Syntheses, Crystal Structures and DNA-Binding Properties of Zn(II) and Mn(II) Complexes Based on Imidazole Derivatives and Carboxylic Acid
GUAN Hui-Chao SONG Xiao-Tong
LIU Gui-Bao YUE Shu-Mei①
(College of Chemistry, Changchun Normal University, Changchun 130032, China)
ABSTRACT Complexes [Zn(pbm)(5-hip)3] (1), [Zn(pbm)(5-nip)3] (2), [Mn(pbm)(H3btc)2(H2O)] (3) and[Mn(pbm)(5-nip)3] (4), where H2HIPA = 5-hydroxyisophthalic acid, H2nip = 5-nitroisophthalic acid, H3btc = trimesic acid and pbm (pyridine benzene chelate material) = 2-(2-pyridyl)benzimidazole, were identified via single-crystal XRD analyses. 1, 2 and 4 pertain to the monoclinic space group C2/c, while 3 belongs to the triclinic space group P1. The interplay of CT-DNA with those complexes was delineated using ultraviolet, fluorescence, and circular dichroism (CD) spectroscopy and viscosity measurements. Complexes 1, 2, 3 and 4 interact with CT-DNA in an electrostatic or grooving mode. We wish to offer a theory-wise foundation for developing anti-tumor medicines.
Keywords: single-crystal structures, spectroscopy, CT-DNA binding, 2-(2-pyridyl)benzimidazole;
1 INTRODUCTION
The researches on the designing and synthesis of metallic complexes have aroused extensive academic interest, which focus on the studies on worldwide coordination chemistry.Those derivatives of imidazole heterocyclic N-donor ligands have attracted substantial attention due to their frameworks and reactivity in the complex synthesis process as well as their magnetic and luminescent properties[1-6]. Nitrogen-containing heterocyclic metal complexes with special chemical structures and unique physicochemical features have been applied in functional materials, medicines and other fields[7-13]. At present, the antitumor activity of transition metal complexes is studied from the interaction modes between transition metal complexes and DNA. Researches on the DNA binding of metallic complexes, which is a vital and major challenge in life science, are particularly important for the exploration of DNA molecular probe and new therapeutic reagents. The interactions of metal complexes with DNA mainly include noncovalent interactions, covalent interactions and cutting action intercalation. Among them, the possible binding modes of the complexes to DNA include noncovalent interactions[14-18]. At present, substantial research about the interplay process of metallic ion metal complexes and DNA has been finished[19-22]. Sama has reported that mononuclear Cu complexes have the apparent binding ability towards CT-DNA[23]. Bian Lin adopted agarose gel electrophoresis for researching PBR322 DNA cleavage with complexes[24]. The interplay of metal compounds with DNA has been broadly researched. Zhou Qing-Huaet al.reported imidazole metal complexes. Meanwhile, the interplay of compounds and calf thymus DNA was explored[25-27]. Thus, the binding modes of metal complexes and DNA have become an academic hotspot.
Our research team has committed to exploring the synthetic method of metallic complexes and their DNA-binding properties[28]. Qi Shuang et al. reported the success in synthesizing transition metal compounds with 2-(2-pyridyl)benzothiazole as ligands in 2018. Besides, a study revealed the binding properties exhibited by these complexes with DNA[29].
In this research, our team reported the synthetic process and structural characterization of Zn(II) and Mn(II) complexes having 2-(2-pyridinyl)benzimidazole as a ligand. Interactions between four complexes and calf thymus DNA were investigated by means of spectroscopy (ultraviolet spectrum, fluorescence spectrum and circular dichroism spectrum)and viscometric analysis.
2 EXPERIMENTAL
2. 1 Materials
All chemicals and solvents were applied without purification unless noted. Aladdin provided 5-hydroxyisophthalic acid, 5-nitroisophthalic acid, 2-(2-pyridyl)benzimidazole,trimesic acid, tris-HCl and CT-DNA. The CT-DNA was refrigerated at 2~8 °C and a TU-1901 spectrophotometer was used to measure visible and ultraviolet photoelectron absorption spectra. Besides, fluorescence was measured by a RF-5301PC spectrometer.
2. 2 Synthetic processes of complexes 1~4
2. 2. 1 Synthetic process of [Zn(pbm)(5-hip)3] (1)
The mixture with ZnCl2·2H2O (0.1 mmol, 0.0197 g), DMF(4 mL), 2-(2-pyridyl)benzimidazole (0.2 mmol, 0.3900 g), H2O(2 mL), and 5-hydroxyisophthalic acid (0.1 mmol, 0.0182 g)was placed into a stainless-steel autoclave which was heated under 80 °C for seventy-two hours. When the reactor was cooled towards room temperature, the pale yellow crystals of complex 1 were obtained with 61% productivity on the basis of pbm. Anal. Calcd. (%) in C22H13N4O5Zn: H, 2.72; C, 55.23; N,11.72. Found (%): H, 2.70; C, 55.14; N, 11.43.
2. 2. 2 Synthesis process of [Zn(pbm)(5-nip)3] (2)
A mixture of 2-(2-pyridyl)benzimidazole (0.3900 g, 0.2 mmol) and 5-nitroisophthalic acid (0.0211 g, 0.1 mmol) was added into 5 mL water solution of ZnCl2·2H2O (0.1 mmol,0.01757 g). The mixture was put into the aforesaid reactor and heated under 160 °C for three days. The reactor was cooled towards room temperature to produce yellow crystals of complex 2 with 62% yield on the foundation of pbm. Anal.Calcd. (%) for C21H12N3O6Zn: H, 2.57; C, 53.88; N, 8.98.Found (%): H, 2.46; C, 53.54; N, 8.63.
2. 2. 3 Synthetic process of [Mn(pbm)(H3btc)2(H2O)] (3)
To his dismay and astonishment1 he found a Giant lying at the entrance of the wood; he was about to run off as fast as his legs could carry him, when the Giant called out: Don t be afraid, I won t harm you
2-(2-Pyridyl)benzimidazole (0.3900 g, 0.2 mmol) and trimesic acid (0.0214 g, 0.1 mmol) were supplemented into 5 mL water of MnCl2·2H2O (0.1 mmol, 0.0197 g). The mixture was put into the aforesaid reactor and heated under 160 °C for 72 hours. The reactor was cooled to room temperature,and the colorless crystals of complex 3 were acquired with 60% yield based on pbm. Anal. Calcd. (%) for C21H14N3O7Mn: H, 2.95; C, 53.02; N, 8.84. Found (%): H,2.46; C, 52.14; N, 8.23.
2. 2. 4 Synthetic process of [Mn(pbm)(5-nip)3] (4)
Complex 4 was obtained in roughly the same way as complex 2. ZnCl2·2H2O (0.01757 g, 0.1 mmol) was utilized by taking the place of MnCl2·2H2O (0.0197 g, 0.1 mmol). It gained light yellow crystals of complex 4 with 62% yield on the foundation of pbm. Anal. Calcd. (%) for C20H12N4O6Mn:H, 2.61; C, 52.26; N, 12.19. Found (%): H, 2.46; C, 52.14; N,12.09.
2. 3 X-ray crystallographic analysis
Single-crystal XRD data for complexes 1~4 were documented via the Bruker Apex CCD diffractometer apparatus,and the graph item is monochromatism radiation with 296(2)K. Multistage technology was used for absorption correction.All structures were settled by Direct Method of SHELXS-97,and specified with the full-matrix least-squares method using the SHELXL-97 program in wings[30]. Table 1 shows the summary of experimental details, crystal data as well as refinement findings. Tables 2~5 list the selected bond lengths together with the bond angles.
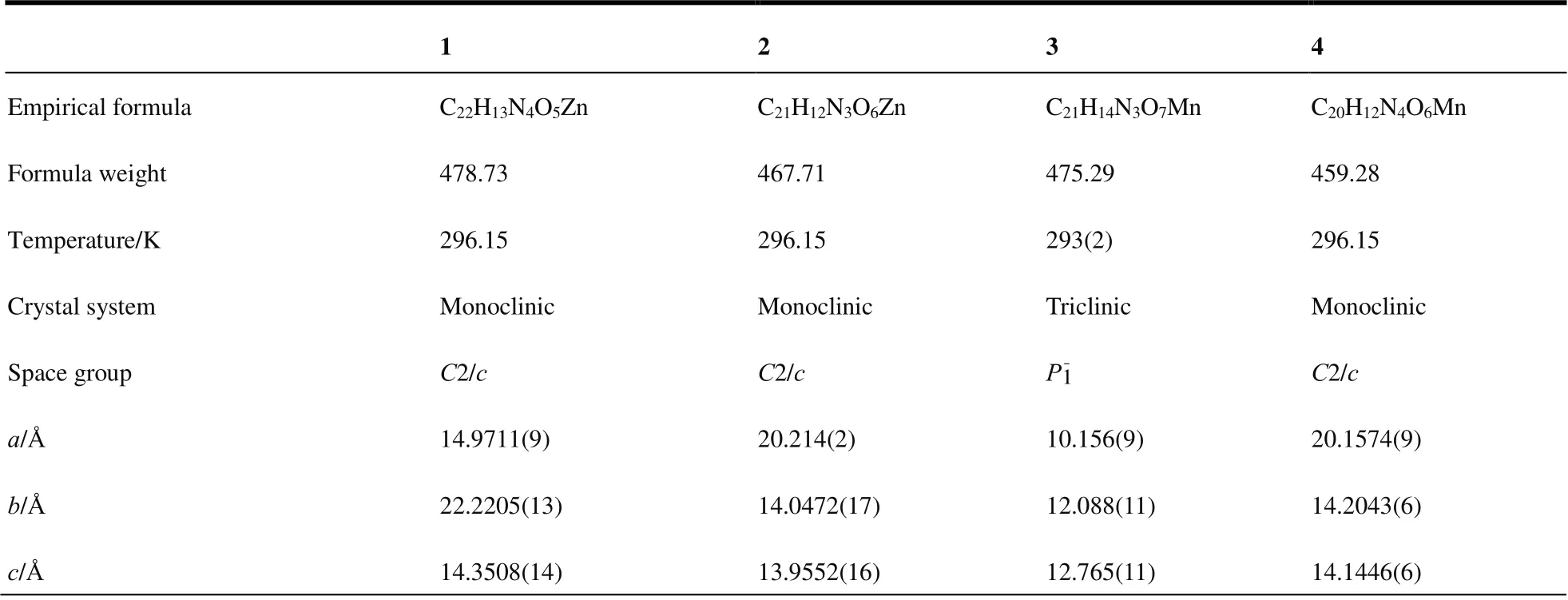
Table 1. Crystal Data and Structure Refinement for Complexes 1~4

α/° 90 90 112.668(15) 90 β/° 105.0120(10) 113.863(2) 102.560(17) 114.0330(10)γ/° 90 90 96.464(17) 90 Volume/?3 4611.1(6) 3623.8(7) 1377(2) 3698.8(3)Z 8 8 2 8 ρcalc (g/cm3) 1.379 1.715 1.146 1.649 μ/mm-1 1.104 1.405 0.516 0.764 F(000) 1944.0 1896.0 484.0 1864.0 Crystal size/mm3 0.25 × 0.2 × 0.2 0.12 × 0.11 × 0.1 0.14 × 0.13 × 0.1 0.13 × 0.11 × 0.1 Radiation MoKα (λ = 0.71073) MoKα (λ = 0.71073) MoKα (λ = 0.71073) MoKα (λ = 0.71073)2θ range for data collection/° 3.666 to 52.744 3.642 to 62.534 3.742 to 55.59 3.622 to 56.54 Reflections collected 14728 15504 7023 13478 Independent reflections 4714 (Rint = 0.0397,Rsigma = 0.0440)4580 (Rint = 0.0178,Rsigma = 0.0195)2524 Data/restraints/parameters 4714/42/318 5730/4/292 5991/394/305 4580/0/328 Goodness-of-fit on F2 1.085 1.064 1.119 1.105 5730 (Rint = 0.0456,Rsigma = 0.0626)5991 (Rint = 0.0719,Rsigma = 0.1668)Final R indexes (I > 2σ(I)) R = 0.0534 R = 0.0562 R = 0.1353, R = 0.0347 Final R indexes (all data) R = 0.0718 R = 0.1042 R = 0.2209 R = 0.0461 wR (all data) 0.1721 0.1771 0.3940 0.1131 Largest diff. peak/hole / e·?-3 1.29/-0.39 0.87/-0.87 1.42/-1.11 0.40/-0.38

Table 2. Selected Bond Lengths (?) and Bond Angles (°) of Complex 1
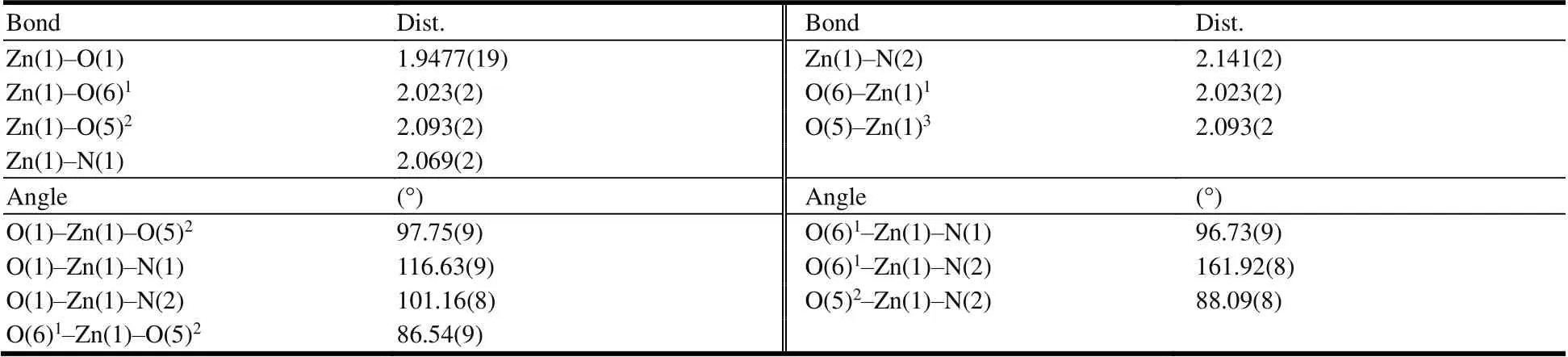
Table 3. Selected Bond Lengths (?) and Bond Angles (°) of Complex 2

Table 4. Selected Bond Lengths (?) and Bond Angles (°) of Complex 3

Table 5. Selected Bond Lengths (?) and Bond Angles (°) of Complex 4
2. 4 DNA binding studies
DNA binding experiments included ultraviolet, fluorescent,and CD spectroscopic analyses and viscometric analyses. The level of CT-DNA should be continuously increased and was added into the mixed solution when measuring the ultraviolet spectroscopy and fluorescence spectrum. The measuring of viscosity was completed via an Ubbelohde viscometric device in the water bath sustained at 20 °C to increase the concentration of the metal complex.
3 RESULTS AND DISCUSSION
3. 1 Crystal structure description
Single-crystal X-ray diffraction analyses reveal that complex 1 crystallizes in space groupC2/c. The asymmetric unit of 1 contains one central metal atom Zn, three HIPA2-anions,and one pbm ligand, as shown in Fig. 1. The Zn2+center is regarded as a typical five-coordinate environment coordinated with three oxygen atoms from three HIPA2-anions and two nitrogen atoms from a pbm ligand. The Zn-O and Zn-N bonds are various from 1.981(3) to 2.019(3) ? and from 2.071(4) to 2.275(4) ?, which can be compared to the reported complexes. The asymmetric units are linked together through bridging acid ligand with two different coordination modes from two carboxylic groups, i.e.,μ1-?1andμ2-?2bidentate bridging respectively, creating an infinite chain running along with the [101] orientation, as depicted in Fig. 2.Besides, the chelating pbm ligand with a dihedral angle of 2.016(92)° is nearly parallel to each other.
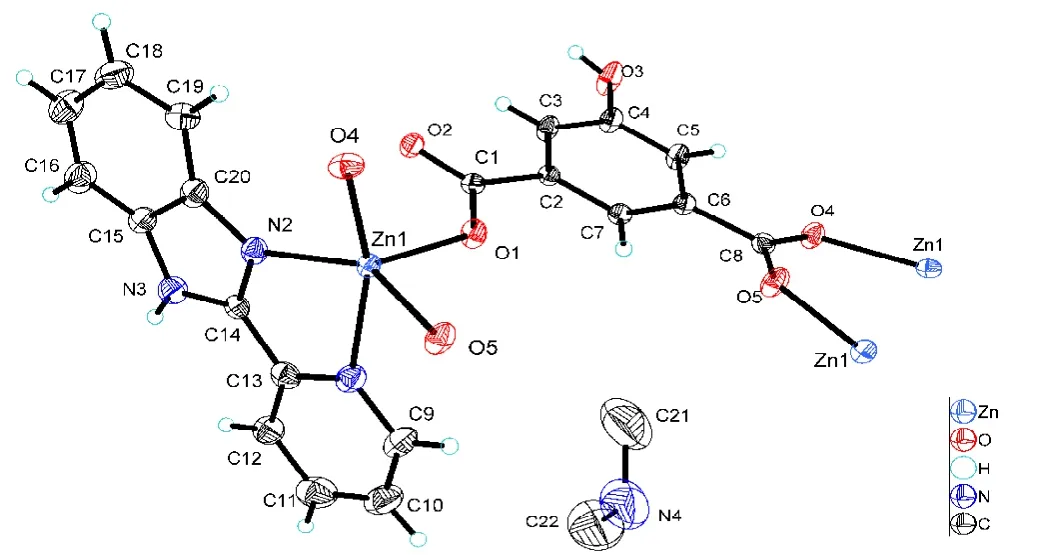
Fig. 1. Molecular structure of complex 1 showing 50% displacement ellipsoids
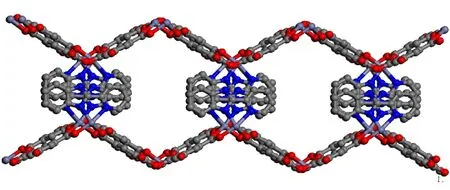
Fig. 2. Packing diagram of complex 1 in a unit cell viewed along the a axis
The geometry of the Zn center is between the ideal square pyramidal (SP) and trigonal bipyramidal (TBP) configuration withτindex of 26.65% (τ= 0% for perfect SP andτ= 100%for ideal TBP geometry). Thus, the Zn center could be regarded as SP coordination geometry with some distortion, in which the O(1) atom acts as the apical axis, and N(1), N(2),O(4) and O(5) atoms as the low plane. The adjacent angles in the equatorial plane atoms are 82.2(2)°~93.45(19)°.

Fig. 3. Molecular structure of complex 2 showing 50% displacement ellipsoids

Fig. 4. Packing diagram of complex 2 in a unit cell viewed along the a axis
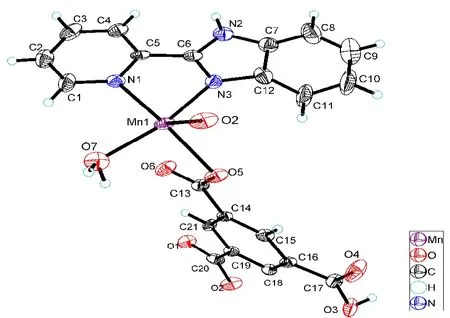
Fig. 5. Molecular structure of complex 3showing 50% displacement ellipsoids

Fig. 6. Molecular structure of complex 4 showing 50% displacement ellipsoids
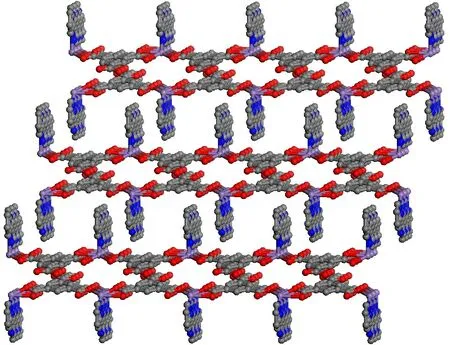
Fig. 7. Packing diagram of complex 3 in a unit cell viewed along the a axis

Fig. 8. Packing diagram of complex 4 in a unit cell viewed along the a axis
Complexes 3 and 4 crystallize in monoclinic space groupP1andC2/c,respectively. The asymmetric units of them contain one pbm ligand. Besides, complex 3 consists of one crystallographically unique Mn(II) atom, one pbm ligand,two carboxylic acid ligands and one coordinated water molecule. Complex 4 contains one center metal atom Mn, three nip2-anions and one pbm ligand, as shown in Figs. 5 and 6.As a typical five-coordinate environment, Mn2+coordinates with three nip2-anions, two nitrogen atoms, and three oxygen atoms in a pbm ligand. For 3, the Mn-O bonds are various from 2.180(8) to 2.237(7) ?, and the Mn-N bonds from 2.209(8) to 2.351(8) ?. For complex 4, the Mn-O bonds change from 2.0291(14) to 2.1415(15) ? and the Mn-N bonds fall in the 2.1853(14)~2.2446(15) ?. In fact, the oxygen atoms on two carboxyl groups connect two Mn(II) ions in order to form the 1D chain. In addition, neighboring one-dimensional chains are linked with a nitrogen atom to generate a two-dimensional plane (Figs. 7 and 8).
3. 2 Characterization of the complex
3. 2. 1 Thermal analysis
The thermostability of complexes 1~4 was investigated by TGA measurement (Fig. 9), finished using specimens comprising substantial single crystals of complexes 1~4 in N2environment at a heating rate of 10 °C·h-1. For 1, no apparent weight loss was observed before the decomposition of the framework occurring at ca. 325 °C, with the remaining weight corresponding to the formation of ZnO. Complex 1 shows a weight loss of 83.08%. For 2, the framework decomposes at ca. 340 °C. The remaining weight corresponds to the formation of ZnO. For 3, the organic composition is decomposed completely at ca. 380 °C, and the remaining weight comes from the formation of MnO. Complex 3 shows a weight loss of 84.5%. Complex 4 experienced a two-step weight loss. The first one at about 296 °C with a weight loss from 296 to 416 °C is due to the decomposition of carboxylic acid ligand with a weight loss of 46.44% (theoretical value:46.83%). The second one between 416 and 513 °C is 35.61%,corresponding to the decomposition of nitrogen-containing ligand (theoretical value: 37.78%).
3. 2. 2 XRD analysis
The experimental and simulated powder XRD patterns of complexes 1~4 are shown in Fig. 10, indicating the practical powder of XRD patterns is consistent with the powder XRD patterns simulated according to the structural data, and the pure phase of the synthesized product is determined.

Fig. 9. TG curves of complexes 1~4

Fig. 10. Simulated and experimental XRPD patterns of complexes 1~4
3. 3 Absorption spectral studies of DNA binding
Ultraviolet spectroscopy plays an essential role in detecting the interaction mode between complexes with DNA[31]. Under normal circumstances, the absorption peak of the complex will show a decreased intensity, and the wavelength will exhibit an obvious red shift phenomenon after adding the DNA[32]. In the meantime, the energy level decreased after coupling leads to the weakening ofπ-π* transition and the red shift phenomenon. In addition, when molecules react with DNA in the groove mode, the wavelength is red-shifted, and the color decrease role is not distinct[33,34]. Meanwhile, with the increase of DNA concentration, the DNA helical structure is destroyed due to the combination of the complex, and its absorption spectrum is increased artificially with the rise of DNA concentration[35]. The UV-visible spectra were measured after the interaction of complexes 1~4 of DNA, as shown in Fig. 11. The picture shows that the maximum absorption peak of the complex increases with increasing the amount of DNA. The complexes have an excellent absorption band at 310 nm, but there is no blue or red shift. Hence, these complexes may have electrostatic effects on DNA.

Fig. 11. Absorption spectra of complexes 1~4, corresponding to (a) to (d) in turn, in tris-HCl buffer (pH 7.0)in the absence and presence of increasing amount of DNA at room temperature
3. 4 Fluorescent fluorescence measurements of DNA binding
The interplay between the complex and CT-DNA was studied through emission titration. In complex solution with a specific concentration, the emission spectra of complexes 1~4 are shown in Fig. 12. With increasing the concentration of CT-DNA, the complex shows strong fluorescence at 395 nm when excited at 285 nm at room temperature. In addition,when the concentration of CT-DNA is increased to a certain amount, the fluorescence emission intensity increases continuously due to the environment of the metal complex changes and the different degree of the internal hydrophobic environment when the compound interacts with DNA, but the location of the emission band does not change significantly. It avoids the quenching effect of solvent water molecules and limits the fluidity of compounds at the binding site, thus reducing the relaxation vibration mode and increasing the emission intensity.
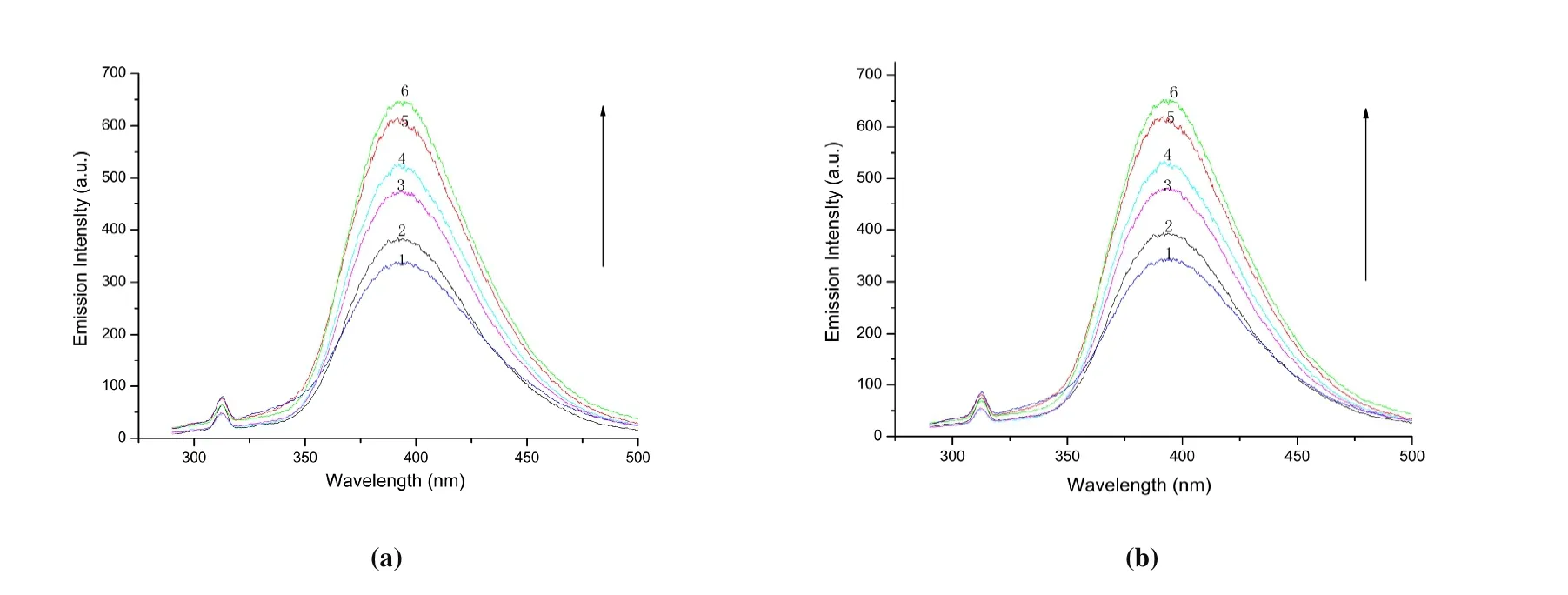

Fig. 12. Emission spectra of complexes 1~4, corresponding to (a) to (d) in turn, in tris-HCl buffer (pH 7.0)in the absence and presence of increasing amount of DNA at room temperature
3. 5 Viscosity studies of DNA binding
To study the interplay of the complexes with CT-DNA,viscosity tests were carried out. This method is more convincing than the spectroscopic method, which can better indicate whether the interaction model between the complexes and DNA is in an intercalated manner[36]. If the complex is inserted into DNA and interacted with it, the distance between the base pairs will increase to allow the ligand to enter,therefore resulting in an increase in the solution viscosity.When the complex binds to DNA in a non-insertion mode,the DNA spirals away, which shortens the length and results in little change in the viscosity of DNA at different concentrations[37,38]. The viscometer was placed in a constant temperature water bath at 30.0 ± 0.1 °C. The blank solution was 15 mL tris and the flow time (t0) was determined. Then measure the flow time (t) of 15 mL CT-DNA and metal complex mixture solution through the capillary tube in different proportions ([complex]/[DNA] = 0.0~1.0). The relative viscosity was calculated according to the following formula:?=(t - t0)/t0.
The viscosity curve of the interplay between complexes 1~4 and DNA is depicted in Fig. 13. It fluctuates up and down in a straight line, indicating that the interplay mode between the complex and DNA is electrostatic interaction or groove binding and it is very similar to the results obtained by spectral analysis.

Fig. 13. Relative viscosity of CT-DNA upon the addition of increasing amounts of complexes 1~4 (r = 0.0~1.0). η is the viscosity of DNA in the presence of complex, and η0 is the viscosity of DNA alone

Fig. 14. Circular dichromatic spectra of interactions between complexes 1~4 and DNA
3. 6 Circular dichromatic (CD) spectroscopy
CD spectroscopy is one of the methods to study the conformational change of DNA[39]. The CT-DNA has two apparent peaks in the CD spectrum. The negative peak at 246 nm is mainly caused by the right-handed helicity of DNA, and a positive peak at 272 nm is due to base stacking[40]. The interaction model of DNA and metal complex is determined by the change of peak position of the CD spectrum. If the binding model of the complex and DNA displays an insertion manner, the parts of the two peaks will change obviously. In case of electrostatic interaction or groove binding, the parts of the two peaks will not change significantly[41]. The concentration of CT-DNA is fixed at 100 μmol·L-1, and the concentration of the complex gradually increases. The reaction system is placed at room temperature for 1 h, and the circular dichroism spectrum of DNA is determined. With or without complexes 1~4, the CD spectra are shown in Fig. 14, where the negative and positive peaks at 246 and 272 nm respectively do not change significantly after interacting with DNA. The results show that complexes 1~4 only have a weak effect on the helicity and base pair stacking of DNA, and the interaction between these complexes and DNA can be determined as groove binding or electrostatic interaction.
4 CONCLUSION
In this study, four new complexes with 2-(2-pyridyl)benzimidazole as the main ligand were synthesized and their crystal structures were studied. The interaction between CT-DNA and the complex was studied by absorption spectrum, fluorescence spectrum, circular dichromatic spectroscopy and viscosity measurement. The results showed that the interaction mode between CT-DNA and the complex is electrostatic interaction or groove bonding. As these findings harbor certain potential implications for researches on anticancer drugs, we wish to offer some meaningful enlightenment for the exploration of anti-tumor medicines.
- 結(jié)構(gòu)化學(xué)的其它文章
- Two Polynuclear Fe Complexes with Boat-like Core:Syntheses, Structures and Magnetic Properties①
- A Robust Heterometallic Cd(II)/Ba(II)-Organic Framework with Exposed Amino Group and Active Sites Exhibiting Excellent CO2/CH4 and C2H2/CH4 Separation①
- Synthesis, Crystal Structure, Spectroscopy and Hirshfeld Analysis of 4,6-Diamino-2-cyclopropylaminopyrimidine-5-carbonitrile with Different Solvents: N,N-dimethylformamide, Methanol and Water①
- CoMFA Study on Anti-proliferative Activity of Fluoroquinolone Amide Derivatives①
- Synthesis and Properties of Dinuclear Europium(III)Complex Containing 2-Benzoylbenzoic Acid①
- Mechanism Study of Aliskiren and Its Analogues by Molecular Dynamic Simulation①

