Radiomics signature: A potential biomarker for β-arrestin1 phosphorylation prediction in hepatocellular carcinoma
Feng Che, Qing Xu, Qian Li, Zi-Xing Huang, Cai-Wei Yang, Li Ye Wang, Yi Wei, Yu-Jun Shi, Bin Song
Abstract
Key Words: Hepatocellular carcinoma; Sorafenib resistance; β-Arrestin1 phosphorylation; Radiomics;Computed tomography; Overall survival
INTRODUCTION
Hepatocellular carcinoma (HCC) was the sixth most common cancer and the third leading cause of cancer-related death worldwide in 2020 [1 ]. Liver resection and transplantation are considered potentially curative methods for early-stage patients with well-preserved liver function. For advancedstage HCC, systemic therapies such as multikinase inhibitors and immune checkpoint inhibitors,represented by sorafenib, have shown the potential to confer a survival advantage of 2 -3 mo[2 ,3 ].However, patients undergoing sorafenib treatment have a high resistance rate, which is still the greatest challenge and leads to a discouraging prognosis[3 ]. Hence, identifying patients who are more likely to benefit from sorafenib treatment and discovering related biomarkers associated with sorafenib treatment response are urgently needed.
β-Arrestins, including β-arrestin1 and β-arrestin2 , are important regulators of seven-transmembrane domain G-protein-coupled receptors. They can block subsequent G protein activation and result in receptor desensitization by phosphorylation/dephosphorylation of β-arrestin1 at a carboxyl-terminal serine, Ser-412 [4 ,5 ]. The development of sorafenib resistance includes primary and secondary resistance,and the phosphorylation of ERK has been widely accepted to play an important role in both[6 -8 ].Activation of AKT and epithelial-mesenchymal transition (EMT) also participates in acquired resistance and the signaling pathways mentioned above have strong relationships with β-arrestin1 [9 -11 ]. Wuet alrevealed that β-arrestin1 enhances hepatocellular carcinogenesis by inflammation-mediated Akt signaling[12 ] and promotes HCC invasion and metastasis through p-ERK1 /2 to mediate EMT[13 ]. The phosphorylation status of β-arrestin1 influences its function in activating downstream receptors such as ERK1 /2 , forming a negative feedback loop[14 ]. All these signals are highly related to sorafenib resistance[9 ,15 ], which indicates that the expression of phosphorylated β-arrestin1 (p-β-arrestin1 ) may correlate with sorafenib resistance in HCC patients. Thus, the preoperative prediction of β-arrestin1 phosphorylation may help identify patients who could benefit from sorafenib treatment.
Radiomics is a newly emerging computational medical imaging method that allows for the quantitative analysis and translation of medical images[16 ,17 ]. Additionally, radiomics studies can provide insights into the depth and comprehensive characterization of tumor heterogeneity, with the underlying hypothesis that radiomics can better characterize tumor heterogeneity[18 -20 ]. Preliminary studies have suggested that radiomics features can be useful for tumor lesion detection[18 ,21 ] and are potentially predictive of the microenvironment and molecular status of tumors[16 ,22 ,23 ]. Xu et al[24 ]extracted radiomics signatures from contrast-enhanced computed tomography (CECT) images to build a risk model that showed good performance in microvascular invasion stratification and could well predict the clinical outcomes of HCC patients. To the best of our knowledge, the value of radiomics based on CECT images in predicting β-arrestin1 phosphorylation in HCC has not yet been reported.
The purpose of this study was therefore to develop and validate a radiomics-based model combining visual imaging and clinical features for the preoperative noninvasive prediction of β-arrestin1 phosphorylation and to further investigate its association with prognostic outcomes in HCC patients.
MATERIALS AND METHODS
Patients
This retrospective study was approved by the Institutional Review Board of West China Hospital and the requirement for informed consent was waived. Patients who had histologically proven HCC and received systemic treatment with sorafenib after surgery between January 2013 and April 2017 were retrospectively reviewed and consecutively recorded. The inclusion criteria were as follows: (1 ) Age ≥18 years; (2 ) Pathologically confirmed HCC; (3 ) Interval between CECT imaging and surgery less than four weeks; (4 ) Treatment naive [i.e., no hepatectomy, transcatheter arterial chemoembolization (TACE) or radiofrequency ablation (RFA) before CECT]; and (5 ) Administration of 400 mg sorafenib twice a day after surgery with up to two dose reductions allowed (from 400 mg once daily to 400 mg every 2 d) for drug-related adverse events. The exclusion criteria were as follows: (1 ) Incomplete or poor-quality CT images; (2 ) Interrupted sorafenib treatment for longer than 48 h between the initiation of sorafenib and the first follow-up time point; and (3 ) Death or loss to follow-up. Among the 146 eligible patients, 47 patients were excluded because CECT imaging was performed more than 4 wk before surgery (n = 13 ),the CT images were incomplete or of poor quality (n= 11 ), sorafenib treatment was interrupted for longer than 48 h between the initiation of sorafenib and the first follow-up time point (n = 8 ), or the patient was lost to follow-up (n= 15 ). Therefore, 99 patients were ultimately enrolled in this study. In addition, the investigated laboratory data within 7 days of the CT examination and clinical conditions were recorded, as shown in Figure 1 .
In this study, consecutive patients who underwent surgery between January 2013 and March 2016 comprised the training cohort and were used to construct the nomograms, and patients who underwent surgery from April 2016 to April 2017 comprised the validation cohort.
Imaging techniques
CT imaging was performed by using multidetector CT scanners (Revolution, GE Healthcare,Milwaukee, United States; SOMATOM definition, Siemens Healthcare, Erlangen, Germany). Precontrast images were first obtained before contrast agent (iodine concentration, 300 -370 mg/mL; volume, 1 .5 -2 .0 mL/kg of body weight; contrast type, iopromide injection, Bayer Pharma AG) injection. Then, the arterial phase and portal venous phase were obtained with the following parameters: tube voltage, 100 -120 kVp; tube current, 450 mA; slice thickness, 0 .625 mm; pitch, 0 .992 :1 ; rotation speed: 0 .5 s/rot; and ASIR-V: 30 %. The arterial phase and portal venous phase were obtained at 25 s and 60 s after contrast injection.
Imaging evaluation
Three abdominal radiologists who were blinded to the histopathological results, clinical data, and survival outcomes reviewed all the CT images. The following imaging features were assessed by these readers: (1 ) Tumor margin, defined as a non-smooth margin with budding portion protruding into the liver parenchyma or infiltrative appearance at the tumor periphery, otherwise as smooth margin; (2 )Tumor size, defined as the maximum diameter, measured on arterial phase transverse images or portal venous phase images; (3 ) Pseudocapsule, defined as a complete capsule with a uniform border around most or all of the tumor, unequivocally thicker or more conspicuous than the fibrotic tissue around background nodules, otherwise as incomplete integrity or not applicable; (4 ) Multifocality; (5 ) Arterial phase hyperenhancement; (6 ) Portal venous/delay phase hypoenhancement; (7 ) Radiologic evidence of necrosis; (8 ) Radiologic evidence of cirrhosis; and (9 ) Portal vein tumor thrombosis invasion. All examinations were performed using a workstation and recorded on a picture archiving and communication system
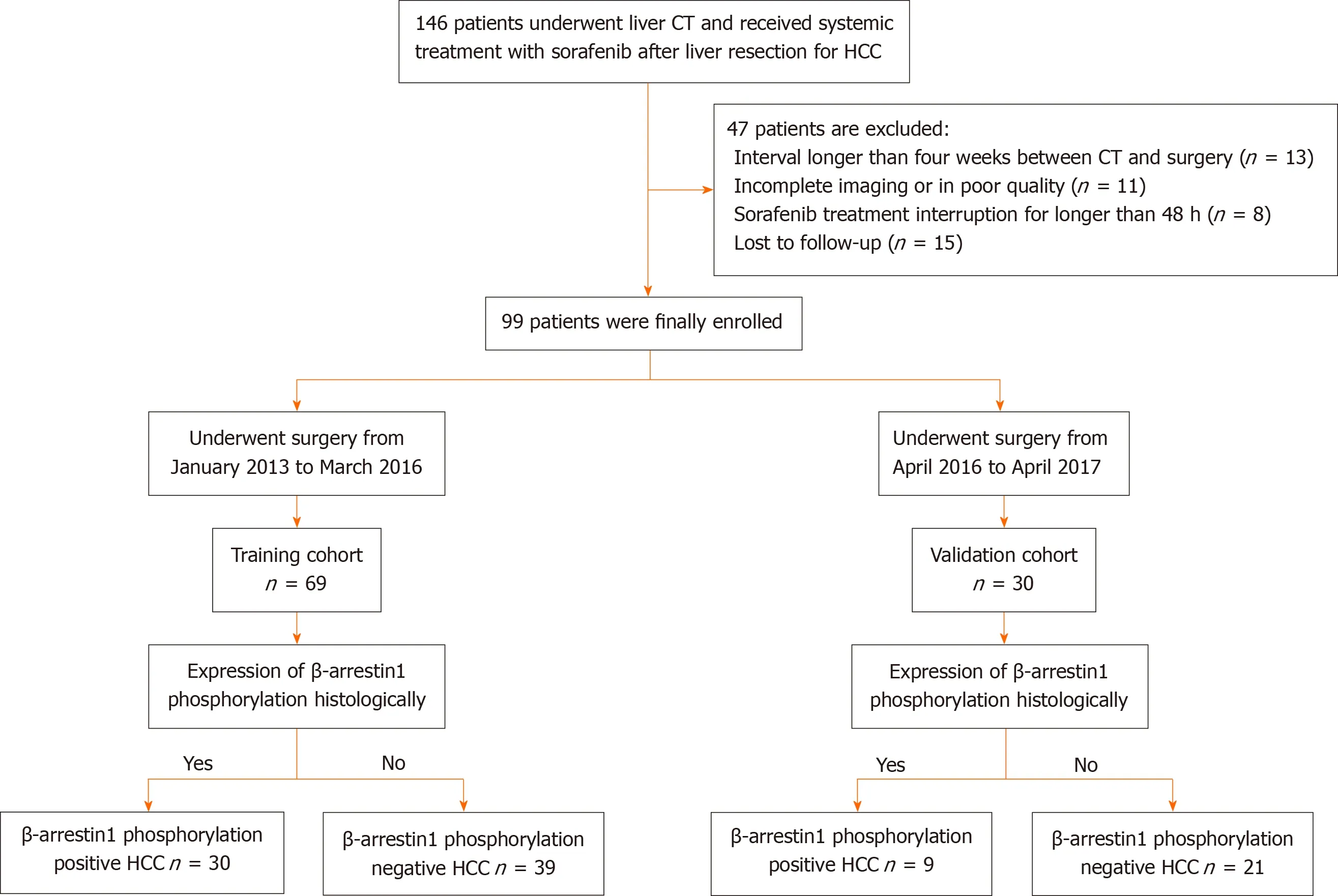
Figure 1 Patient recruitment process.
Immunohistochemistry
Surgically resected specimens embedded in paraffin were cut into 4 μm-thick sections dewaxed,hydrated, and subjected to antigen retrieval. Subsequently, the tissue slides were incubated with primary antibodies using rabbit anti-human p-β-arrestin1 polyclonal antibody (Abcam Biotechnology,ab247229 ; diluted, 1 :200 ) at 4 °C overnight, followed by incubation with secondary antibodies (cat #K5007 ; Dako). Staining was performed with 3 ,3 ’-diaminobenzidine (DAB) and counterstained with hematoxylin. Two senior pathologists who were blinded to all radiological and clinical results independently selected five nonoverlapping and discontinuous regions to calculate the mean for statistical analysis. Variations in the results within a range of 5 % were reassessed, and a consensus decision was made. With the threshold value of 5 % (p-β-arrestin1 tumor cells/total tumor cells), cases with expression higher than 5 % were considered p-β-arrestin1 positive.
Follow-up surveillance after surgical resection
The patients were consistently followed-up after liver resection at intervals of 3 to 6 mo based on αfetoprotein and imaging examinations, including ultrasound, CT or magnetic resonance imaging (MRI),and the time of disease-specific progression (local recurrence or distant organ metastasis) and time of death were recorded. These survival data were collected by one radiologist using electronic medical records and follow-up imaging studies until June 30 , 2020 . Overall survival (OS) was measured as the interval from the date of surgery to the date of death from a disease-related cause or the latest followup. For patients who were alive at the latest follow-up, the data were censored.
Radiomics workflow
Regions of interest (ROIs) were manually delineated around the outline of the tumor slice by slice using ITK-SNAP software (version 3 .6 .0 ) and excluded necrosis and calcification in the tumors. Radiomics features were generated from the images using in-house scientific research 3 D analysis software(Analysis Kit, version V3 .0 .0 . R, GE healthcare). Two classes of feature extraction methods were extracted as follows: the original feature class and 14 filter classes (boxmean, additiveGaussiannoise,binomialblurimage, curvatureflow, boxsigmaimage, log, wavelet, normalize, laplaciansharpening,discreteGaussian, mean, specklenoise, recursiveGaussian and shotnoise). A total of 2600 features were extracted from the tumors. Two radiologists (readers 1 and 2 ) performed ROI segmentation in a blinded manner to assess interobserver reliability. Reader 1 repeated the feature extraction twice during a 1 -wk period to evaluate intraobserver reliability. The interobserver reliability and intraobserver reliability were assessed by obtaining the intraclass correlation coefficient (ICC). Features with ICC values > 0 .75 were selected for subsequent investigation. The feature selection process comprised the following three steps in the training group: variance analysis, Spearman correlation, and Lasso regression analysis. The radiomics score of each patient was calculated using this determined multivariable logistic regression model.
Prediction models of β-arrestin1 phosphorylation
For CT radiographic and clinical factors, predictors withP< 0 .05 in the univariate logistic analysis (P<0 .05 ) were included. Multivariate logistic analysis, which was used to identify significant predictors based on a backward stepwise selection process with the Akaike information criterion, was employed to develop a clinical-radiological (CR) model. In addition, a clinical-radiological-radiomics (CRR) model was constructed by multivariate logistic regression analysis, tests of the association with radiomics scores, clinical factor evaluations and CT imaging findings based on a backward stepwise selection process with the Akaike information criterion.
Statistical analysis
Categorical variables are summarized as frequencies and proportions, while continuous variables are expressed as the means and standard deviations or medians and interquartile ranges (IQRs). The differences in characteristics between groups were evaluated using Student’sttest (normal distribution)and the Mann-WhitneyUtest (skewed distribution) for continuous variables and the chi-squared test or Fisher’s exact test for categorical variables. OS curves were drawn by using the Kaplan-Meier method,and the difference in OS between groups was compared using the log-rank test. Inter-observer agreement was applied to assess the reliability of imaging analysis using the Kappa test; 0 -0 .2 represents slight, 0 .21 -0 .40 : fair, 0 .41 -0 .60 : moderate, 0 .61 -0 .80 : substantial, 0 .81 -1 : excellent.
The discriminative performance of the prediction models was quantified by the area under the curve(AUC) of receiver operator characteristic (ROC) curves. Differences in the ROC curves were compared by using the DeLong test. Calibration curves were generated to assess the calibration of the prediction model with the Hosmer-Lemeshow test. The probabilities of net benefits were quantified by decision curve analysis to evaluate the clinical application value of the prediction models.
The statistical analyses were implemented usingRstatistical software (version 3 .4 .2 , http://www.Rproject.org) and SPSS software (version 22 .0 , IBM), and two-sided P values < 0 .05 were considered significant.
RESULTS
Patient characteristics
Of the 99 patients [male/female: 88 /11 ; mean age, 51 .53 ± 12 .62 years, range 21 to 78 years) included in the study (training (n= 69 ) and validation (n = 30 )], p-β-arrestin1 was identified in 39 (39 .4 %) patients.The 3 -year survival rates of p-β-arrestin1 -positive and p-β-arrestin1 -negative HCC patients were 38 .5 %and 31 .7 %, respectively. The Kaplan-Meier method showed that p-β-arrestin1 -positive patients lived longer than p-β-arrestin1 -negative patients (P < 0 .05 with the log-rank test). The clinical, pathological,and imaging characteristics of patients in the training and validation cohorts are summarized in Table 1 .
Development of the radiomics score
Variance analysis usingttest identified 15 radiomics features, assessed by Spearman rank correlation,and 4 features (boxsigmaimage_glrlm_RunLengthNonUniformity, wavelet_firstorder_wavelet-HLLSkewness, wavelet_glcm_wavelet-HLH-Correlation, and wavelet_ngtdm_wavelet-LHL-Busyness) were chosen for logistic regression analysis (P> 0 .05 ). Variables with P < 0 .1 in the univariable logistic regression analysis were included in the multivariable regression model with backward stepwise selection using the Akaike information criterion. The radiomics score was calculated with the following formula: radiomics score = -0 .3527 + 0 .4748 × boxsigmaimage_glrlm_RunLengthNonUniformity +0 .7046 × wavelet_firstorder_wavelet-HLL-Skewness-0 .5697 × wavelet_glcm_wavelet-HLH-Correlation +0 .6471 × wavelet_ngtdm_wavelet-LHL-Busyness.
Development of the predictive models
In total, 2 clinical characteristics [alanine aminotransferase (ALT) and aspartate aminotransferase (AST)levels], 2 imaging features (tumor size and tumor margin on portal venous phase images) and the radiomics score were identified by univariate analysis (allP< 0 .1 ). In the multivariable logistic regression analysis, radiomics score [odds ratio (OR), 3 .412 ; 95 %CI: 1 .562 -7 .453 , P = 0 .002 ], ALT level(OR, 0 .159 ; 95 %CI: 0 .038 -0 .673 , P < 0 .012 ), tumor size (OR, 0 .243 ; 95 %CI: 0 .059 -1 .003 , P = 0 .05 ) and tumor margin (OR, 0 .170 ; 95 %CI: 0 .044 -0 .664 , P = 0 .011 ) significantly predicted β-arrestin1 phosphorylation(Table 2 ). Thus, the CR and CRR models were constructed by using the above aggressive features and the nomograms of the above multiparametric models are shown in Figure 2 A and B. Excellent interobserver agreement was observed for the imaging feature evaluation, with Kappa values of 0 .890 for tumor size and 0 .789 for smooth tumor margin (Figure 3 ).
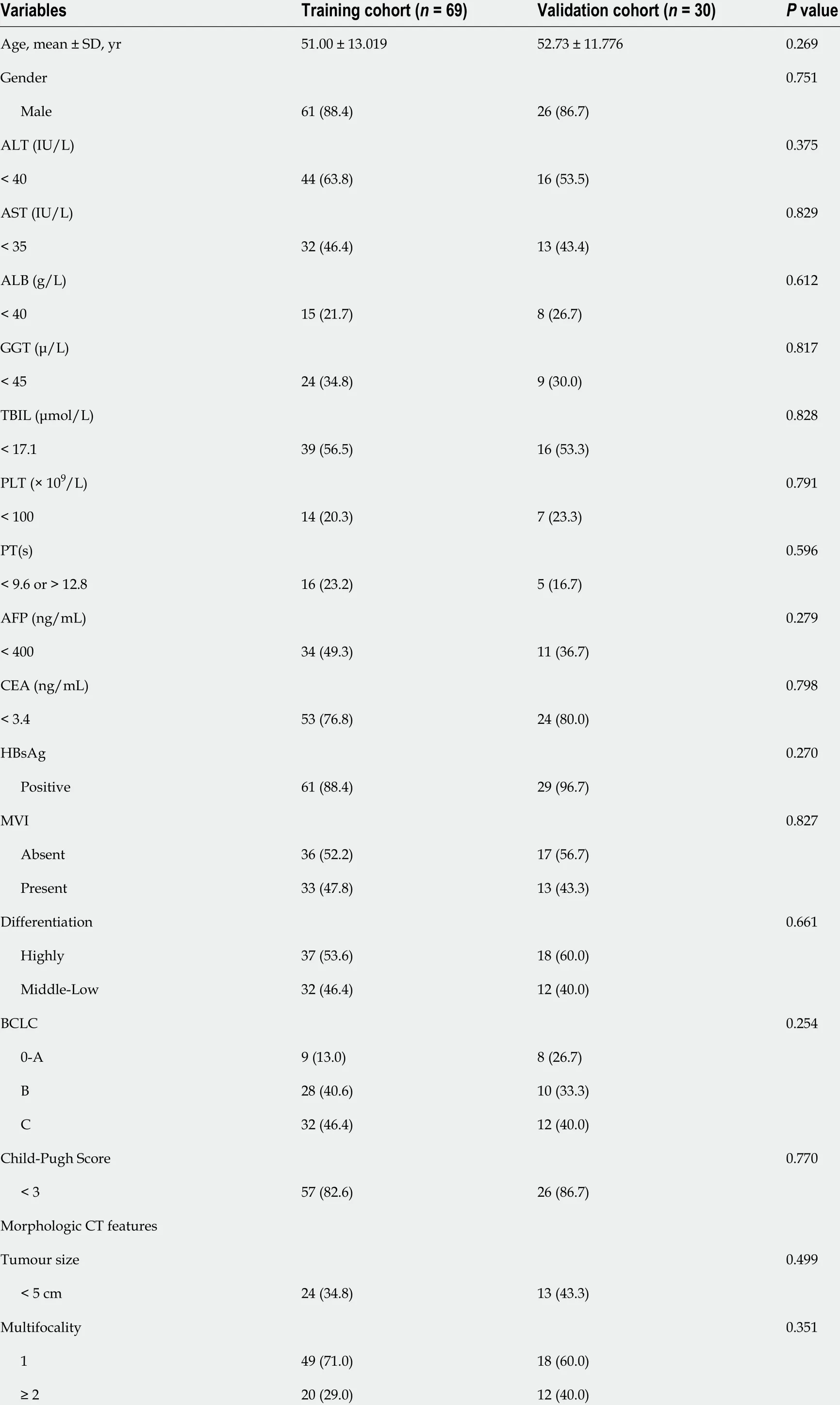
Table 1 Baseline characteristics of the patients in the training and validation cohorts
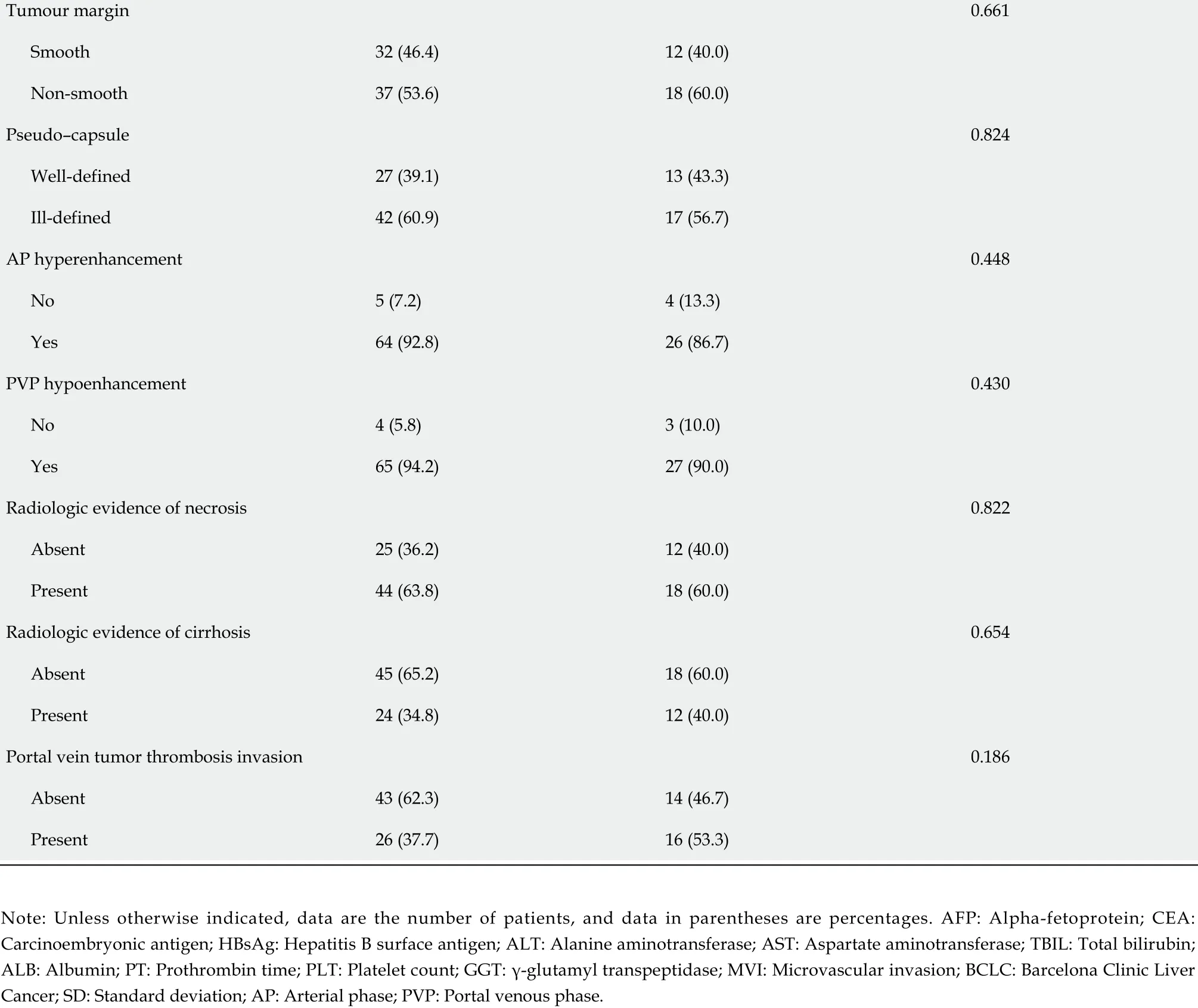
Tumour margin 0 .661 Smooth 32 (46 .4 )12 (40 .0 )Non-smooth 37 (53 .6 )18 (60 .0 )Pseudo-capsule 0 .824 Well-defined 27 (39 .1 )13 (43 .3 )Ill-defined 42 (60 .9 )17 (56 .7 )AP hyperenhancement 0 .448 No 5 (7 .2 )4 (13 .3 )Yes 64 (92 .8 )26 (86 .7 )PVP hypoenhancement 0 .430 No 4 (5 .8 )3 (10 .0 )Yes 65 (94 .2 )27 (90 .0 )Radiologic evidence of necrosis 0 .822 Absent 25 (36 .2 )12 (40 .0 )Present 44 (63 .8 )18 (60 .0 )Radiologic evidence of cirrhosis 0 .654 Absent 45 (65 .2 )18 (60 .0 )Present 24 (34 .8 )12 (40 .0 )Portal vein tumor thrombosis invasion 0 .186 Absent 43 (62 .3 )14 (46 .7 )Present 26 (37 .7 )16 (53 .3 )Note: Unless otherwise indicated, data are the number of patients, and data in parentheses are percentages. AFP: Alpha-fetoprotein; CEA:Carcinoembryonic antigen; HBsAg: Hepatitis B surface antigen; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; TBIL: Total bilirubin;ALB: Albumin; PT: Prothrombin time; PLT: Platelet count; GGT: γ-glutamyl transpeptidase; MVI: Microvascular invasion; BCLC: Barcelona Clinic Liver Cancer; SD: Standard deviation; AP: Arterial phase; PVP: Portal venous phase.
Predictive performance of the models
In the training cohort, the AUCs of the radiomics score, CR model and CRR model were 0 .754 (95 %CI:0 .640 -0 .868 ), 0 .794 (95 %CI: 0 .686 -0 .901 ) and 0 .898 (95 %CI: 0 .820 -0 .977 ), respectively. The CRR model had a significantly higher AUC than the radiomics score (P= 0 .007 ) and the CR model (P = 0 .011 ). In the validation cohort, the AUCs of the radiomics score, CR model and CRR model were 0 .704 (95 %CI: 0 .454 -0 .953 ), 0 .646 (95 %CI: 0 .411 -0 .880 ) and 0 .735 (95 %CI: 0 .505 -0 .966 ), respectively. The diagnostic performance of the radiomics score and two models is shown in Table 3 and Figure 2 C and D. The calibration curve of all the models showed excellent agreement between the predictions and observations in both the training and validation cohorts (allP> 0 .05 ) (Figure 2 E and F). The decision curve showed that the CRR model had the largest overall net benefit compared with the treat-all-patients as p-β-arrestin1 positive and treat-none patients as p-β-arrestin1 negative across the full range of reasonable threshold probabilities (Figure 4 ).
Risk stratification with p-β-arrestin1 predicted by the CRR model
According to the risk of β-arrestin1 phosphorylation predicted by the CRR model, patients with p-βarrestin1 positivity lived longer than those with p-β-arrestin1 negativity using the log-rank test (P<0 .05 ) in both the training and validation cohorts (Figure 5 ).
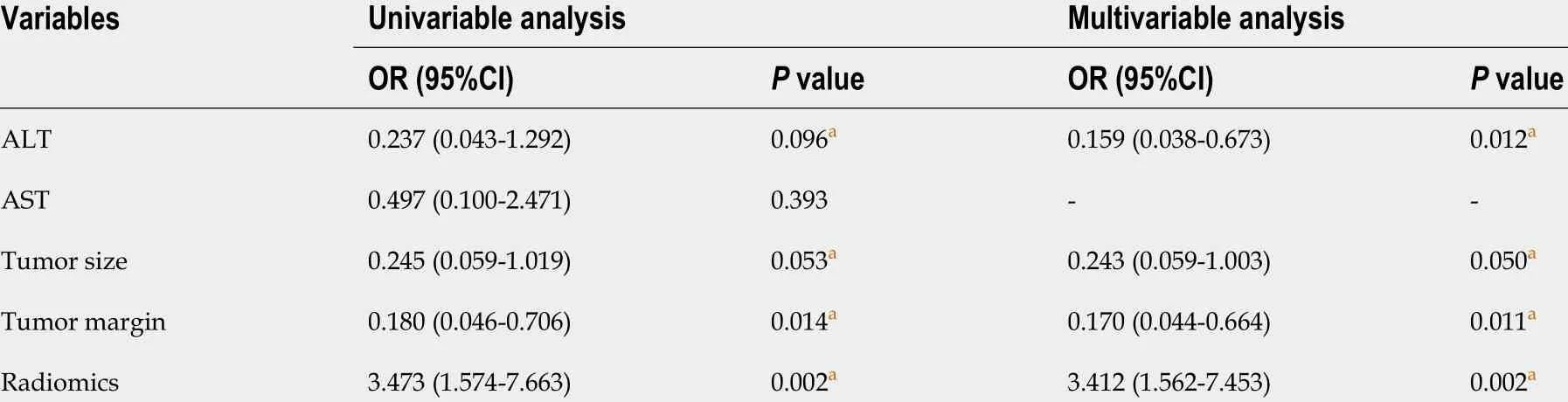
Table 2 Univariate and multivariate regression analyses of the p-β-arrestin1 -positive and p-β-arrestin1 -negative groups in the training cohort
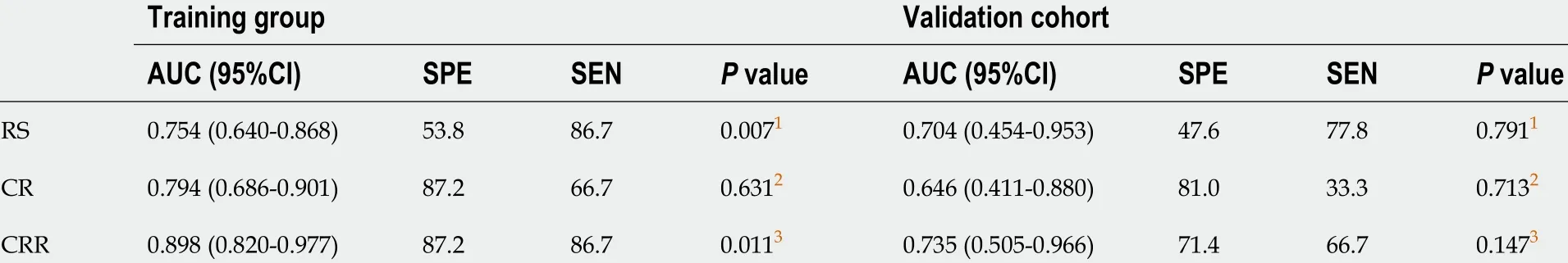
Table 3 Diagnostic performance of the three models for predicting β-arrestin1 phosphorylation-positive hepatocellular carcinoma
DISCUSSION
In this retrospective study, a CT image-based model incorporating qualitative imaging features, clinical characteristics and quantitative radiomics features for predicting β-arrestin1 phosphorylation in HCC was generated. In addition, in patients treated with sorafenib, we found that p-β-arrestin1 -positive HCC patients predicted by the CRR model were associated with better prognosis. The CRR model may serve as a noninvasive and effective tool to predict HCC patients β-arrestin1 phosphorylation status and help select patients who are suitable for sorafenib treatment.
For predicting β-arrestin1 phosphorylation in HCC patients, radiomics features provided increased power (AUC = 0 .754 ) and were indicated to be independent predictors for p-β-arrestin1 in the final CRR model (P= 0 .005 ). Utilizing the radiomics method, the proposed CRR model yielded an improved diagnostic performance in the training cohort (AUC from 0 .794 to 0 .898 ) and validation cohort (AUC from 0 .646 to 0 .735 ), indicating that the combined radiomics approach may have greater value in preoperative β-arrestin1 phosphorylation prediction than clinico-radiological features. The reason why the CRR model achieved the best predictive performance can be explained by the fact that the final model includes both qualitative and quantitative imaging features to provide a comprehensive overview of the correlations of radiomics features with HCC pathological status and genomics characteristics[25 ]. The radiomics signature includes shape, intensity, and texture information, which can reflect the complexity of the properties of the target tissue. Previous studies have shown that imaging features, including texture features, are informative of the gene expression profiles of HCC lesions,which parallels the diversity of molecular activities[26 ]. Hectorset alfound that MRI radiomics features are highly associated with HCC immuno-oncological characteristics and can serve as noninvasive predictors of its status[27 ]. A predictive nomogram incorporating a radiomics signature and other clinico-radiological factors showed a significantly improved diagnostic performance in cytokeratin19 stratification of HCC[28 ]. However, to our knowledge, no other studies have investigated the possible value of quantitative analysis integrating clinical factors in predicting β-arrestin1 phosphorylation in HCC. Developed in the training cohort and applied to the validation cohort, the radiomics score based on CECT images combined with clinico-radiological factors could correctly identify the β-arrestin1 phosphorylation status of more than 86 .7 % of the patients in the training cohort and was well validated to serve as a quantitative multiple-feature parameter for the β-arrestin1 phosphorylation-based risk stratification of HCC patients. Our study investigates the predictive aspects of computational-assisted models for the preoperative prediction of β-arrestin1 phosphorylation status, which currently can now only be attained by invasive biopsy or surgery. This computational method can guide clinical management by identifying patients for targeted therapy, as most patients recommended for systematic treatment according to the Barcelona Clinic Liver Cancer algorithm are not candidates for surgery due to their poor condition[29 ].
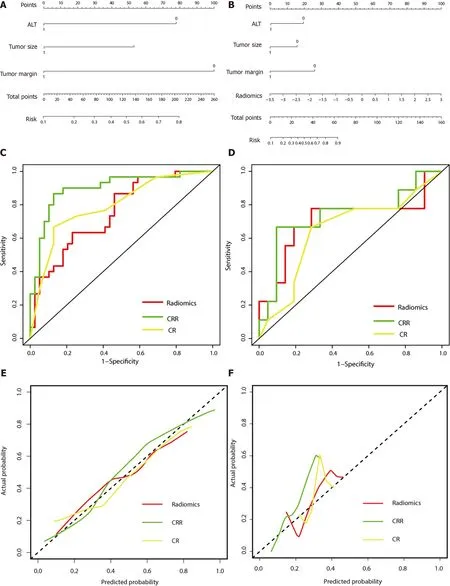
Figure 2 Performance of the three models. A: The developed clinico-radiological (CR) nomogram; B: The developed clinico-radiological-radiomics (CRR)nomogram. Predictor points are found on the uppermost point scale that corresponds to each variable. On the bottom scale, the points for all variables are added and translated into a β-arrestin1 phosphorylation positivity probability. C: Comparison of receiver operating characteristic (ROC) curves of the radiomics model, CR model and CRR model in the training cohort; D: Comparison of receiver operating characteristic (ROC) curves of the radiomics model, CR model and CRR model in the validation cohort. E: Calibration curves of the three models in the training cohort; F: Calibration curves of the three models in the validation cohort. The actual high expression of p-β-arrestin1 is represented on the y-axis, and the predicted probability is represented on the x-axis. The closer fit of the solid line to the ideal black dotted line indicates a better calibration.
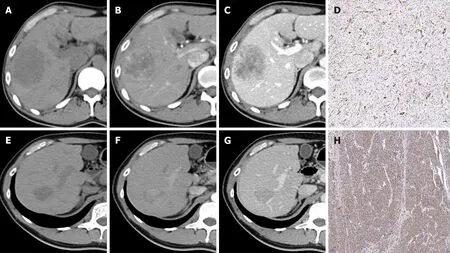
Figure 3 Representative images of contrast-enhanced computed tomography and β-Arrestin1 phosphorylation (magnification, × 100 ). A:CT images of a 45 -year-old man with a 6 .3 -cm hepatocellular carcinoma (HCC) in the right liver lobe in the plain phase; B: The tumor shows heterogeneous hyperenhancement in the arterial phase; C: The tumor shows washout at the portal venous phase with intratumor necrosis, an ill-defined capsule and a non-smooth tumor margin. D: Immunohistochemical staining shows a β-arrestin1 phosphorylation-negative status at 100 × magnification.
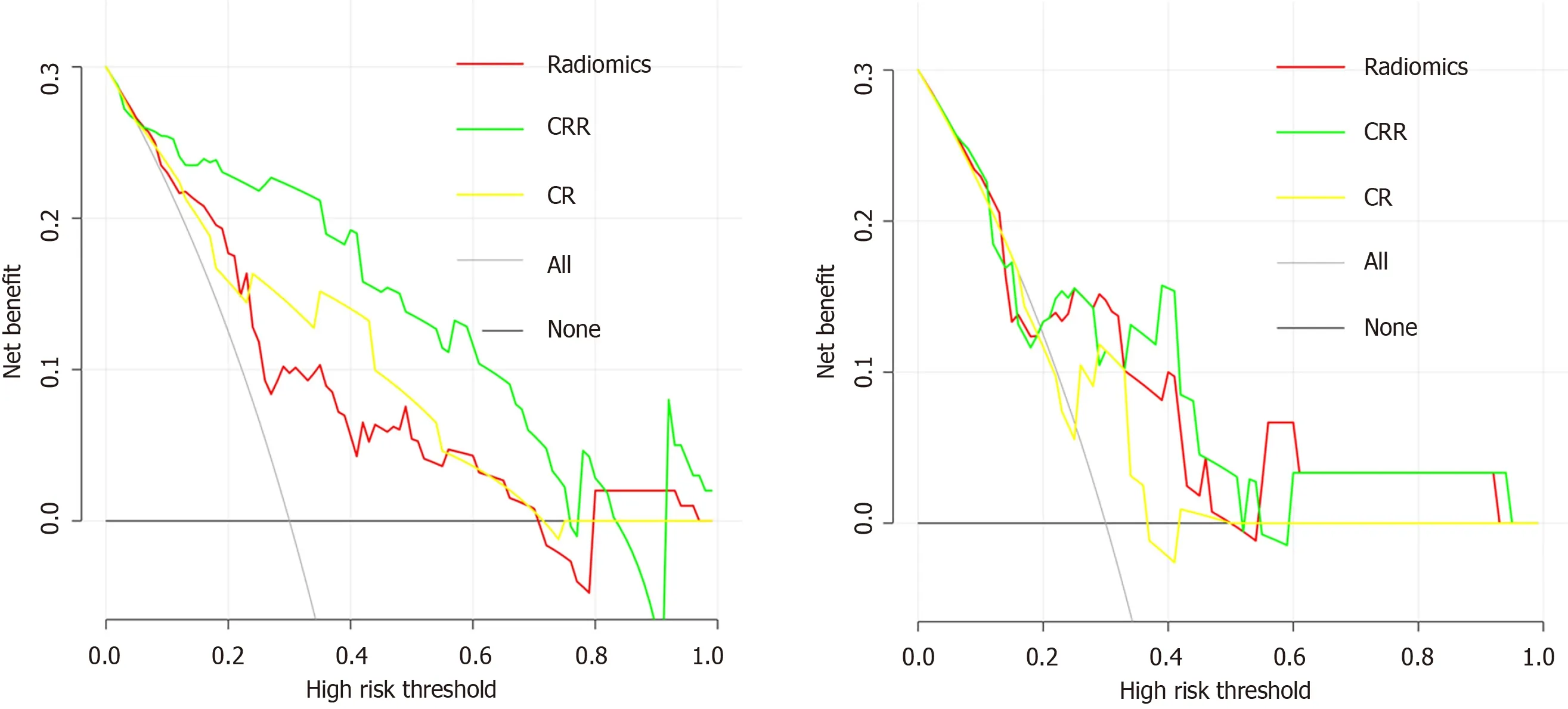
Figure 4 Decision curve analysis for each model. A: Decision curve analysis in the training cohort; B: Decision curve analysis in the validation cohort. The yaxis measures the net benefit, and the x-axis is the threshold probability. The gray line represents the hypothesis that all patients are β-arrestin1 phosphorylationpositive. The black line represents the hypothesis that all patients are β-arrestin1 phosphorylation-negative. Among the three models, the clinico-radiologicalradiomics (CRR) model provided the highest net benefit compared with the radiomics and clinico-radiological (CR) models.
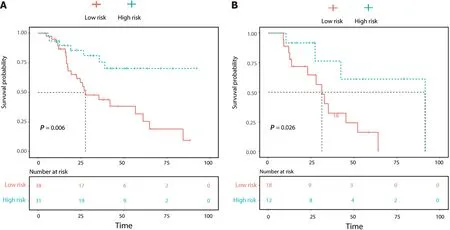
Figure 5 Overall survival (OS) curve analysis. A: The OS curve estimates by clinic-radiological-radiomics model in patients with β-Arrestin1 phosphorylation positive and β-Arrestin1 phosphorylation negative in the training cohort; B: The OS curve estimates by clinic-radiological-radiomics model in patients with β-Arrestin1 phosphorylation positive and β-Arrestin1 phosphorylation negative in the validation cohort.
The clinicopathologic features of preoperative serum ALT levels were significantly different in the p-β-arrestin1 -positive and p-β-arrestin1 -negative groups in this study. In line with the findings of previous studies, serum ALT is an important hepatic inflammation marker that is correlated with liver function.Hepatitis infection can simultaneously induce serum ALT increases and β-arrestin1 upregulation, and higher serum ALT levels are a feature commonly associated with this subtype of HCC[12 ]. In clinical practice, serum ALT levels can be easily obtained and incorporated into a radiomics model for individualized risk estimation. A larger tumor size and nonsmooth tumor margins were also shown to be associated with p-β-arrestin1 expression. This finding is in accordance with previous studies showing that β-arrestin1 can promote hepatocellular proliferationviathe Akt pathway, and HCCs with higher pβ-arrestin1 levels are more likely to have an infiltrative growth pattern[13 ]. Serum AST levels were associated with p-β-arrestin1 in the univariate analysis but not the multivariate models, probably because of a lack of statistical power due to the insufficient number of patients.
We also found that patients who were p-β-arrestin1 -positive lived longer than those who were p-βarrestin1 -negative. Previous studies have revealed that high expression of β-arrestin1 contributes to tumor survival, proliferation, angiogenesis, invasion and metastasis and is associated with the prognosis of epithelial ovarian cancer, prostate cancer and lung cancer[30 -34 ]. Although the correlation of β-arrestin1 with HCC prognosis has not been investigated, β-arrestin1 has been shown to be positively related to HCC carcinogenesis and metastasis[12 ,13 ]. Evidence has shown that the sorafenib response is impaired in HCC with dysregulated phosphorylated ERK (p-ERK) and AKT (p-AKT)activation and that suppression of ERK1 /2 increases sorafenib sensitivity in several HCC cell lines[35 -37 ], while β-arrestin1 can activate PI3 K/Akt signaling by Akt phosphorylation and trigger ERK1 /2 phosphorylation-mediated EMT in HCC. Moreover, hyperactive PI3 K/AKT signaling has been reported to be one of the primary causes of EMT in HCC resistance to sorafenib[35 ,36 ,38 ]. These studies indicate that PI3 K/AKT signaling and p-ERK1 /2 -mediated EMT signal hyperactivity may function in βarrestin1 -induced HCC resistance to sorafenib and further influence the prognosis of HCC patients treated with sorafenib, which was consistent with a series of studies recently showing that β-arrestin1 expression had some correlation with resistance to therapy in several types of cancers, such as breast[39 ]ovarian[40 ,41 ] and non-small-cell lung cancer[42 ]. Increased phosphorylation of β-arrestin1 Leads to decreased levels of dephosphorylated β-arrestin1 , which influences its function in the activation of downstream factors, such as p-ERK and p-AKT. Therefore, the phosphorylation status of β-arrestin1 has a critical role in HCC sorafenib resistance. Predicting p-β-arrestin1 can help to identify patients who are sensitive to this treatment and prevent unnecessary side effects.
There were some limitations in our study. First, this was a retrospective longitudinal cohort study and selection bias may exist due to the strict inclusion criteria. Although we performed internal validation, additional external validation is needed to facilitate the wider use of this predictive model.Second, our study was performed at a single institution, and the CT scanner in this study was not fixed in their protocol. However, this could be a strength in terms of the generalizability of the findings by reflecting actual clinical practice. Third, p-β-arrestin1 positivity was defined as a cutoff of 5 % for tumor cells to avoid false-positive results. The association between our predictive model and the graded degree of p-β-arrestin1 immunopositivity should be further assessed.
CONCLUSION
In conclusion, CECT-based radiomics combining clinico-radiological factors achieved desirable results in the prediction of β-arrestin1 phosphorylation in HCC which showed prognostic value in patients treated with sorafenib. This finding suggests that CT radiomics may provide promising and noninvasive biomarkers for the evaluation of p-β-arrestin1 expression and may help identify the subset of HCC patients who are more sensitive to sorafenib treatment, thus potentially guiding personalized treatment strategies.
ARTICLE HIGHLIGHTS

FOOTNOTES
Author contributions:Che F, Xu Q, Shi YJ and Song B designed the research; Che F, Li Q and Xu Q conducted literature search and analysis; Yang CW, Huang ZX, Wang LY and Wei Y provided material support; Song B provided funding for the article; Che F and Xu Q wrote the paper; Che F and Xu Q contributed equally to this work.
Supported bythe Science and Technology Support Program of Sichuan Province, No. 2021 YFS0144 and No.2021 YFS0021 ; China Postdoctoral Science Foundation, No. 2021 M692289 ; and National Natural Science Foundation of China, No. 81971571 .
Institutional review board statement:This study was approved by the Ethics Committee of West China Hospital.
Informed consent statement:Patients were not required to give informed consent to the study because this retrospective study used anonymous clinical data that were obtained after each patient agreed to treatment by written consent.
Conflict-of-interest statement:We have no financial relationships to disclose.
Data sharing statement:No additional data are available.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4 .0 ) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4 .0 /
Country/Territory of origin:China
ORCID number:Feng Che 0000 -0002 -8008 -2696 ; Qing Xu 0000 -0001 -9438 -5535 ; Qian Li 0000 -0001 -8330 -7654 ; Zi-Xing Huang 0000 -0001 -7967 -5948 ; Cai-Wei Yang 0000 -0003 -3335 -3948 ; Li-Ye Wang 0000 -0002 -5535 -4514 ; Yi Wei 0000 -0003 -3993 -9747 ; Yu-Jun Shi 0000 -0003 -0494 -6023 ; Bin Song 0000 -0002 -7269 -2101 .
S-Editor:Wu YXJ
L-Editor:A
P-Editor:Yu HG
 World Journal of Gastroenterology2022年14期
World Journal of Gastroenterology2022年14期
- World Journal of Gastroenterology的其它文章
- Comment on review article: Chronic hepatitis C virus infection cascade of care in pediatric patients
- Viral hepatitis: Past, present, and future
- Syngeneic implantation of mouse hepatic progenitor cell-derived three-dimensional liver tissue with dense collagen fibrils
- Osteosarcopenia in autoimmune cholestatic liver diseases: Causes, management, and challenges
- Endoluminal vacuum-assisted therapy to treat rectal anastomotic leakage: A critical analysis
- Comments on “Effect of type 2 diabetes mellitus in the prognosis of acute-on-chronic liver failure patients in China”
