca. 1700 cm?1in the FT-IR curve of PET/ZnO mixture almost disappears;meanwhile,the vibration peak intensity of conjugated double bonds at ca. 1600 cm?1significantly increases, compared to PET. The possible reason is that ZnO with ample Lewis acid sites catalyzes the dehydration and decarboxylation of PET to promote the generation of vinyl-terminated chain fragments, which is advantageous for the subsequent crosslinking reaction to form smaller micropores. Importantly,ZnO acts as an efficient catalyst for the controlled carbonization of other polyesters(e.g.,polycarbonate)into porous carbon(Figure S3).
2.2. Phase Structure and Elemental Composition

Figure 2. SEM images of PC-x: a) PC-0.5, b) PC-1, c) PC-2, and d) PC-3. e) TEM and f) HRTEM images of PC-1; micropores were indicated by red circles.g) N2 physisorption isotherms of PC-x and their pore size distribution plots by using h) DFT and i) BJH models.
XRD pattern of PC-0 (Figure 3a) reveals two weak diffraction peaks at 2θ = 20° and 43°, which are owing to graphitic planes (002) and(101), respectively.[47,48]Compared to PC-0, the intensities of(002) and (101) diffraction peaks in PC-1 decrease noticeably,indicating a lower graphitization degree of PC-1. As displayed in Figure 3b, the positions of D and G bands in Raman spectra of PC-1 and PC-0 are close, but PC-1 exhibits a larger ID/IGratio(2.73, Figure S4) than PC-0 (1.67), which means the presence of more defects and/or disorder carbon,[49,50]likely due to the highdensity nanopores of PC-1. XPS served as the method to investigate the surface elemental composition. The surface elements of PC-0 or PC-1 are consisted of oxygen and carbon (Figure S5). To characterize the chemical component and functional group, highresolution C 1s XPS spectra were curve-fitted into four individual peaks (Figure 3c and d): graphitic carbon (284.5–284.6 eV),C-OH (285.6–285.7 eV), C=O (286.9–287.1 eV), and COOH(288.9 eV).[46,51,52]On basis of XPS characterization, the relatively less oxygen-containing groups of PC-1 (e.g., C-OH and COOH) probably result from the catalytic effect of ZnO on removing part of functional groups during carbonization at 550 °C.
2.3. Light Absorbability, Solar-to-Heat Conversion, and Hydrophilicity
UV-Vis-NIR spectrometer was employed to characterize the optical absorption of PCW-1, PC-1, and wood. Wood exhibits a moderate absorbance of ca. 73–88% in the solar spectrum of 300–2500 nm(Figure 4a), while PC-1 exhibits a broadband and strong absorbance with an average value of ca. 90%. This is attributed to the abundant nanopores of PC-1 that serve as optical microcavities to enhance the light absorption by improving incident light path and multiple scattering. Notably, under the synergy of porous carbon and wood, PCW-1 presents an extremely high solar absorbance(ca.99%).Meanwhile,the thermal conductivity of PCW-1 is as low as 0.196 W m?1K?1,which can effectively reduce the conductive heat loss during solar evaporation.It is acknowledged that the combination of a broadband and strong optical absorption with a low thermal conductivity is advantageous to achieve high photothermal effect. Thereby, the photothermal performance of PCW-1 was investigated under 1 kW m?2irradiation.As can be seen, the surface temperature of dry PCW-1 grows up promptly from room temperature to ca.62.0 °C(Figure 4b–d),obviously higher than that of wood(ca.48.5 °C).A similar phenomenon is observed in wet PCW-1 and wood(Figure S6).
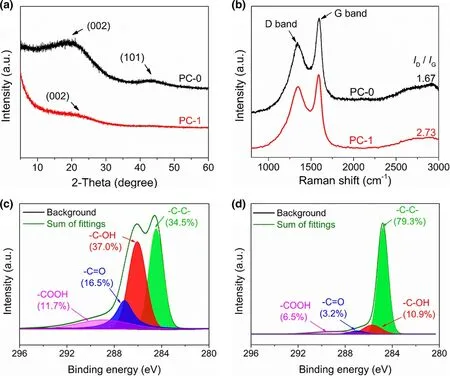
Figure 3. a) XRD curves and b) Raman spectra of PC-0 and PC-1. Highresolution C 1s XPS curves of c) PC-0 and d) PC-1.

Figure 4. a) UV-Vis-NIR absorption spectra of PCW-1, PC-1, and wood. b)Surface temperature of PCW-1, wood, and water. Infrared images of c)wood and d) PCW-1.
The hydrophilic property of evaporators was investigated by contact angle measurement. When a water droplet is placed on the surface of wood, the initial contact angle is 91° at 0.01 s, and the water droplet is totally absorbed by wood at 1.58 s (Figure 5a). Strikingly, the contact angle of PCW-1 is 26° at 0.01 s and quickly goes down to 0° at 0.12 s, which is probably attributed to the abundant nanopores and oxygen-containing groups in PC-1. The results above demonstrate the superhydrophilicity and super-wetting of PCW-1, which are ideal for water supply and vapor escaping.
2.4. Interfacial Solar Steam Generation
High optical absorption, good photothermal conversion, superhydrophilicity, super-wetting, and 3D interconnected nanostructures make PCW-1 a very promising choice for interfacial solar steam generation. The evaporation performance of PCW-1 was studied by employing a laboratory-made measurement system (Figure S7). The water mass change is approximately proportional to the irradiation time(Figure 6a). The evaporation rate of PCW-1 reaches up to 2.38 kg m?2h?1(Figure 6b, Video S1), which is 15.4, 3.8, and 1.6 times higher than that of water without evaporators in the dark(0.15 kg m?2h?1)or under 1 kW m?2irradiation(0.63 kg m?2h?1),and using wood under 1 kW m?2irradiation (1.45 kg m?2h?1),respectively. The enhancement factor of PCW-1 (3.78) is obviously higher than that of wood (2.30). Likewise, when waste PET-derived porous carbon (W-PC-1) is employed, the evaporation rate of W-PC-1/wood (i.e., W-PCW-1) bilayer solar evaporator is up to 2.31 kg m?2h?1(Figures S8 and S9). We additionally measured the evaporation rate of PCW-1 at different solar intensities (Figure S10),that is, 1.81 kg m?2h?1(0.5 kW m?2), 3.07 kg m?2h?1(1.5 kW m?2), 3.72 kg m?2h?1(2 kW m?2), 4.43 kg m?2h?1(2.5 kW m?2), and 4.45 kg m?2h?1(3 kW m?2). Overall, PCW-1 presents excellent evaporation performance and overperforms most of previous efficient evaporators (Figure 6c and Table S2), such as attapulgite/polyacrylamide(1.2 kg m?2h?1),[10]carbonfoam(1.26 kg m?2h?1),[25]wood aerogel (1.351 kg m?2h?1),[28]MXene/wood(1.465 kg m?2h?1),[31]MOF-based hierarchical structure (1.50 kg m?2h?1),[8]Ag/polypyrrole (1.55 kg m?2h?1),[14]N-dopedcarbon(1.62 kg m?2h?1),[53]porouscarbon(1.68 kg m?2h?1),[42]and sandwich absorber(1.87 kg m?2h?1).[30]
To measure the water evaporation enthalpy,a control experiment of water loss in the dark within 1 h was carried out (Figure 6d). The water mass change without absorbers is 109 mg, lower than that of wood(156 mg)or PCW-1(186 mg).Since the energy obtained from the environment is same under these conditions, the water equivalent enthalpy (Eequ) can be calculated according to the water loss and the known theoretical evaporation enthalpy value of liquid water(2434 J g?1)by using identical power input(Uin)as follows:

where E0and m0refer to the evaporation enthalpy and water mass change in the dark without evaporators, respectively; mgis the water mass change using wood or PCW-1. The water evaporation enthalpy using PCW-1 is calculated to be 1427 J g?1, which is significantly lower than that of wood (1701 J g?1) or without absorbers (2434 J g?1). Accordingly, the alliance of wood and PC-1 displays a synergistic effect on reducing the water evaporation enthalpy during solar steam generation.

Figure 5. Time evolution of the water contact angles of a) wood and b) PCW-1.

Figure 6. a) Time-dependent water mass change curves and b) evaporation rates without absorbers in the dark or under 1 kW m?2 irradiation (blank), or using wood and PCW-1 under 1 kW m?2 irradiation. c) Comparison of PCW-1 with previous evaporators (Table S2). d) Water mass loss in the dark and equivalent enthalpy without absorbers or by using wood or PCW-1.
In light of the analyses and results above,we put forward the possible mechanism on the interfacial solar steam generation using PCW-1(Figure 7). Firstly, wood block owns vertically aligned tubular structures with a diameter of ca. 35 μm (Figure S11), which provide 3D microchannels to realize continuous water supply. Secondly,the abundant hydrophilic hydroxyl groups from wood, which mainly consists of cellulose and lignin, form hydrogen bonds with water molecules to reduce the water evaporation enthalpy by destroying part of hydrogen bonds network of bulk water (Figure 7b). A similar phenomenon is observed in the corn stalk evaporator,[54]in which a part of water molecules form hydrogen bonds with hydrophilic groups of stalk to weaken the van der Waals force between water molecules, leading to the reduction of the water evaporation enthalpy by ca.60%.Thirdly,as a high-efficiency photothermal material,PC-1 effectively heats the interfacial region of water and air, which reduces the heat loss (ca. 11.6%,Note S1 in supporting information);meanwhile,the plentiful and multiscale interconnected micro-/meso-/macropores inside PC-1 ensure sufficient water supply for solar vapor generation (Figure 7c). Lastly,the abundant oxygen-containing functional groups of PC-1 form numerous hydrogen bonds with water molecules,which are beneficial to form water clusters in the nanopores of PC-1,due to the space confinement effect. Compared to bulk water, the number of hydrogen bonds among water clusters in the nanopores is supposed to be reduced.[54]This is the reason for the lower energy demand of vapor release from PCW-1 to elevate the evaporation rate.
2.5. Effect of Pore Structures in PC-x on Solar Steam Generation
To elucidate the influence of pore structures in porous carbon on solar steam generation performance,PC-x with different SBETobtained from catalytic carbonization of PET by ZnO was coated on the top surface of wood to fabricate PCW-x. The water mass change is almost linear to the irradiation time(Figure 8a).The evaporation rates of PCW-0,0.5, 1, 2, and 3 are measured as 1.60, 1.82,2.38, 2.24, and 1.98 kg m?2h?1, respectively, higher than that of wood(1.45 kg m?2h?1,Figure 8b).Consequently,the pore structures of PC-x indeed affect the solar steam generation performance. To better interpret the function of porous structures,the nanopores of PC-x are divided into micropores and meso-/macropores. As displayed in Figure 8c, PC-0 contains a great deal of micropores (Smicro= 568.1 m2g?1) and a small number of mesopores and macropores(Smeso+macro= 82.2 m2g?1).By contrast,PCx (x = 0.5, 1, 2, and 3) possess a slightly smaller number of micropores but greatly more mesopores and macropores, especially PC-1 and PC-2,of which Smicroand Smeso+macroare 492.5 and 671.2 m2g?1(PC-1), and 444.6 and 683.4 m2g?1(PC-2), respectively(Table S1). Subsequently, we performed linear fittings of the evaporation rate with SBET,Smeso+macro,and Smicroto reveal the correlation between porous structures and evaporation performance. Linear correlation coefficients (R2) between the evaporation rate and SBETand Smeso+macroare 0.9005 and 0.8471, respectively (Figure 8d and e),proving their good linear fittings.Therefore,it could be concluded that the meso-/macropore structures of porous carbon play the significant roles in solar steam generation. It is likely because mesopores and macropores serve as buffer channels for a large number of water molecules transported from the wood cavities and provide the water transportation highways as well(Figure 8h and i).
By comparison,the linear fitting of Smicroand evaporation rate shows a quite low degree of correlation(Figure 8f),implying that there is no direct linear relationship of Smicrowith the evaporation rate. Nonetheless, it is worthy to be noted, considering the size of water molecule(ca.0.33 nm),micropores(0.3–2 nm)of PC-x facilitate the formation of water clusters (Figure 8g), which is important to lower down the water evaporation enthalpy.A similar phenomenon is observed in previous studies.[55,56]Dong et al.proved that the formation of water clusters in UiO-66-COOH nanochannels drastically reduced the water evaporation enthalpy by ca. 45%.[55]In this scenario, with the combined merits of meso-/macropores and micropores, the coated-porous carbon layer along with the wood matrix not only accelerates the water transportation and vapor escaping, but also reduces the water evaporation enthalpy to maximize the evaporation rate.
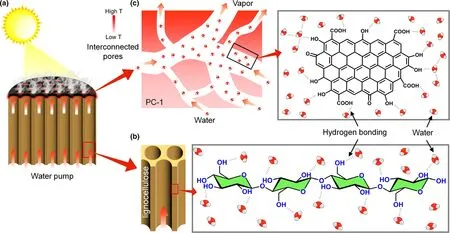
Figure 7. a) Scheme of the mechanism on the interfacial solar steam generation by using PCW-1. Schemes of reducing water evaporation enthalpy using b) wood and c) PC-1.
2.6. Solar Steam Generation Using other Water Sources
The interfacial solar steam generation of PCW-1 was also investigated by using dye-polluted water and seawater. Firstly, methylene blue or rhodamine B with a concentration of 20 mg L?1was used as a model dye contaminant to investigate the evaporation of dye-polluted water.The strong absorption peaks around 664 and 554 nm are the characteristic absorption peaks of methylene blue and rhodamine B,respectively.The disappearance of absorption peaks in condensed water demonstrates a high dye removal efficiency (>99.9%, Figure 9a). Moreover,the dye removal efficiency is not affected by the concentration of methylene blue (Figure S12). Secondly, compared to seawater, the Na+, Mg2+, K+, and Ca2+concentrations in freshwater are reduced by nearly two or three orders of magnitude(Figure 9b).Noteworthy,the freshwater fully meets the potable water standards stipulated by the World Health Organization(WHO)and U.S.Environmental Protection Agency(EPA).Thirdly,the long-term desalination of PCW-1 was tested continuously using seawater for 10 cycles (60 min for one cycle, Figure 9c). The evaporation rate of PCW-1 fluctuates between 2.1 and 2.4 kg m?2h?1. No accumulated salts are observed on the surface of PCW-1 (Figure 9d). The good self-desalting capability appears to arise from two aspects.On one hand,the micron-sized cavities of wood and nanopores of PC-1 guarantee the quick diffusion of water through microchannels and nanochannels toward the evaporator surface to replenish the vaporized salt water. On the other hand, the salt concentration gradient,which is formed due to the diverse hydraulic conductivity, drives the dissolution of salt in the microchannels back to the bulk water, realizing spontaneous inter-channel salt interchange. To sum up,the timely self-renewal of PCW-1 ensures the long-term durability and excellent reusability.
2.7. Outdoor Solar Desalination
To assess the evaporation performance of PCW-1 for practical applications, a large-scale solar desalination device was constructed, which is mainly composed of evaporation chamber, PCW-1 (diameter =16.5 cm),vapor condenser,and water collector troughs(Figure 10a–d and Figure S13).The transparent poly(methyl methacrylate)cover condenses the evaporated water and subsequently converges freshwater to bottom outlet pipe. The desalination device was placed on the roof of East Chemistry Building at Huazhong University of Science and Technology (October, 2020). During solar evaporation, a layer of water mist appears on the cover after 10 min,and numerous water beads are formed after 40 min (Figure 10e–g). The solar flux goes up from 0.10 kW m?2at 8:00 to 0.469 kW m?2at 12:00, remains almost unchanged (0.43–0.47 kW m?2) during 12:00–15:00, and finally decreases to 0.04 kW m?2at 18:00 (Figure 10h). The outdoor temperature is approximately 16–25 °C with the highest value achieved at 13:00–16:00. Besides, the freshwater production rate raises from 0.05 kg m?2
h?1at 8:00 to 0.79 kg m?2h?1at 14:00, and subsequently decreases to 0.18 kg m?2h?1at 18:00 (Figure 10i). During this daytime(8:00–18:00), the mass of accumulated freshwater produced by the device goes up rapidly and reaches 3.65 kg m?2(Figure 10f). The freshwater produced from a 1 m2device is enough for the one person’s daily water consumption(2.5 L). Importantly,the cost of PCW-1 is as low as ca. ¥ 7.7 (Table S3). Overall, the outdoor results above demonstrate that the strong water purification ability and good scalable capability of PCW-1 under natural light condition for practical solar evaporation and desalination applications.Likewise,the performance of W-PCW-1 is tested for outdoor solar desalination.The maximum freshwater production rate and the accumulated freshwater production are measured to be 0.70 kg m?2h?1and 3.71 kg m?2, respectively (Figure S14).

Figure 8. a) Time-dependent mass change curves and b) evaporation rates of wood and PCW-x (x = 0, 0.5, 1, 2 and 3) under 1 kW m?2 irradiation.c) Summary of Smicro and Smeso+macro of PC-x. Linear fittings of the evaporation rate with d) SBET, e) Smeso+macro, and f) Smicro. g–i) Schemes of water transportation in nanochannels of different pores.
3. Conclusion
In summary, we have fabricated low-cost, high-performance woodbased bilayer solar evaporators by dip coating the surface of wood block with porous carbon from the controlled carbonization of PET.ZnO acts as both physical template and catalyst to convert PET into porous carbon with high specific surface areas (1164 m2g?1), wellcontrolled micro-/meso-/macropores, abundant oxygen-containing functional groups, and low thermal conduction property. The resultant porous carbon not only improves light absorption via increasing incident light path and multiple scattering to realize broadband and strong optical absorption and high photothermal effect, but also promotes the formation of water clusters in nanochannels, leading to the reduction of the water evaporation enthalpy by ca. 41%. Consequently, the porous carbon-coated wood bilayer solar evaporator shows high evaporation rate (2.38 kg m?2h?1), excellent long-term stability, and good salt-resistance behavior, which surpasses the stateof-the-art carbon-based photothermal materials. More importantly, a large-scale solar desalination device based on porous carbon-coated wood bilayer solar evaporator is designed for outdoor experiments.The daily drinkable freshwater production amount is up to 3.65 kg m?2. This study provides a new strategy to build up scalable,cost-efficient interfacial solar vapor generation systems, which makes a significant step toward addressing the global freshwater shortage.Since slightly reduced carbonization temperature is favorable to preserve a large number of oxygen-containing groups, further studies will be conducted on the low-temperature carbonization of polyesters(e.g., at 300 °C) to control the structure and surface chemical components of porous carbon.

Figure 9. a) UV-Vis spectra of dye-polluted water and condensed water after evaporation; insets show the corresponding photographs. b) Concentrations of several metallic ions in seawater and freshwater.c) Long-term stability and d) salt excretion property of PCW-1 during seawater desalination for 600 min.
4. Experimental Section
Materials and chemicals: ZnO, Rhodamine B, hydrochloride acid, chitosan,acetic acid, and methylene blue were provided by Sinopharm Chemical Reagent Company (China). PET was supplied by Shandong Usolf Company (China). PET bottle was provided by a recycling store (Wuhan) and clipped into small sheets.Pine block was supplied by Leshuo Home Decoration Materials Company(China).Seawater was obtained from Bohai Sea(Figure S15).
Preparation of PC-x: PET was blended with ZnO powder at the ZnO/PET mass ratio(x)of 0.5,1,2,or 3(Figure 1).The resultant mixture was added to an alumina crucible, pre-heated at 300 °C for 10 min with a heating rate of 10 °C min?1, and carbonized at 550 °C for 8 min. After cooling, the obtained product was purified by HCl (0.5 mol L?1) solution and then water, dried and named as PC-x (Note: PC and x represent porous carbon and ZnO/PET mass ratio,respectively).For comparison,PC-0 was obtained by carbonizing PET in the absence of ZnO. Likewise, waste PET was carbonized at the ZnO/PET mass ratio of 1 to prepare porous carbon (marked as W-PC-1,Figure S16). It is worth noting that ZnO is easily reclaimed for the next carbonization experiment (Figures S17 and S18).
Preparation of PCW-x: Firstly, 0.4 g chitosan was dissolved in acetic acid (60 mL, 11.6 mol L?1) at 60 °C. An optimized dosage of PC-x powder (40 mg,according to our primary results as shown in Figure S19) was then put into 0.5 mL chitosan solution and stirred for 10 min.The mixture was evenly coated on the top surface of wood block (diameter =30 mm, thickness = 10 mm) and naturally dried at room temperature to obtain PCW-x(Figure S20).Similarly, W-PC-1 and PC-0 were coated on the top surface of wood to prepare W-PCW-1 and PCW-0,respectively.
Characterization: N2physisorption isotherms at 77 K were operated on Micromeritics ASAP 2460.Pore size distribution was characterized by the quench solid density functional theory (DFT) model or Barret–Joyner–Halenda(BJH) model. Specific surface area(SBET) was calculated by using Brunauer–Emmett–-Teller(BET)equation.Field-emission scanning electron microscope (SEM) was conducted on SU8010. Transmission electron microscope (TEM) and highresolution TEM (HRTEM) were performed on Tecnai G2 F30 microscope. Crystalline structure was investigated by X-ray diffraction (XRD, SmartLab-SE) and a confocal Raman microscope(in Via Reflex).X-ray photoelectron spectroscopy (XPS) was conducted on a VG ESCALAB MK II spectrometer. UV-Vis-NIR spectrophotometer was performed on PerkinElmer LAMBDA 750S. Functional groups were recorded by FT-IR spectroscopy (BRUKER Vertex 80 FT-IR). Thermal conductivity was studied by hot disk method(Hot Disk TPS 2500s). Inductively coupled plasma optical emission spectrometer (ICP-OES) was performed on Agilent 720ES. Contact angle was characterized by a contact angle measuring instrument(Dataphysics OCA15EC).
Interfacial solar steam evaporation: Solar steam evaporation experiments were performed by using a xenon light (CEL-S500L, CEAULIGHT, Figure S7).PCW-x was placed onto the surface of water in a beaker. The water mass reduction was real-time monitored by an electronic balance (JA2003, Soptop).Subsequently, we calculated the evaporation rate(m, kg m?2h-1), conversion efficiency (η, %), and enhancement factor(E.F.)by using Equation(2–4):

where Δm represents water mass reduction in 1 h(kg),S represents evaporator area(m2),m’stands for evaporation rate under irradiation after subtracting dark evaporation rate(kg m?2h?1), t stands for irradiation time (i.e., 1 h), hLvrepresents the latent heat of water vaporization(J g?1),and Pinrepresents solar power(kW m?2).
Acknowledgements
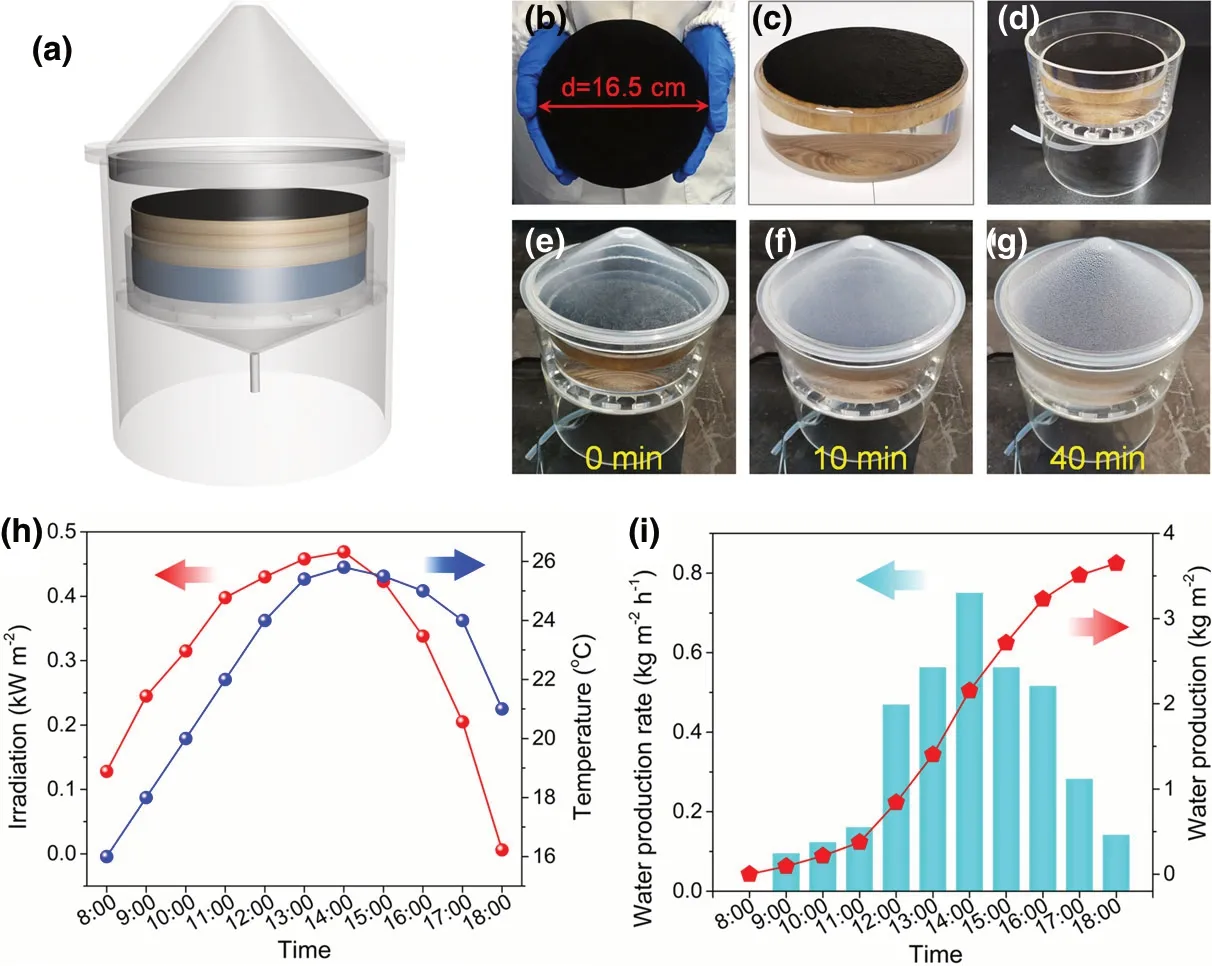
Figure 10. a) Scheme of a large-scale solar desalination device for outdoor experiments.Photographs of b) the large-scale PCW-1 and c) its floating on seawater or d) placing in chamber. e?g) Photographs of evaporation chamber for freshwater generation. h) Solar power density and outdoor temperature curves. i) Freshwater production rate and accumulated freshwater production under natural irradiation.
We would like to thank the reviewers for their kind and valuable comments.This research is financially supported by the National Natural Science Foundation of China (No. 51903099 and 51991353), Huazhong University of Science and Technology (No. 3004013134), the 100 Talents Program of the Hubei Provincial Government, and the Innovation and Talent Recruitment Base of New Energy Chemistry and Device(No.B21003).We are grateful to the Analytical and Testing Centre of HUST,and the Analytical and Testing Centre of the School of Chemistry and Chemical Engineering (HUST) for access to their facilities. We would like to thank Prof.Qiang Zhao for access to his facilities.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
Supporting Informationis available from the Wiley Online Library or from the author.
Keywords
solar steam generation, waste polyester, hierarchically porous carbon,desalination, freshwater production
Received: February 10, 2021
Revised: March 23, 2021
Published online: April 5, 2021
[1] X. Zhou, Y. Guo, F. Zhao, G. Yu, Acc. Chem. Res. 2019, 52, 3244.
[2] T. Ding, Y. Zhou, W. L. Ong, G. W. Ho, Mater. Today 2021, 42, 178.
[3] M. Gao, L. Zhu, C. K. Peh, G. W. Ho, Energy Environ. Sci. 2019, 12, 841.
[4] P. Tao, G. Ni, C. Song, W. Shang, J. Wu, J. Zhu, G. Chen, T. Deng, Nat.Energy 2018, 3, 1031.
[5] W. Wang, Y. Shi, C. Zhang, S. Hong, L. Shi, J. Chang,R. Li, Y. Jin, C. Ong, S. Zhuo, P. Wang, Nat. Commun.2019, 10, 3012.
[6] M.-Q. Yang, C. F. Tan, W. Lu, K. Zeng, G. W. Ho, Adv.Funct. Mater. 2020, 30, 2004460.
[7] H. Liang, Q. Liao, N. Chen, Y. Liang, G. Lv, P. Zhang, B.Lu, L. Qu, Angew. Chem. Int. Ed. 2019, 58, 19041.
[8] Q. Ma, P. Yin, M. Zhao, Z. Luo, Y. Huang, Q. He, Y.Yu, Z. Liu, Z. Hu, B. Chen, H. Zhang, Adv. Mater.2019, 31, 1808249.
[9] F. Zhao, X. Zhou, Y. Shi, X. Qian, M. Alexander, X.Zhao, S. Mendez, R. Yang, L. Qu, G. Yu, Nat. Nanotechnol. 2018, 13, 489.
[10] J. Jia, W. Liang, H. Sun, Z. Zhu, C. Wang, A. Li, Chem.Eng. J. 2019, 361, 999.
[11] L. Zhou, Y. Tan, D. Ji, B. Zhu, P. Zhang, J. Xu, Q. Gan,Z. Yu, J. Zhu, Sci. Adv. 2016, 2, e1501227.
[12] X. Chen, C. Meng, Y. Wang, Q. Zhao, Y. Li, X.-M.Chen, D. Yang, Y. Li, Y. Zhou, ACS Sustainable Chem.Eng. 2020, 8, 1095.
[13] Y. Xu, J. Wang, F. Yu, Z. Guo, H. Cheng, J. Yin, L. Yan,X. Wang, ACS Sustainable Chem. Eng. 2020, 8, 12053.
[14] Y. Xu, J. Ma, Y. Han, H. Xu, Y. Wang, D. Qi, W. Wang,Chem. Eng. J. 2020, 384, 123379.
[15] S. Cao, P. Rathi, X. Wu, D. Ghim, Y.-S. Jun, S. Singamaneni, Adv. Mater. 2021, https://doi.org/10.1002/adma.202000922
[16] F. Jiang, T. Li, Y. Li, Y. Zhang, A. Gong, J. Dai, E. Hitz,W. Luo, L. Hu, Adv. Mater. 2018, 30, 1703453.
[17] S. He, C. Chen, Y. Kuang, R. Mi, Y. Liu, Y. Pei, W.Kong, W. Gan, H. Xie, E. Hitz, C. Jia, X. Chen, A. Gong,J. Liao, J. Li, Z. J. Ren, B. Yang, S. Das, L. Hu, Energy Environ. Sci. 2019,12, 1558.
[18] M. Zhu, Y. Li, G. Chen, F. Jiang, Z. Yang, X. Luo, Y. Wang, S. D. Lacey, J.Dai, C. Wang, C. Jia, J. Wan, Y. Yao, A. Gong, B. Yang, Z. Yu, S. Das, L.Hu, Adv. Mater. 2017, 29, 1704107.
[19] H. Liu, C. Chen, G. Chen, Y. Kuang, X. Zhao, J. Song, C. Jia, X. Xu, E.Hitz, H. Xie, S. Wang, F. Jiang, T. Li, Y. Li, A. Gong, R. Yang, S. Das, L.Hu, Adv. Energy Mater. 2018, 8, 1701616.
[20] H.Jang,J.Choi,H.Lee,S.Jeon,A.C.S.Appl,Mater.Interfaces 2020,12,30320.
[21] G. Xue, K. Liu, Q. Chen, P. Yang, J. Li, T. Ding, J. Duan, B. Qi, J. Zhou, A.C. S. Appl, Mater. Interfaces 2017, 9, 15052.
[22] Z. Chen, B. Dang, X. Luo, W. Li, J. Li, H. Yu, S. Liu, S. Li, A. C. S. Appl,Mater. Interfaces 2019, 11, 26032.
[23] T. Chen, Z. Wu, Z. Liu, J. T. Aladejana, X. A. Wang, M. Niu, Q. Wei, Y.Xie, A. C. S. Appl, Mater. Interfaces 2020, 12, 19511.
[24] Y. Ito, Y. Tanabe, J. Han, T. Fujita, K. Tanigaki, M. Chen, Adv. Mater.2015, 27, 4302.
[25] P. Qiu, F. Liu, C. Xu, H. Chen, F. Jiang, Y. Li, Z. Guo, J. Mater. Chem. A 2019, 7, 13036.
[26] D. Guo, X. Yang, Sci. China Mater. 2019, 62, 711.
[27] T. Li, H. Liu, X. Zhao, G. Chen, J. Dai, G. Pastel, C. Jia, C. Chen, E. Hitz,D. Siddhartha, R. Yang, L. Hu, Adv. Funct. Mater. 2018, 28, 1707134.
[28] W. Chao, X. Sun, Y. Li, G. Cao, R. Wang, C. Wang, S.-H. Ho, A. C. S.Appl, Mater. Interfaces 2020, 12, 22387.
[29] K. Kim, S. Yu, C. An, S.-W. Kim, J.-H. Jang, A. C. S. Appl, Mater. Interfaces 2018, 10, 15602.
[30] C. Tian, J. Liu, R. Ruan, X. Tian, X. Lai, L. Xing, Y. Su, W. Huang, Y. Cao,J. Tu, Small 2020, 16, 2000573.
[31] N. Ma, Q. Fu, Y. Hong, X. Hao, X. Wang, J. Ju, J. Sun, A. C. S. Appl,Mater. Interfaces 2020, 12, 18165.
[32] M. Zhu, Y. Li, F. Chen, X. Zhu, J. Dai, Y. Li, Z. Yang, X. Yan, J. Song, Y.Wang, E. Hitz, W. Luo, M. Lu, B. Yang, L. Hu, Adv. Energy Mater. 2018,8, 1701028.
[33] Z. Wang, Y. Yan, X. Shen, C. Jin, Q. Sun, H. Li, J. Mater. Chem. A 2019,7, 20706.
[34] L. Zhang, B. Tang, J. Wu, R. Li, P. Wang, Adv. Mater. 2015, 27, 4889.
[35] X. Jie, W. Li, D. Slocombe, Y. Gao, I. Banerjee, S. Gonzalez-Cortes, B.Yao, H. AlMegren, S. Alshihri, J. Dilworth, J. Thomas, T. Xiao, P.Edwards, Nat. Catal. 2020, 3, 902.
[36] T. Tang, X. Chen, X. Meng, H. Chen, Y. Ding, Angew. Chem. Int. Ed.2005, 44, 1517.
[37] J. Gong, J. Liu, Z. Jiang, X. Wen, X. Chen, E. Mijowska, Y. Wang, T. Tang,Chem. Eng. J. 2013, 225, 798.
[38] J. Gong, J. Feng, J. Liu, Z. Jiang, X. Chen, E. Mijowska, X. Wen, T. Tang,Chem. Eng. J. 2014, 248, 27.
[39] J. Gong, J. Liu, Z. Jiang, X. Chen, X. Wen, E. Mijowska, T. Tang, Appl.Catal. B: Environ. 2014, 152–153, 289.
[40] J. Gong, J. Liu, X. Chen, Z. Jiang, X. Wen, E. Mijowska, T. Tang, J. Mater.Chem. A 2015, 3, 341.
[41] J. Gong, X. Chen, T. Tang, Prog. Polym. Sci. 2019, 94, 1.
[42] B. Zhang, C. Song, C. Liu, J. Min, J. Azadmanjiri, Y. Ni, R. Niu, J. Gong,Q. Zhao, T. Tang, J. Mater. Chem. A 2019, 7, 22912.
[43] C. Song, L. Hao, B. Zhang, Z. Dong, Q. Tang, J. Min, Q. Zhao, R. Niu, J.Gong, T. Tang, Sci. China Mater. 2020, 63, 779.
[44] N. Liu, L. Hao, B. Zhang, R. Niu, J. Gong, T. Tang, Sustain. Energy Fuels 2020, 4, 5522.
[45] L. Hao, N. Liu, B. Zhang, R. Niu, J. Gong, T. Tang, J. Taiwan Inst. Chem.Eng. 2020, 115, 71.
[46] C. Shu, Z. Gan, Y. Hou, T. Zhu, J. Ma, W. Tang, Y. Wu, Energy Environ.Mater. 2021, 4, 81.
[47] Y. Feng, S. Chen, D. Shen, J. Zhou, B. Lu, Energy Environ. Mater. 2021,https://doi.org/10.1002/eem2.12126
[48] J. Xu, J. Zhu, X. Yang, S. Cao, J. Yu, M. Shalom, M. Antonietti, Adv.Mater. 2016, 28, 6727.
[49] L. Liu, T. Ma, W. Fang, Y. Liu, K. Konstantinov, J. Wang, H.-K. Liu, Energy Environ. Mater. 2021, https://doi.org/10.1002/eem2.12177
[50] Q. Liu, J. Shen, X. Yu, X. Yang, W. Liu, J. Yang, H. Tang, H. Xu, H. Li, Y.Li, J. Xu, Appl. Catal. B: Environ. 2019, 248, 84.
[51] X. Wu, T. Gao, C. Han, J. Xu, G. Owens, H. Xu, Sci. Bull. 2019, 64, 1625.
[52] P. Meng, A. Brock, Y. Xu, C. Han, S. Chen, C. Yan, J. McMurtrie, J. Xu, J.Am. Chem. Soc. 2020, 142, 479.
[53] C. Song, B. Zhang, L. Hao, J. Min, N. Liu, R. Niu, J. Gong, T. Tang, Green.Energy Environ. 2021, https://doi.org/10.1016/j.gee.2020.10.002
[54] Z. Sun, W. Li, W. Song, L. Zhang, Z. Wang, J. Mater. Chem. A 2020, 8,349.
[55] Z. Dong, C. Zhang, H. Peng, J. Gong, Q. Zhao, J. Mater. Chem. A 2020,8, 24493.
[56] J. Tang, T. Zheng, Z. Song, Y. Shao, N. Li, K. Jia, Y. Tian, Q. Song, H. Liu,G. Xue, A. C. S. Appl, Mater. Interfaces 2020, 12, 18504.
 Energy & Environmental Materials2022年2期
Energy & Environmental Materials2022年2期
- Energy & Environmental Materials的其它文章
- Editor’s Note on “Frontier Outlook” Column
- Graphite Anode for Potassium Ion Batteries: Current Status and Perspective
- Green Synthesis of Nitrogen-to-Ammonia Fixation: Past,Present, and Future
- Photo-assisted Rechargeable Metal Batteries for Energy Conversion and Storage
- 3D Printing of Next-generation Electrochemical Energy Storage Devices: from Multiscale to Multimaterial
- The Transference Number
