Effects of ultra-high-pressure treatment on the structural and functional properties of buckwheat 13S globulin
Yiming Zhou, Boya Ouyang, Lina Du, Yun Wei, Xiaoli Zhou,*, Ying Xiao, Yifen Wang
a State Key Laboratory of Dairy Biotechnology, Shanghai Engineering Research Center of Dairy Biotechnology, Dairy Research Institute,Bright Dairy & Food Co. Ltd., Shanghai 200436, China
b School of Perfume and Aroma Technology, Shanghai Institute of Technology, Shanghai 201418, China.
c Department of Biosystems Engineering, Auburn University, Alabama 36879-5417, USA
Keywords:
Ultra-high-pressure
Buckwheat 13S globulin
Structure
Functional characteristics
A B S T R A C T
Components with strong adsorption capacity for cholates from buckwheat proteins were screened, separated and purified by several methods, and the effects of ultra-high-pressure (UHP) on the structure and function of buckwheat 13S globulin (BW13SG) were studied. Samples were treated by UHP at different pH (3.0 and 7.0) value(s) and at 100-500 MPa for 10-30 min. The results showed that the tertiary structure of BW13SG was partially denatured and aggregated. The decrease in the unordered structure indicated that UHP resulted in a looser secondary structure of BW13SG. UHP treatment also increased solubility, emulsion activity and stability, foaming capacity and stability. The samples treated at 500 MPa, pH 3.0 for 30 min had the most enhanced functionality. Moreover, under this condition, the sodium cholate and sodium deoxycholate adsorption capacities of BW13SG were both higher than 98% and the adsorption capacity of sodium taurocholate, which can be difficult to adsorb, was higher than 60%.
1. Introduction
Buckwheat (Fagopyrum esculentumandFagopyrum tataricum)is a dicotyledonous crop of the Polygonaceae family. Buckwheat(F. tataricum) is a minor crop and specially grown in China [1].B uckwheat has high protein content and amino acid composition is reasonable, among which water-soluble albumin and salt-soluble globulin account for more than 50% of the total protein [2,3], with the most representative being 280 kDa of 13S globulin [4]. Buckwheat Protein (BWP) has recently become a major research focus due to its high nutritional content and disease-preventing effects.
H owever, common BWP does not contain gluten, which limits its application as a gelling agent, emulsifier, or emulsion stabilizer [5,6].It has been reported that protein modification can change its properties to meet the needs of food processing [7]. Therefore, in order to overcome the shortcomings of common BWP, physical modification of BWP, through ultra-high-pressure (UHP) has been widely used [8].UHP treatment has been reported to lead to structural changes in protein molecules due to different extents of unfolding and denaturation, which causes modification of their functional properties [9].
UHP treatment is a non-heat treatment technology that sterilizes and modifies foods in vessels at room temperature and 100-1 000 MPa.In recent years, effects of UHP on the physicochemical properties of more than 25 proteins found in wheat, corn, rice, potato, pea and broad bean have been evaluated [10-12]. Compared with untreated proteins, proteins treated with hydrostatic pressure had higher hydrophobicity [13]. After UHP treatment (400-700 MPa,20 min), the immune response activity of egg protein decreased with increasing pressure, as well as the protein structure and functional properties (including foaming and emulsifying properties) [14]. This change was proportional to the magnitude of the pressure [15]. I t has been reported in the literature that UHP treatment is widely used in foreign food processing, as its cold sterilization technology induces less harm to a product in terms of the food nutrition and sensory quality relative to heat treatment [16].
The objective of this study, therefore, was to investigate physicochemical, functional, and structural properties of buckwheat 13S globulin (BW13SG) modified by UHP.
2. Materials and methods
2.1 Materials
Buckwheat flour was purchased from Yanmen Qinggao Food Co., Ltd. (Shanxi, China). BWP (65.4% protein) was made in our laboratory, 8-anilino-1-napthalenesulfonic acid ammonium salt (ANSA, ≥ 97%),β-mercaptoethanol, sodium phosphate monobasic dihydrate (≥ 99%), sodium phosphate dibasic (≥ 99%), diaminoethane tetraacetic acid (EDTA),tris(hydroxymethyl) aminomethane (Tris), potassium chloride(KCl), 2,4-dinitrophenylhydrazine (DNPH), trichloroacetic acid (TCA), sodium dodecyl sulfate (SDS) and guanidine hydrochloride were obtained from Sinopharm Chemical Reagent(Shanghai, China).
2.2 Preparation of BW13SG
2.2.1 Preparation of BWP
Buckwheat flour was dissolved in distilled water at a ratio of 1 : 10, adjusted to pH 8.0, with 1 mol/L NaOH. The slurry was stirred with a magnetic stirrer at 4 °C and 50 ×gfor 2 h, and then centrifuged at 5 000 ×gfor 10 min. The supernatant liquid was collected, adjusted to pH 4.5, with 1 mol/L HCL solution, and then centrifuged again at 5 000 ×gfor 10 min. The resulting deposit was collected and then lyophilized.
2.2.2 DEAE-Sepharose Fast Flow
A specific amount of DEAE-Sepharose Fast Flow medium was taken and loaded in a 1.6 cm × 50 cm chromatographic column, with a filling height of about 45 cm. The buffer was equilibrated to a fixed amount of buckwheat dry samples of water-soluble protein, which were soluble in a fixed volume of pH 6.5 phosphate buffer. These were then centrifuged at 5 000 ×gfor 10 min, and the supernatant was loaded on the ion exchange column. The column was washed with 10 volumes of pH 6.5 of phosphate buffer, 0.2 mol/L and 0.5 mol/L NaCl-phosphate were used as an eluent, and the flow rate was 1.0 mL/min. The column was run at room temperature, and a collection tube was filled every 10 min, using a UV detector at 280 nm to detect protein. The main peaks were collected, dialyzed overnight and freeze-dried for testing.
2.2.3 Adsorption of cholate by purified BWP
2.2.3.1 Cholate standard curve
A series of cholate standards, containing 0, 20, 40, 60, 80, 100,200 or 400 mg cholate, respectively, were dissolved in distilled water in a 50 mL volumetric flask and added to a scale. 1 mL of sample solution or standard solution was transferred into a tube with a stopper, and 6 mL of 45% sulfuric acid was added, followed by mixing. Next, 1 mL of 0.3% (V/V) furfural was added, the solution was mixed, and then placed in a constant temperature water bath at 65 °C for 30 min, followed by cooling to room temperature. The absorbance was measured at 620 nm and then compared to a standard curve [17].
2.2.3.2 Determination of cholate adsorption
Aqueous solutions of 4 mg/mL sodium cholate, sodium deoxycholate and sodium sulfonic cholate were prepared, respectively.Purified materials from BWP were then added and each was incubated at 37 °C for 1 h and then centrifuged at 5 000 ×gfor 10 min. 1 mL of supernatant was removed, the absorbance was measured at 620 nm and the cholic acid concentration was determined based on the cholate standard curve. According to the concentration difference of cholate in the solution before and after the reaction, the adsorption capacity of purified BWP for cholate was calculated.
2.2.4 Sephadex G-75 gel filtration chromatography
Sephadex G-75 gel was loaded into a 1.6 cm × 50 cm column with a packing height of 45 cm. The column was rinsed with phosphate buffer until it was equilibrated. A fixed amount of each freeze-dried sample was taken, dissolved in a given volume of pH 6.5 phosphate buffer and centrifuged at 5 000 ×gfor 10 min. The supernatant from this was then added to the gel column, eluted with phosphate buffer at a rate of 0.3 mL/min, with a tube being collected every 20 min, and the main separation peak was detected by a UV-vis spectrophotometer at 280 nm. Samples were dialyzed overnight and then freeze-dried for testing.
2.2.5 SDS-polyacrylamide gel electrophoresis (SDS-PAGE)
SDS-PAGE is used to determine the molecular weight of purified buckwheat protein. The specific configuration method is shown in Table 1. After the electrophoresis, the gel was taken out, placed in Coomassie Brilliant Blue R-250 staining solution,and gently shaken at about 40 °C for about 1 h. Rinse the gel with distilled water, put it in Coomassie Brilliant Blue Decolorizing Solution, and shake it gently at 40 °C until the blue background on the gel is removed [18].
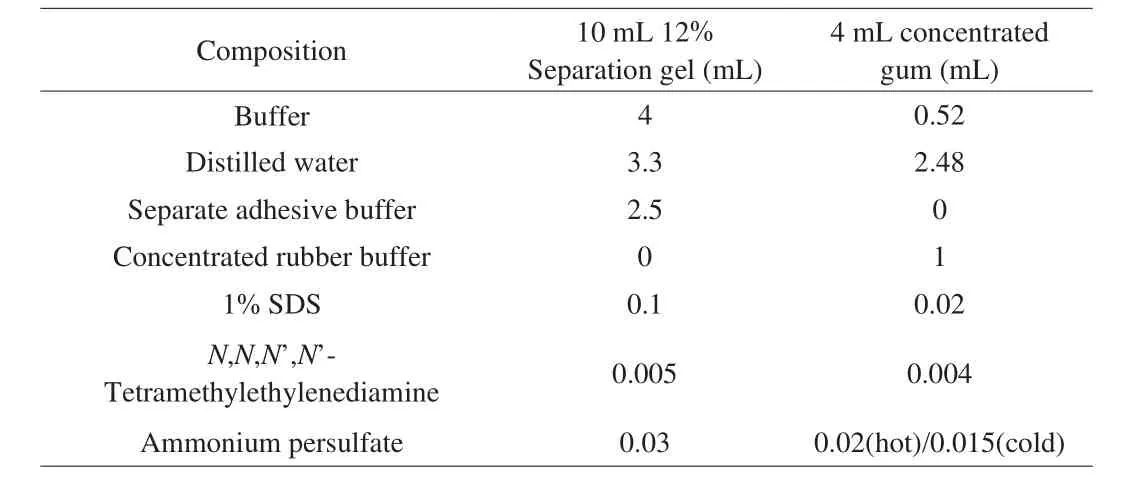
Table 1SDS-PAGE solution preparation.
2.3 UHP treatment
BW13SG solutions (1%m/V) were prepared by dissolving BW13SG powders in 0.05 mol/L Gly-HCl buffer (pH 3.0) or 0.05 mol/L Tris-HCl buffer (pH 7.0) at 25 °C overnight under constant stirring of 120 r/min. As most food products have pH values ranging from acidic to neutral, two representative pH, namely 3.0 and 7.0 were chosen to study the effects of pH on the functional and structural properties of BW13SG. Prior to pressure processing, equivalent volume of BW13SG solutions were vacuum-packed in polyethylene bags. In the laboratory high pressure vessel (600 MPa-5 L UHP, Baotou, China), the samples were treated under ultra-high pressure with water as the transmission medium, the temperature in the high pressure chamber was (30 ± 1) °C, the pressure range was (100 ± 10)–(500 ± 10) MPa, 25 °C, treatment for 10–30 min.Untreated samples (0.1 MPa) were used as controls. Afterward, all samples were freeze-dried for 24 h, and the obtained powders were collected for analysis.
2.4 Determination of structure changes in BW13SG
The structure changes of BW13SG can be re flected from physical and chemical characteristics, such as free sulfhydryl content, surface hydrophobicity, fluorescence spectra, Fourier transform infrared spectra [19,20].
2.4.1 Free sulfhydryl (SHF) content
SHFwas detected using Ellman’s reagent as reported with some modifications. Brie fly, 0.015 g of protein powder was solubilized in 5 mL of Tris-Glycine buffer (pH 8.0). The mixture was incubated at 25 °C for 1 h, and mixed with 0.05 mL of Ellman’s reagent solution(4 mg/mL DTNB solution prepared with buffer) immediately. The solution was left to stand at 20 °C for 15 min and then centrifuged at 10 000 ×gfor 15 min at 4 °C. The absorbance of the supernatant was detected at 412 nm (A412nm) using a UV-vis spectrophotometer.The buffer was used instead of a protein solution as a reagent blank.A protein blank was used in which 0.05 mL of the buffer was used in place of the Ellman’s reagent solution. The SHFcontent was calculated as follows.

where SHFmeans the free sulfhydryl content, 73.53 =106/(1.36 × 104), 1.36 × 104means the molar absorption coefficient of Ellman’s reagent,A412nmmeans the wavelength of the spectrophotometer,Dmeans the dilution factor 5.05 andCmeans the protein concentration (5 mg/mL).
2.4.2 Surface hydrophobicity
Using BW13SG at a concentration of 2 mg/mL. 20 μL 8-anilino-1-naphthalene sulphonic acid solution (ANS, 15 mmol/L, pH 7.0)was vortexed with 4 mL of BW13SG (2 mg/mL). Keep the reaction mixture in the dark for 20 min, then centrifuge at 5 000 ×gfor 5 min at 4 °C, take the supernatant to determine the protein concentration by Lowry method, and dilute the sample to the same concentration with the same phosphate buffer, and then use Spectra Max M2 spectrophotometer (Shimadzu, Japan) measures the fluorescence intensity. Using an excitation wavelength of 375 nm, the intensity in arbitrary units (a.u.) was recorded at emission wavelengths ranging from 430 nm to 610 nm.
2.4.3 Fluorescence spectroscopy measurement
BW13SG samples before and after UHP were diluted to a concentration of 0.1 mg/mL in phosphate buffer saline (10 mmol/L).Fluorescence measurements were carried out using a fluorescence spectrophotometer (F-7000, Shimadzu, Japan). Fluorescence emission spectra for BW13SG were recorded with an excitation wavelength of 334 nm and emission wavelengths of 350–500 nm, with a slit width of 10 nm.
2.4.4 Fourier transform infrared spectroscopy (FTIR)
FTIR spectra were obtained in transmission mode from 4 000 cm?1to 600 cm?1with a resolution of 0.44 cm?1at room temperature using a VERTEX-70 Fourier transform infrared spectroscope (Bruker Co., Germany). Samples were diluted with KBr(1 : 100,V/V) before acquisition.
2.5 Measurement of the solubility of BW13SG
The solubility of BW13SG with and without UHP treatment were determined. The solubility of native and UHP treated BW13SG were determined by the method reported by Manassero et al. [21]and Lan et al. [22]. BW13SG (50 mg) was transferred into dry centrifugal tubes, weighed, and mixed with distilled water (5 mL). The tubes were incubated in a shaking water bath at 70 °C for 30 min, cooled to room temperature, and then centrifuged at 1 000 ×gfor 15 min.The supernatant was carefully decanted and the resulting tubes with their contents were weighed. The residue obtained after drying the supernatant represented the amount of protein dissolved in water, as given by the following equation.

2.6 Measurement of foaming properties of BW13SG
Foaming capacity (FC) and foaming stability (FS) were determined according to the method described by Qin et al. [23]with a few modifications. Volumes of 50 mL (V1) UHP treated or UHP untreated BW13SG solutions (1%m/V) were stirred for 1 min using an Ultra-Turrax T25 high-speed blender (IKA, Staufen, Germany) at 10 000 ×gat room temperature. Volumes of the whole mixture were recorded before and after whipping. FC and FS were calculated using the following equations, respectively.

whereV1is the volume of the initial mixture,V2is the volume of the mixture after whipping andV3is the volume of the mixture at 30 min after whipping.
2.7 Measurement of the emulsifying properties of BW13SG
The emulsifying activity index (EAI) and emulsifying stability index(ESI) were measured following the method described by Hou et al. [24]with a slight modification. Dispersions containing 5 mL of 0.5 g/100 g protein were homogenized with 5 mL of corn oil at 7 200 ×gfor 5 min. The sample collected from the bottom of the emulsion was diluted in sodium phosphate buffer containing 0.1% SDS at pH 7. The absorbance of the diluted emulsion was then measured at 500 nm. EAI and ESI were calculated by the following equations.
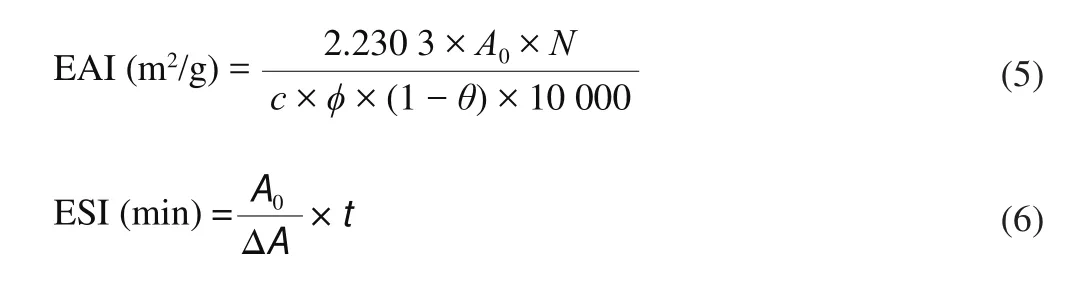
where EAI is emulsifying activity (m2/g), ESI is emulsifying stability (min),A0is the absorbance of the diluted emulsion immediately after homogenization,DFis the dilution factor (× 150),cis the weight of protein per volume (g/mL),φis the optical path(0.01 m),θis the fraction of oil used to form the emulsion (0.25), ΔAis the change in absorbance between 0 and 10 min, andtis the time interval (10 min).
2.8 Statistical analysis
Data were analyzed using Excel 2003 (Microsoft, Redmond, WA,USA) and SPSS 18.0 (SPSS Inc., Chicago, IL, USA). Significant differences were identified with Duncan’s multiple range test(P< 0.05). All experiments were performed at least in triplicate using randomly selected samples.
3. Results and discussion
3.1 DEAE-Sepharose fast flow
DEAE-Sepharose fast flow ion exchange chromatography on BWP was successively eluent with phosphate buffer (pH 7.2,10 mmol/L) and 0.2, 0.5 mol/L NaCl (phosphate buffer con figuration),respectively. Three main components were obtained, named BWSP1,BWSP2 and BWSP3, respectively. The elution curve is shown in Fig. 1A, and the effects of each component on the adsorption of cholatein vitrowere then determined.

Fig. 1 DEAE-Sepharose FF chromatography of BWP (A) and Sephadex G-75 chromatography of BWP P3 (B), SDS-PAGE of P32 (C). (a) Molecular weight marker; (b) P32.
3.2 DEAE-Sepharose FF separation components to adsorb cholate in vitro
Cholesterol decomposition is the product of cholic acid salt, the human body through the study of the adsorption of bile salts and is discharged through the intestinal cholesterolin vitrocan promote the metabolism, the activity of active substance evaluation method for the determination of cholic acid salt adsorption of cholic acid with bile acid sodium salt, sodium deoxycholic acid and taurocholic acid sodium, the taurocholic acid sodium is the most difficult to adsorption,so choose the typical three bile salts for testing [25]. Therefore, it was representative to select sodium cholate, sodium deoxycholate and sodium taurocholate as adsorption proxies in these experiments.
As can be seen from Table 2, the purified BWP adsorbed all three representative cholates selected. The adsorption capacity of P3 was greater than the other two peaks, the adsorption capacity of sodium cholate and sodium deoxycholate was above 90%, and the adsorption capacity of sodium sulphurate, which was difficult to be adsorbed,was also higher than 50%.
3.3 Sephadex G-75 separation of P3

Table 2The binding capacity of bile salts by purified protein from BWP and purified BWP P3.
According to the elution curve shown in Fig. 1B, there were four main separation peaks of P3 in BWP after separation by Sephadex G-75, which we named P31, P32, P33 and P34, respectively. The effects of each component on the adsorption of cholatein vitrowere then determined.
3.4 Sephadex G-75 separation of P3 component to adsorb cholate
As can be seen from Table 2, P3 purified from BWP adsorbed all three cholate salts, and among the fractions found in P3, the capacity of P32 was greater than the other three peaks. The adsorption capacity of sodium cholate was up to 96%, while the adsorption capacity of sodium deoxycholate was up to 73%, and the adsorption capacity of sodium taurocholate, a difficult substrate for adsorption, was higher than 50%.
3.5 SDS-PAGE purity detection
P32 was electrophoresed on a SDS-PAGE to obtain a single band, indicating that P32 was a single component. According to the relative molecular weight and relative mobility of the standard protein marker used, the relative molecular weight of the protein band was 38 000 kDa, as shown in Fig. 1C.
3.6 Effects of UHP on the structure of BW13SG
3.6.1 Fluorescence spectra
When BW13SG was treated at pH 3.0 for 0–30 min, the fluorescence peak intensity of BW13SG increased, while the pressure increased from 100 MPa to 300 MPa, with further increases in the pressure to 500 MPa leading to lower fluorescence intensity. This variation tendency suggested that UHP pressures ranging from 100 MPa to 300 MPa might result in the exposure of the tryptophan residues of BW13SG. Higher pressures led the exposed hydrophobic groups to reassociate or aggregate, forming a more stable structure,which caused the refolding of the aromatic amino acid residues [26].Fig. 2A shows that the fluorescence peak intensity of BW13SG was consistently increased with increasing pressure at pH 7.0, and the intensity did not decrease until the treatment was held at 500 MPa for 30 min. This phenomenon may be attributed to the more stable conformation of BW13SG at neutral pH values, which was evident from the circular dichroism studies of others [27]. Franco et al. [28]also revealed that the fluorescence spectra of BW13SG did not show any differences in theλmaxafter UHP at 20 °C compared to an untreated protein, which was in agreement with the results of this study.

Fig. 2 (A) Effect of different levels of UHP on BWP fluorescence at different pH values for 30 min. (B) Effect of different levels of UHP on BWP FT-IR spectra at different pH values for 30min. (C) Effect of different levels of UHP on BWP surface hydrophobicity at different pH values. (D) Effect of different levels of UHP on BWP sulfhydryl contents at different pH values.
3.6.2 FT-IR spectra
FT-IR spectra of BW13SG subjected to different treatments are displayed in Fig. 2B. The amide I band (1 700–1 600 cm-1) is due mainly to the C=O streching vibration (approximately 80%) of the amide groups coupled with little in-plane N H bending. Using peak fit 4.12 software to deconvolute the protein secondary structure content of the amide I band of the product under high pressure. The change of the secondary structure content of the sample is shown in Table 3. The secondary structure of the protein in the sample is mainlyβ-folding andβ-turning, and the content ofβ-folding andβ-turning is mainly changed after high pressure. Compared with the soybean globulin without ultra-high-pressure treatment,the content ofα-helix andβ-fold in the secondary structure of the sample decreased, and the content ofβ-corner and disorder structure increased, indicating that the secondary structure of the soybean globulin changed due to ultra-high-pressure. The possible reason is that the original high-level structure of the sample is depolymerized, the peptide chain is broken, some peptide chains are diffused, and some of theα-helix andβ-fold structures change into disordered structure after the high pressure. It was found that the content ofα-helix andβ-fold structure decreased after UHP,which was consistent with the result of Tang et al. [29].

Table 3Content of secondary structures of BW13SG by UHP treatment.
3.6.3 Surface-exposed hydrophobicity
Alterations ofH0and SH contents are good indicators of tertiary conformational changes of certain proteins, denoting the extent of protein folding/unfolding [30]. It can be seen from Fig. 2C that in a pH 3.0 Gly-HCl buffer solution, the surface hydrophobicity (SH0) of BW13SG increased significantly with the increase of UHP treatment pressure. After 500 MPa treatment, the relative surface hydrophobic index of BW13SG reached 205.9%, and theSH0increased more than twice. However, in Tris-HCl in a pH 7.0 buffer solution, the effect of UHP treatment on theSH0of BW13SG was much less obvious, and the BW13SGSH0at steady state and with increasing pressure after a pressure of 300 MPa started to present a tendency of slow increase.At 500 MPa for 30 min, after processing, the relative index ofSH0reached a maximum value (123.8%).
Xue et al. [31]also found a similar effect of UHP (300 MPa)treatment on soybean protein in soybean milk. The surface hydrophobicity of BWP in a pH 3.0 buffer solution could be significantly improved with a treatment time of 10 min, and theSH0continued to rise with increasing treatment time, reaching a maximum value at 30 min, where the relativeSH0index was 170.8%. Similar to the effect of pressure, the effect of treatment time on theSH0of BW13SG in a pH 7.0 buffer solution was far less significant than that in a pH 3.0 buffer solution. TheSH0index of BW13SG in a pH 3.0 buffer solution was much higher than that in a pH 7.0 buffer solution, which re flected the presence of different forms of BW13SG conformation after dissolution in different pH buffer solutions.
Puppo et al. [32]considered that UHP causes the unfolding of protein molecules, which was accompanied by the exposure of hydrophobic groups to the surrounding medium. Xu et al. [33]and Li et al. [34]suggested that UHP treatment caused the destruction of weak hydrogen bonds and van der Waals forces in protein molecules,and thus, changed the structure of the protein molecules, along with the exposure of hydrophobic groups in the process.
3.6.4 Free sulfhydryl content
Sulfhydryl groups ( SH) are highly reactive and can be converted to each other through an exchange reaction creating a sulfhydryl-disulfide bond [35]. This exchange reaction can occur both within and between molecules, and plays a role in the functional properties of proteins. It also plays other important roles, such as gluten formation [36]and gel protein film formation [37]. As can be seen from Fig. 2D, with the increase of UHP treatment pressure,the content of free sulfhydryl on the surface of BW13SG decreased significantly. The free sulfhydryl content on the surface of BW13SG in the two buffer solutions decreased by 26.7% (pH 3.0) and 32.8%(pH 7.0), respectively, at a treatment pressure of 500 MPa. The UHP treatment of soy protein isolates, except that the sulfhydryl level of soy protein isolates in a pH 3.0 buffer solution increased slightly at 200 MPa [32]. From Fig. 2D, we also found that the content of free sulfhydryl of BW13SG in a pH 3.0 buffer solution was much higher than that in a pH 7.0 buffer solution, which should be caused by the prolonged exposure of protein molecules to acidic pH.
In a pH 3.0 Gly-HCl buffer solution at 200 MPa, 10 min of UHP significantly reduce the free sulfhydryl content on the surface of BW13SG compared with 0 min, and this was slightly different from a pH 7.0 Tris-HCl buffer solution at 200 MPa for 10 min. When the content of free sulfhydryl on the protein surface after UHP treatment was compared with 0 min, there was a slight decrease. Zhou et al. [38]in their analysis of the causes of the decrease in free sulfhydryl groups on protein surfaces caused by UHP treatment, indicated that UHP treatment causes the protein structure to unfold, which was accompanied by the formation of disul fide bonds, thus reducing the content of free sulfhydryl.
3.7 Solubility
Solubility is the most practical measure of protein denaturation and aggregation, and it is, therefore, a reliable index of protein functionality [39]. Here, we investigated the effect of UHP treatment on the solubility of buckwheat protein in two buffer solutions with different pH values (pH 3.0, Gly-HCl buffer solution and pH 7.0,Tris-HCl buffer solution). The experimental results are shown in Fig. 3A. The solubility of BW13SG in a Tris-HCl buffer solution at pH 7.0 was much higher than that in a Gly-HCl buffer solution at pH 3.0 because of the aggregation of proteins near the isoelectric point. At pH 3.0, when the holding time was 30 min, the solubility of BW13SG increased with increasing pressure. The highest solubility was observed when BW13SG was subjected to UHP at 500 MPa(63.2%). One possibility was that the protein molecules partially unfolded, and this increased the interaction between the proteins and water molecules. When the pressure increased, the BW13SG molecules expanded, which exposed hydrophobic groups and buried intramolecular sulfhydryl groups [40]. At pH 7.0, the solubility of BW13SG could be increased to 91.8% with a pressure above 500 MPa. This may have been due to the proteins being treated with UHP, the condensation of the globular protein solution and the gradually extended association of proteins. Protein molecules thus become depolymerized to smaller subunit units, globular protein molecules, internal polar groups and hydrophobic group become exposed, making the surface charge distribution of protein molecules strengthen, and the combined water around the newly exposed polar groups increase protein hydration, thus increasing its solubility [41].

Fig. 3 Effect of different levels of UHP on BWP solubulity at different pH values (A). Effect of different levels of UHP on BWP emulsions at different pH values (B). Effect of different levels of UHP on the physical stability of BWP emulsions at different pH values (C). Effect of different levels of UHP on BWPfoaming properties at different pH values (D). Effect of different levels of UHP on BWP foaming stability at different pH values (E).
3.8 Emulsifying properties
The EAI refers to a molecule’s ability to dissolve or disperse in two immiscible liquids, and the ESI is the ability to maintain an emulsion and its resistance to rupture [22]. As can be seen in Figs.3B and 3C, in a Gly-HCl buffer solution with a pH 3.0, the EAI and ESI of BW13SG were improved with increasing UHP treatment pressure, reaching a maximum value of 67.3 m2/g at 500 MPa for 30 min. The effect of treatment time on EAI and ESI was similar to that of pressure, and a treatment time of 10 min yields good improvement. In a Tris-HCl buffer solution at pH 7.0, the case was a different story. The BW13SG EAI was almost not affected by the processing time and pressure, but the processing time and pressure change had certain influence on the ESI. The ESI after 100 MPa reached a maximum, and then the ESI slowly declined with the increase of pressure variation trend. Through this, the ESI of BW13SG after 500 MPa processing, was still better than that of untreated samples. The improvement of the ESI of the protein under weak alkaline conditions was not as significant as that under acidic conditions with UHP treatment time. A 10 min treatment time could make it reach a maximum value, and no significant difference could be observed at other treatment time points.
Molina et al. [42]found that in neutral solution conditions and pH (pH 6.5 or 7.5), soybean protein isolate 7S after UHP treatment at 400 MPa showed their highest EAI andSH0, and the specific provisions of the 11S group showed their highest after dealing with a pressure of 200 MPa. The EAI andSH0of soy protein isolated at 400 MPa had the highest EAI, although when it was low, in terms of theSH0view of this phenomenon, the authors believe that a pressure treatment of 400 MPa enabled the 7S group of soybean protein isolate to decompose into partially denatured or completely denatured monomers, thus improving their surface activity. Therefore, UHP-treated proteins could be useful in the food industry for emulsion formation and prolong the shelf life of foods.
EAI is a functional property and relates to the ability to retard bile acid adsorption in the small intestine, thus leading to the degradation of cholesterol in the liver and further reducing its levels in the blood [43].Therefore, the addition of UHP-BW13SG to foods might not only be beneficial for food quality but also better for human health.
3.9 Foaming properties
Based on Figs. 3D and 3E, in a Gly-HCl buffer solution with a pH 3.0,the FC and FS of BW13SG were improved with increasing UHP treatment pressure. The FC and FS of a protein are able to deal with a pressure of 500 MPa, reaching a maximum of 83%, and 20 min of UHP treatment time can improve the protein FC and FS. In a Tris-HCl buffer solution with a pH 7.0, the influence of pressure on the FC of BW13SG reached a maximum value at 200 MPa, and then the FC of the BW13SG decreased with increasing pressure. However, the FC of the sample treated at 500 MPa was still better than that of the untreated sample. The effects of pressure and treatment time on the FS of BW13SG were very similar to those under acidic conditions,but the extent of improvement was far less significant than that under acidic conditions.
BW13SG showed good FC in weakly alkaline conditions, but it showed better FS in acidic conditions. The emergence of this phenomenon was because when the pH of the solution was in or close to a protein isoelectric point, a lack of repulsion interactions between protein molecules occurs, which is beneficial to the protein-protein interactions at the interface and the formation of thick film [44,45].Here, it was precisely because of protein solubility problems led to our experiment in the acidic conditions of FC higher than weak alkali conditions, because only the soluble part of protein is involved in the formation of bubbles, but FS is opposite, because of the insoluble protein particles adsorption increased protein membrane adhesion force, thus enhancing the FS.
3.10 In vitro adsorption of cholate from isolated components
As can be seen from Table 4, the adsorption capacity of BW13SG increased with increasing treatment pressure. At 500 MPa (30 min,Gly-HCl buffer pH 3.0), the adsorption capacity of BW13SG sodium cholate and sodium deoxycholate was both higher than 98%, and the adsorption capacity of sodium taurocholate was higher than 60%, in terms of BWP purification (%).

Table 4The binding capacity of bile salts by BW13SG of UHP treatment.
4. Conclusion
UHP treatments effectively increased the UHP treatments effectively increased the solubility, emulsion capacity and stability,foaming capacity and stability of BW13SG at 500 MPa (30 min, Gly-HCl buffer pH 3.0). In addition, the UHP treatment is lead to the exposure of hydrophobic groups and the formation of disulfide bonds in BW13SG. UHP treatment make BW13SG restricting in the Gly-HCl buffer (pH 3.0), then gather to form the soluble macro molecular aggregate, while UHP treatment caused the depolymerization of soluble macro molecular aggregate in the Tris-HCl buffer (7.0).Accordingly, these findings provide a better understanding of BW13SG and the effects of UHP on modified proteins. It can be concluded that BW13SG can be used as a protein-rich ingredient in functional foods.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgements
The authors grateful acknowledge the financial support by the Open Project Program of State Key Laboratory of Dairy Biotechnology (No. SKLDB2018-002), National Natural Science Foundation of China (No. 31871805 & No. 31501437), Shanghai Municipal Education Commission (Plateau Discipline Construction Program) and China Agriculture Research System (CARS-08-D2).
- 食品科學(xué)與人類健康(英文)的其它文章
- Pomegranate peel polyphenols alleviate insulin resistance through the promotion of insulin signaling pathway in skeletal muscle of metabolic syndrome rats
- Sucrose-free hawthorn leathers formulated with fructooligosaccharides and xylooligosaccharides ameliorate high-fat diet induced inflammation,glucose and lipid metabolism in liver of mice
- Roles of Adinandra nitida (Theaceae) and camellianin A in HCl/ethanol-induced acute gastric ulcer in mice
- Polygonatum sibiricum polysaccharides protect against obesity and non-alcoholic fatty liver disease in rats fed a high-fat diet
- Trehalose ameliorates autophagy dysregulation in aged cortex and acts as an exercise mimetic to delay brain aging in elderly mice
- Deep eutectic solvents and alkaline extraction of protein from seabuckthorn seed meal: a comparison study

