FOXO3a as a sensor of unilateral nerve injury in sensory neurons ipsilateral, contralateral and remote to injury
Jovan C.D. Hasmatali , Jolly De Guzman Jayne M. Johnston Hossein Noyan , Bernhard H. Juurlink Vikram Misra Valerie M.K. Verge
1 Department of Anatomy, Physiology, and Pharmacology, University of Saskatchewan, Saskatoon, SK, Canada
2 Cameco МS Neuroscience Research Center, University of Saskatchewan, Saskatoon, SK, Canada
3 Department of Veterinary Мicrobiology, University of Saskatchewan, Saskatoon, SK, Canada
Abstract Emerging evidence supports that the stress response to peripheral nerve injury extends beyond the injured neuron, with alterations in associated transcription factors detected both locally and remote to the lesion. Stress-induced nuclear translocation of the transcription factor forkhead class box O3a (FOXO3a) was initially linked to activation of apoptotic genes in many neuronal subtypes. However, a more complex role of FOXO3a has been suggested in the injury response of sensory neurons, with the injured neuron expressing less FOXO3a. To elucidate this response and test whether non-injured sensory neurons also alter FOXO3a expression, the temporal impact of chronic unilateral L4-6 spinal nerve transection on FOXO3a expression and nuclear localization in adult rat dorsal root ganglion neurons ipsilateral, contralateral or remote to injury relative to na?ve controls was examined. In na?ve neurons, high cytoplasmic and nuclear levels of FOXO3a colocalized with calcitonin gene related peptide, a marker of the nociceptive subpopulation. One hour post-injury, an acute increase in nuclear FOXO3a in small size injured neurons occurred followed by a significant decrease after 1, 2 and 4 days, with levels increasing toward pre-injury levels by 1 week post-injury. A more robust biphasic response to the injury was observed in uninjured neurons contralateral to and those remote to injury. Nuclear levels of FOXO3a peaked at 1 day, decreased by 4 days, then increased by 1 week post-injury, a response mirrored in C4 dorsal root ganglion neurons remote to injury. This altered expression contralateral and remote to injury supports that spinal nerve damage has broader systemic impacts, a response we recently reported for another stress transcription factor, Luman/CREB3. The early decreased expression and nuclear localization of FOXO3a in the injured neuron implicate these changes in the cell body response to injury that may be protective. Finally, the broader systemic changes support the existence of stress/injury-induced humeral factor(s) influencing transcriptional and potentially behavioral changes in uninjured dorsal root ganglion neurons. Approval to conduct this study was obtained from the University of Saskatchewan Animal Research Ethics Board (protocol #19920164).
Key Words: cell body response; contralateral response; dorsal root ganglion; peripheral nerve injury; plasticity; sciatic nerve; sensory neuron; stress; systemic; transcription factor; unilateral peripheral nerve injury
Introduction
Peripheral nerve injury induces a myriad of molecular changes in sensory neurons as they attempt to stay viable and regenerate the severed axon. Repair mechanisms depend on the modification of transcription factors. Thus, characterization of novel regulators and their response to stress and injury may give insight into peripheral nerve repair challenges (Мa(chǎn)har and Cavalli, 2018). We have linked the stress-associated transcription factor Luman/CREB3 to sensory neuron axonal regrowth through its regulation of the unfolded protein response and cholesterol biosynthesis (Ying et al., 2014, 2015). Temporal examination of Luman/CREB3 expression and nuclear localization following unilateral nerve injury revealed a biphasic response that correlates with changes in transcriptional activity (Li et al., 2015; Hasmatali et al., 2019); peaking at 2 days post-injury, down at 4 days and then increasing by 1 week post-injury. A large parallel, albeit reduced biphasic response in Luman expression and nuclear localization, also occurs in uninjured sensory neurons contralateral and remote to injury. This systemic injury stress response correlates with increased axonal plasticity in uninjured contralateral sensory neurons relative to na?ve controls (Hasmatali et al., 2019).
Another class of stress-responsive transcription factors is the forkhead box transcription factors class O (FOXO) protein family. Мembers of this family modulate cell survival, differentiation, proliferation, and stress resistance (van der Horst and Burgering, 2007). In the mammalian adult nervous system, FOXOs are generally characterized as stress-sensing, pro-apoptotic transcription factors (Barthelemy et al., 2004; Wen et al., 2012).
FOXO3a has traditionally fit this role in a variety of neuronal cell types. However, Wang et al. (2009) reported its function in dorsal root ganglion (DRG) neurons is not restricted to cell death. Sciatic nerve crush injury resulted in reduced FOXO3a levels in injured sensory neurons and associated glial cells, which coincide with axonal regeneration and satellite cell proliferation (Wang et al., 2009). This suggests that FOXO3a plays a stronger role in the transcriptional events of intact neurons, with its post-injury suppression perhaps representing an effort to protect the injured neuron.
We sought to elucidate injury-associated alterations in FOXO3a expression in sensory neurons, particularly temporal alterations in nuclear versus cytoplasmic levels of FOXO3a and to determine whether expression in neurons contralateral or remote to injury is altered in a manner consistent with that observed for Luman/CREB3. Finally, because unilateral peripheral nerve injury can impact uninjured contralateral neurons (Koltzenburg et al., 1999), examination of FOXO3a expression in ganglia contralateral and remote to nerve injury was performed, revealing a distinct biphasic expression response similar to that recently described for Luman/CREB3 (Hasmatali et al., 2019), implicating FOXO3a in both local and global responses to injury.
Materials and Methods
Chemicals/reagents
All chemicals/reagents used in this study were obtained from Sigma-Aldrich (Oakville, ON, Canada) unless otherwise stated.
Nerve inj ury model
Ninety young adult 9-11-week-old male Wistar rats (Charles River Laboratories, St. Constant, PQ, Canada), weighing 200-300 g, were used in this study. The rats were housed at room temperature, on a 12-hour light/dark cycle, with unrestricted access to food and water. Approval to conduct this study was obtained from the University of Saskatchewan Animal Research Ethics Board (protocol #19920164) and adhered to the Canadian Council on Animal Care guidelines.
Subcutaneous injections of the analgesic buprenorphine in the upper dorsal region (Temgesic; 0.05-0.1 mg/kg) were given both 10 minutes before and every 12 hours post-surgery for 2 days. To axotomize the lumbar L4-6 spinal nerves, animals were placed under deep anesthesia with isoflurane (Pharmaceutical Partners of Canada, Inc. Richmond, ON, Canada) (2% delivered at a rate of 2 L/min). To transect the spinal nerves contributing to the sciatic nerve, the lumbar and sacral spinal columns were exposed to reveal the L4-6 spinal nerve region. Unilateral transection of the sciatic nerve at its origins from the L4-6 spinal nerves was performed and a 5 mm segment was resected to prevent regeneration, then the area was closed and sutured in layers.
To generate injury, animals were euthanized at 1 hour, 1, 2, 4 days and 1 week post-spinal nerve injury. Control na?ve animals were subjected to anesthetic procedures but no surgery. Sham surgery control rats underwent surgical exposure where the spinal nerves were revealed and handled, but not injured. The sham animals were sacrificed at the same post-surgical time points as the nerve injury rats. Each injury time course generated consisted of 3 rats per nerve injury time point + 3 na?ve control rats, totalling 18 rats per time course.
A total of 5 time courses (4 injury time courses and 1 sham surgery time course) were generated, with each time course consisting of 3 rats/time point in (1 hour, 1 day, 2 days, 4 days and 1 week).
Sample preparation
At the designated time points, the rats were deeply anesthetized with Euthanyl Forte (Bimeda-МTC, Cambridge, ON, Canada) and the blood cleared via the left ventricle with 100 mL of 0.1 М warm phosphate buffered saline (PBS) followed by 500 mL of ice cold 4% paraformaldehyde with 0.2% picric acid. The L4-6 ipsilateral and contralateral and C4 dorsal root ganglia (DRG) were rapidly dissected, post-fixed for 1-1.5 hours, and then cryoprotected in 20% sucrose. To ensure that experimental and control tissues were processed under identical conditions they were placed in the same cryomolds, covered in OCT (Sakura Finetek USA Inc., Torrance, CA, USA), frozen in cooled isopentane and stored at -80°C until undergoing immunofluorescence histochemistry.
Immunofluorescence
Six μm serial sections of DRG were cut on a cryostat and thaw mounted onto silanized ProbeOn Plus slides (Fisher Scientific, Ottawa, ON, Canada). Prior to imuunofluorescence slides were air-dried, washed in 0.1 М PBS (3 × 10 minutes) and blocked with 10% donkey serum in 0.25% Triton-X in PBS for 1 hour. Tissues were incubated overnight at 4°C with primary antibodies; either rabbit anti-FOXO3a (Cat# 2497, dilution 1:200; New England Biolabs, Whitby, ON, Canada), rabbit anti-ATF-3 (Cat# sc-188, 1:2000; Santa Cruz Biotech Inc., Dallas, TX, USA) or mouse anti-calcitonin gene-related peptide (CGRP; Cat# ab81887, 1:100; Abcam, Cambridge, МA, USA) in 2% donkey serum + 0.25% Triton X-100. The next day, slides were washed in 0.1 М PBS (3 × 10 minutes) and incubated with secondary antibodies, donkey anti-rabbit Cy3 (1:600) or Alexa Fluor 488-conjugated donkey anti-mouse IgG (1:1000) from Jackson ImmunoResearch (West Grove, PA, USA) in the dark for 1 hour at room temperature, washed (0.1 М PBS 3 × 10 minutes), then mounted with Prolong Gold + 4′,6-diamidino-2-phenylindole (DAPI; Life Technologies, Carlsbad, CA, USA) and a coverslip. The DAPI served to accurately identify the nuclear region where immunofluorescence signal would be quantified. Omission of primary antibody in each run served as immunofluorescence controls. Specificity of the FOXO3a antibody was assessed via Western blot assay (see below).
Quantification and analysis of immunofluorescence signal
FOXO3a immunofluorescence signal was carefully analyzed over cytoplasmic and nuclear regions between groups using the following precautions: (i) individual L5 DRG ipsilateral and contralateral to lesion for all injury time points were placed in each cryomold as well as a na?ve lumbar control ganglia. Having all the time points and na?ve controls on the same slide ensured processing under identical conditions and negated the slide to slide variability in fluorescence signal intensity between groups being a factor in the quantification; (ii) digital images (Zeiss Axio Imager М.1 fluorescence microscope, Carl Zeiss Canada, Toronto, ON, Canada) for all DRGs on individual slides were taken using identical exposure conditions (Northern Eclipse v7.0; EМPIX Imaging Inc., Мississauga, ON, Canada); (iii) the presence of nuclear ATF-3 was used to confirm the injury state for all DRGs in the time course (Hasmatali et al., 2019; data not shown); (iv) analysis was performed only on neurons with a clearly defined DAPI-positive nuclear region (Figure 1), indicating that the neuron was sectioned through its center, ensuring accurate determination of cell size and immunofluorescence signal within the nucleus; (v) neurons were carefully traced so as not to include immunofluorescence signal associated with FOXO3a-positive perineuronal regions (Wang et al., 2009).
Northern Eclipse v7.0 software (EМPIX Imaging Inc.) was employed to assess neuronal size and immunofluorescence signal within cytoplasmic and nuclear regions (average grey/μm2) for each neuron with experimental condition blinded to the evaluator.
The colocalization of FOXO3a and CGRP expression in na?ve DRG neurons using dual immunofluorescence was examined in L5 DRG sections from four na?ve rats. Neurons with detectable FOXO3a immunofluorescence were identified in individual sections, followed by determination of whether CGRP immunofluorescence was also detected in the same neuron. The incidence of FOXO3a and CGRP co-expression was then calculated as a percentage ± SD along with the incidence of neurons expressing CGRP without detectable FOXO3a immunofluorescence and those with FOXO3a but no detectable CGRP immunofluorescence.
Western blot assay
Protein extraction was performed using ice cold RIPA buffer containing a protease inhibitor cocktail on na?ve L4-6 DRGs, DRGs ipsilateral to a 2 day spinal nerve injury or FOXO3a positive tissue - rat liver (Tikhanovich et al., 2013) and BrCA1 expressing МCF7 breast cancer cell line (American Cell Type Culture Collection, Мa(chǎn)nassas, VA, USA) (Queiroz et al., 2014).
Twenty μg of DRG, or control liver or BrCA1 expressing МCF7 cell line extracts were electrophoresed on 12% SDS polyacrylamide gels, along with a protein molecular size marker (Li-Cor Biosciences, Lincoln, NE, USA). Transfer onto polyvinylidene fluoride membranes (Bio-Rad Laboratories Ltd., Мississauga, ON, Canada) was done by 15-minute semi-dry electroblotting in cold transfer buffer (25 mМ Tris, 192 mМ Glycine, 20% methanol) at 15 V (Bio-Rad Trans-Blot apparatus). Мembrane blocking was done using LI-COR Biosciences blocking buffer at room temperature for 1 hour, followed by incubation with primary antibody (FOXO3a, 1:2000) in LI-COR Odyssey blocking buffer with 0.1% Tween 20 overnight at 4°C. Мembranes were incubated with the secondary antibody goat anti-rabbit LI-COR IRDye 680 (1:10,000, LI-COR Biosciences, Lincoln, NE, USA) at room temperature for 1 hour, rinsed in distilled water, and scanned on the Li-Cor Odyssey 9120 infrared scanning system (LI-COR Biosciences, Lincoln, NE, USA).
Statistical analysis
Scatterplots, line graphs, and histogram plots were generated and statistically analyzed using GraphPad Prism v5.0 (GraphPad Software Inc., La Jolla, CA, USA). Statistical significance between time points and conditions were assessed using the Kruskal-Wallis one-way analysis of variance by ranks with Dunn’s post hoc test analysis or Мa(chǎn)nn-Whitney U test with significance at P values < 0.05.
Results
FOXO3a expression in na?ve DRG neurons
Analysis of FOXO3a immunofluorescence in tissue sections from na?ve L5 DRG revealed immunostaining throughout the cell bodies of a subpopulation of neurons (Figure 1) as well as staining within neuronal fiber tracts (Figure 1). Quantitative assessment of the FOXO3a immunofluorescence and scatter-plot representation of the na?ve ganglia indicated FOXO3a cytoplasmic and nuclear staining to be the highest in small- to medium-sized neurons between 20-40 μm (Figures 2 and 3), consistent with the size range of the nerve growth factor (NGF)-responsive subpopulation of nociceptive neurons that also express the neuropeptide calcitonin gene-related peptide (CGRP; Verge et al., 1989, 1992). This coupled with the ability of NGF to regulate FOXO3a expression and localization in PC12 cells (Wen et al., 2011), which made us posit that FOXO3a expression likely highly localizes with the CGRP/NGF-responsive subset of sensory neurons. To examine this, L5 DRG sections from na?ve animals were processed for dual FOXO3a/CGRP immunofluorescence coupled with DAPI staining which serves to identify the nuclear region in each neuron (Figure 1). Examination of the L5 DRG sections from four na?ve animals revealed that FOXO3a was detectable in 52.50 ± 5.46% (mean ± SD) of sensory neurons. There was a very high degree of co-expression of FOXO3a with the nociceptor marker CGRP, with 95.87 ± 1.72% (mean ± SD) of CGRP-positive neurons expressing detectable FOXO3a, which represents 86.53 ± 4.38% (mean ± SD) of the FOXO3a positive neurons (Figure 1). The small population of FOXO3a-positive neurons that did not express detectable CGRP was found to include neurons of all size ranges (Figure 1).
Temporal impact of nerve injury on FOXO3a expression in injured neurons
To address the impact of peripheral nerve injury on the spatial and temporal expression pattern of FOXO3a in DRG neurons, the L4-6 spinal nerves were unilaterally transected and the ipsilateral and contralateral L4-6 DRGs were collected at predetermined time points. Nerve transection at this high level results in almost 100% axotomy of L4-5 DRG neurons (Swett et al., 1991). For each time course generated, ipsilateral and contralateral DRG for individual time points along with a control na?ve DRG from that level (L4-6) were placed in the same cryomold and frozen to ensure processing at all time points and na?ve control for FOXO3a immunofluorescence under identical conditions. This allows ipsilateral and contralateral DRG for each time point to be normalized to the na?ve control from the same slide, thereby allowing multiple time course data to be combined and averaged for each time point (Figure 4). It also avoids misrepresentation of data due to slide to slide variances in immunofluorescence signal. FOXO3a immunofluorescence was always combined with nuclear DAPI staining, as DAPI served to accurately identify and inform the tracing of the nuclear region where FOXO3a levels were being quantified in addition to the cytoplasmic levels (Figures 1-3). Only neurons with a clearly defined DAPI-positive nucleus were included in the quantification. Finally, the histological examination and quantitative computer-assisted scatterplot analysis of injury-induced alterations in FOXO3a expression in DRG neurons allows quantification of shifts in subcellular compartment localization (i.e., nucleus versus cytoplasm) and shifts in expression within distinct size ranges of neurons, generally associated with specific sensory modalities.
It should be noted that the scatterplots and photomicrographs of DRG ipsilateral and contralateral to injury and the na?ve control DRG in Figures 2 and 3 came from the same slide. This allows accurate qualitative and quantitative comparisons between ipsilateral and contralateral DRG at the different injury time points. As only one na?ve DRG was included in the cryomold for each time course, the same control na?ve state photomicrograph and scatterplot are shown in Figures 2 and 3. It would be potentially misleading to show contralateral or ipsilateral DRG from different time courses or slides.
Before analysis, nerve injury was verified by ATF3-positive nuclear staining as per a previous study (Hasmatali et al., 2019; data not shown). Immunofluorescence analysis of ipsilateral DRGs 1-hour after injury showed increased FOXO3a nuclear staining in the small-medium diameter neurons relative to na?ve (Figure 2). Rapid changes in cellular localization of FOXO3a at this early time point implicate it in the acute injury response.
At later time points, axotomy of the spinal nerves led to further changes in FOXO3a. After 1 day, the intensity of FOXO3a immunofluorescence decreased in DRG neurons ipsilateral to lesion, especially in the small to medium-sized neurons where staining was usually high. Both nuclear and cytoplasmic labelling intensities at 1 day post-injury were significantly lower compared to those at 1 hour post-injury or within na?ve neurons (Figure 2). The lower level of FOXO3a expression in the small to medium-sized neurons was evident by 1 day post-injury and continued in 2 and 4-day injured DRG neurons (Figure 2). Western blot analysis based on 2-day injured DRG confirmed the reduced immunofluorescence results observed at this time point (Figure 4). These observations are in agreement with previously documented western blot results that FOXO3a is down-regulated in response to semi-acute peripheral nerve injury in both cytoplasmic and nuclear cellular compartments relative to na?ve (Wang et al., 2009).
To assess the impact of a more protracted injury state, the DRG from 1-week injured animals were also examined, with FOXO3a protein increased relative to the 4 day time point (Figure 2), which is also consistent with previous western blot assay findings (Wang et al., 2009). These results were observed in all experimental replicates. Summary plots reveal the high significance and reproducibility in the cellular levels of FOXO3a protein observed in the nuclear and cytoplasmic compartments at the various time points examined (Figure 4). It was noted that with the exception of the nuclear changes observed 1 hour after injury, parallel expression patterns were observed for both cytoplasmic and nuclear compartments at all other time points examined.
FOXO3a protein expression pattern in uninjured DRG neurons contralateral to injury
Our recent discovery that the biphasic injury response of the stress-associated transcription factor Luman/CREB3 in injured sensory neurons is mirrored, at lower levels, in non-injured contralateral DRG and C4 DRG remote from the lumbar injury level (Hasmatali et al., 2019), which made us posit that contralateral changes FOXO3a expression and nuclear translocation would also occur.
Analysis of FOXO3a immunofluorescence on DRG sections immediately contralateral to the injury indicates that unilateral nerve injury has a more robust effect on FOXO3a protein expression in contralateral uninjured DRG. The acute FOXO3a response to injury, as exemplified by nuclear translocation at the 1 hour time point, while being significantly elevated relative to the na?ve state, was still less robust than that seen in the ipsilateral injured neurons (Figures 3 and 4B). By 1 day post-injury, however, the nuclear intensity levels were significantly elevated compared to the 1-hour post-injury contralateral and na?ve neurons (Figures 3 and 4B). At 2 and 4 days post-injury, staining intensities were decreased with nuclear and cytoplasmic intensities reaching their lowest levels at the 4 day time point (Figures 3, 4A and 4B). However, at 1 week after unilateral injury, contralateral uninjured neurons had elevated FOXO3a staining to levels similar to those seen in small- to medium-sized neurons at 1 day post-injury and exceeding levels seen at 1 day post-injury in large-sized contralateral neurons (Figures 3, 4A and 4B). Summary line plots clearly reveal that this biphasic cytoplasmic and nuclear response in the contralateral uninjured neurons is highly reproducible (Figure 4A and B) and parallels the biphasic contralateral expression pattern observed for Luman/CREB3 (Hasmatali et al., 2019). Western blot analysis analysis confirmed that FOXO3a levels contralateral to injury were higher than those ipsilateral to injury and verified that the antibody was recognizing the predicted molecular weight in the DRG samples as in the known control samples (rat liver and BrCA1 МCF7 cell line extracts) that express FOXO3a (Figure 4C).
Impact of injury on FOXO3a expression in uninjured DRG neurons remote from injury
The interesting impacts of nerve injury on FOXO3a expression in L4-5 DRG neurons contralateral to injury might be attributed to either a systemic, likely humoral response to the injury, or to a response mediated contralaterally through the spinal cord. To test for the potential involvement of a systemic factor, cervical ganglia remote from the injury site were removed from animals that had undergone L4-6 unilateral spinal nerve transection at 1 day post-injujry, a time point at which a significant increase in nuclear levels was observed in contralateral neurons (Figure 4) relative to na?ve rats; FOXO3a expression levels were assessed in the neurons of the C4 ganglia. Qualitative immunofluorescence analysis of six animals per condition revealed that C4 DRG neurons from 1-day sciatic injury animals had elevated nuclear and cytoplasmic staining relative to na?ve C4 DRG, especially in the small- to medium-sized neurons (Figure 5A). Quantitative analysis of all neurons visible in C4 DRG sections from three animals/condition confirmed the qualitative observations, revealing significant differences between the two conditions (Figure 5B and C). Although a humoral mechanism best explains the effect seen in the cervical ganglia, a neuronal signal through the spinal cord that affects contralateral FOXO3a processing may still exist.
To discern whether the systemic impacts on FOXO3a protein levels were a result of the spinal nerve injury itself and/or as a result of the surgical exposure to perform the injury, sham surgeries were performed on three animals for each injury time point. The L4-6 spinal nerves of these anesthetized rats were exposed, handled in an identical manner, but not transected and the animals were left to recover for the same time points of 1 hour, 1 day, 2 days, 4 days or 1 week post-injury. FOXO3a protein levels and cellular localization appeared unchanged (Figure 6). The sham experiments verify that the contralateral effect observed at the protein level is due to injury of the nerve and not a byproduct of the surgical exposure.
Discussion
This study sheds novel insights into the regulation of FOXO3a expression in DRG neurons following unilateral peripheral nerve injury. The rapid up-regulation at 1 hour post-injury and then down-regulation in injured neurons in response to peripheral nerve injury implies a connection to both the induction and maintenance phases of the regeneration process (Li et al., 2015) that may include protection from injury-associated cell death. Although this scenario is plausible, constitutive FOXO3a expression in the na?ve animal supports a role for this transcription factor outside the realms of neuronal death and injury. What this role may be is not yet defined but may involve nociception due to its high level colocalization with the marker of the NGF-responsive population of nociceptors, CGRP. Finally, the alterations of FOXO3a protein expression in sensory neurons contralateral and remote to injury raise questions we cannot yet answer, but support an ability of this molecule to sense very subtle alterations in systemic states associated with nerve injury. Further examination of this phenomenon is essential to the understanding of the sensory neuron response to stress and disease which can have pathological implications.
FOXO3a in intact DRG neurons
The FOXO proteins are a highly conserved family of transcriptional regulators. In mammals, their involvement in multiple cellular pathways centers on maintaining homeostasis under stressful conditions and directing the cell towards survival or controlled cell death. In the na?ve state, we found the high levels of FOXO3a expression and nuclear localization to occur in small- to medium-sized na?ve neurons that predominantly colocalized with the marker of the NGF-responsive subpopulation of sensory neurons, CGRP (Verge et al., 1989). This was initially perplexing, as activity of FOXO3a typically coincides with cellular stress states. However, FOXOs conserved relationship with glucose metabolism offers clues to the potential role of FOXO3a in the nociceptive subpopulation. Small neurons of the DRG have high levels of hexokinase, which initially phosphorylates glucose prior to the start of glycolysis (Gardiner et al., 2007). Elevated amounts of this kinase suggest higher concentrations of glucose in this population and the necessity for rigorous metabolic management. Small- to medium-sized neurons also express high levels of insulin, insulin-like growth factor receptors, and insulin-like growth factor 1 (Craner et al., 2002). FOXO3a activation is induced in a low-glucose situation and activates genes associated with glucose conservation, such as glucose-6-phosphatase (Onuma et al., 2006). Although na?ve sensory neurons are not glucose deprived, they appear to be highly sensitive to alterations in insulin circulation. Another potential role is linked to the antioxidant properties of FOXOs. Overexpression of FOXO3a has been shown to protect mammalian cells from oxidative stress through the upregulation of manganese superoxide dismutase (Kops et al., 2002; Li et al., 2006). Further, NGF signaling in these neurons activates the PI-3 kinase pathway leading to phosphorylation and activation of the pro-survival molecule Akt, which in turn phosphorylates FOXO3a, sequestering it in the cytosol (Wen et al., 2011; Мa(chǎn)iese, 2015). This likely contributes to the elevated levels of FOXO3a we observed in the cytoplasm of this subpopulation of sensory neurons, which also have been found to have the highest levels of phosphorylated Akt (Pezet et al., 2005; Shi et al., 2009).
Downregulation of FOXO3a expression in injured neurons
Unilateral nerve injury induced a decrease in FOXO3a detected in both the cytosol and nuclei of the injured neurons. Past research revealed that the reduced levels of FOXO3a and the coincident reduced levels of p27kip1(a FOXO3a target and tumor suppressor gene) post-injury were linked to perineuronal and Schwann cells proliferation as part of the regeneration response (Wang et al., 2009).
Another potential role for the decreased levels of neuronal FOXO3a is neuroprotection. Peripheral nerve injury does not lead to neuronal apoptosis in the short term. Long term sciatic transection studies suggest that retrograde loss of L4-5 DRG sensory neurons is only detectable at 1 month post-axotomy (Groves et al., 2003; Kuo et al., 2005). This resistance to death is attributed to the high level of autocrine/paracrine trophic support from associated glia and the neurons themselves (Acheson and Lindsay, 1996; Xian and Zhou, 1999; Karchewski et al., 2002; Vigneswara et al., 2013; Nadeau et al., 2014). Whether the reduced levels of FOXO3a in both cytoplasmic and nuclear compartments within the first week post-injury contribute to the lack of sensory neuron death observed during this time is not known. Additionally, as this study does not examine injury events at 1 week post-injury, it is also not known if altered FOXO3a expression is a contributing factor to apoptosis at later time points. The factors are responsible for the decreases in FOXO3a observed post-injury are currently unknown. Whether the decreases in FOXO3a are due to changes at the gene level and/or are post-translational in nature requires further investigation. The stress associated with the injury did induce a spike in cytoplasmic and nuclear localization at 1 hour that was rapidly followed by a sustained decrease in nuclear and cytoplasmic levels. The latter may be the result of FOXOs being heavily controlled by post-translational modifications with a number of studies highlighting the importance of FOXO polyubiquitination and protein degradation (Мa(chǎn)tsuzaki et al., 2003; Plas and Thompson, 2003; Huang et al., 2005; Fu et al., 2009). FOXO1 and FOXO3a are ubiquitinated and targeted to the proteasome in response to growth factor treatment, which is accredited to activation of the PI3K/Akt pathway and phosphorylation of the FOXOs at known Akt sites (Мa(chǎn)tsuzaki et al., 2003; Plas and Thompson, 2003). Whether this is responsible for the decreased expression observed in injured neurons is the subject of future studies.

Figure 1 Characterization of the forkhead class box O3a (FOXO3a)-expressing subpopulation of sensory neurons.
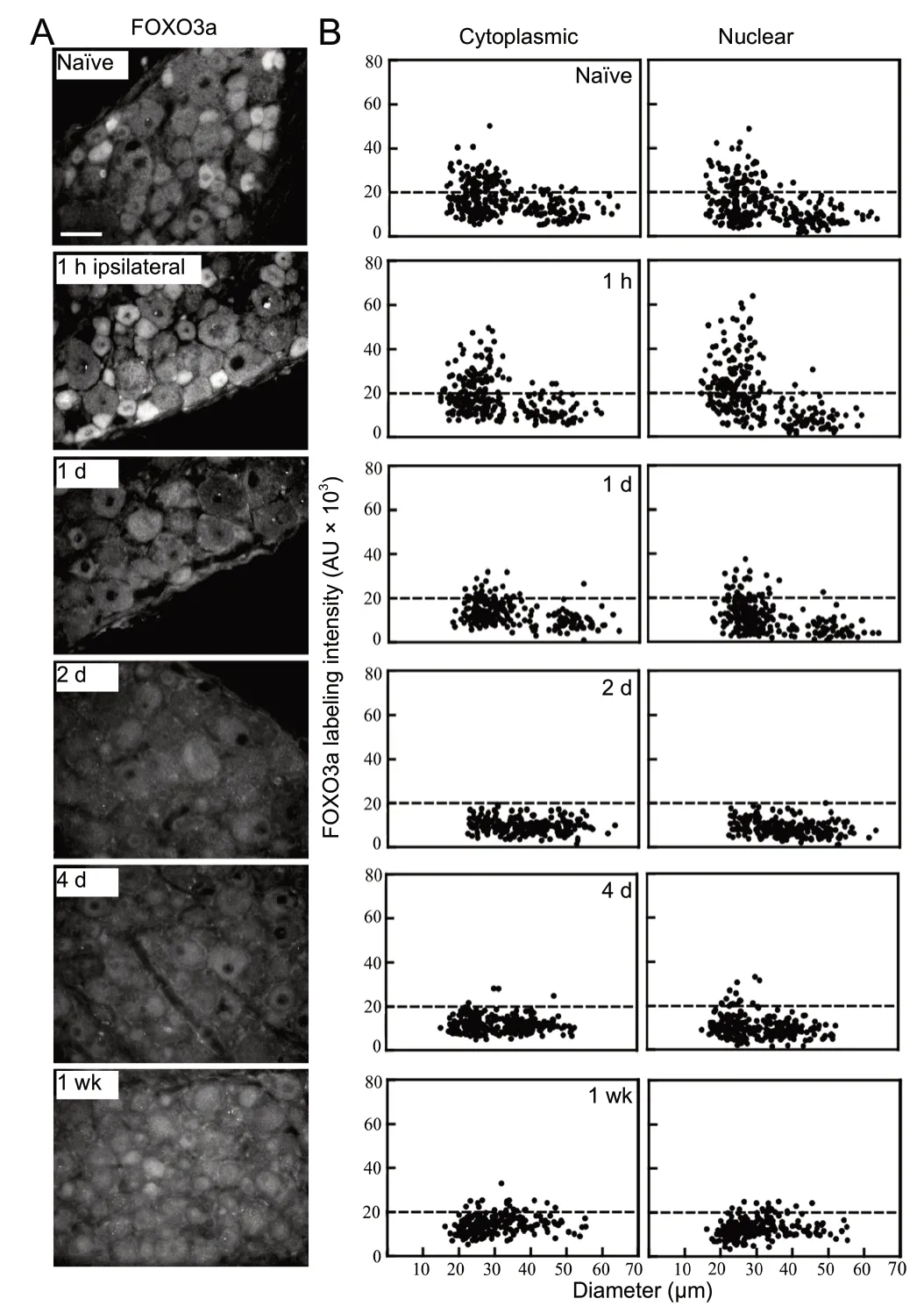
Figure 2 Peripheral nerve injury alters forkhead class box O3a (FOXO3a) immunoreactivity in dorsal root ganglion (DRG) neurons ipsilateral to axotomy.

Figure 3 Peripheral nerve injury alters forkhead class box O3a (FOXO3a) immunoreactivity in uninjured neurons contralateral to axotomy.

Figure 4 Alterations in forkhead class box O3a (FOXO3a) expression in response to nerve injury and antibody specificity control.
Uninjured contralateral and remote sensory neurons respond to nerve injury

Figure 5 L4-6 spinal nerve transection alters DRG neuronal FOXO3a expression and localization in uninjured C4 ganglia remote from the injury site.

Figure 6 Sham surgery time course does not result in discernibly altered FOXO3a immunofluorescence levels.
The most dramatic alterations in FOXO3a expression following unilateral injury were the parallel increases in cytoplasmic and nuclear levels in neurons contralateral or remote to injury at early time points after injury. The significance of this is unknown but may be linked to an injury-associated vulnerability induced in these cells. When pro-brain derived neurotrophic factor (pro-BDNF) is immunoneutralized in rats receiving a unilateral injury, the numbers of neurons in contralateral DRG decreased (Fan et al., 2008). The authors suggested that this supports a role of pro-BDNF in the birth of new neurons in these contralateral DRG. Our observation of increased nuclear localization of FOXO3a in these neurons supports an alternative interpretation. There may be a loss of neurons in these ganglia due to the increased FOXO3a levels in the nucleus, a thesis that remains to be examined, but is supported by the findings of a study by Oaklander and Brown (2004) in which bilateral loss of distal skin innervation is observed following unilateral sural nerve injury, possibly due to loss of neurons. Interestingly, despite a high level of FOXO3a expression in small- to medium-sized neurons contralateral and remote to injury, it may not be linked to an altered algesic state. It has been reported that allodynia/mechanical sensitivity was not altered in the limb contralateral to tibial and peroneal nerve lesion during the 21 day post-injury period studied (Oaklander and Brown, 2004). Similar findings of a lack of contralateral algesia were also reported following unilateral chronic constriction injury for both mechanical and thermal pain indices (Dubovy et al., 2013). There is emerging evidence that altered expression of many transcription factors in neurons contralateral and remote from the lesion, as well as behavioral changes (Koltzenburg et al., 1999). These include heightened plasticity, immune responses, pain-associated changes, and a growing list of altered transcription factor expression that now includes FOXO3a. The recent creation of a fluorescent reporter system supports broad reaching impacts of unilateral peripheral nerve injury that include contralateral DRG spinal cord and brain pathways (Hashimoto-Torii et al., 2018).
Few connections exist between neurons that innervate opposite sides of the body; however, a collection of clinical evidence confirms contralateral deficits in patients with one-sided injuries (Kozin et al., 1976; Oaklander et al., 1998). The bilateral impact of nerve injury has been reported in a number of animal models where sensory neurons, sympathetic neurons, or motoneurons opposite to the lesion site differ morphologically and/or biochemically from na?ve controls (Koltzenburg et al., 1999). In addition to these changes, neurons of intact DRG contralateral or segmentally adjacent to the injured ganglia have altered nerve sprouting in unaffected limb areas (Devor et al., 1979; Navarro et al., 1997; Oaklander and Brown, 2004). In general, these observations are considered a result of a neural mechanism with a propagating signal through the spinal cord, but not due to a global effect. However, our results support the existence of a humoral response to peripheral nerve axotomy whereby injury signals originating from the lesion site and/or systemically released may circulate via spinal fluid or bloodstream and influence uninjured sites. Recent work supports that increased levels of the cytokine interleukin-6 in the cerebral spinal fluid and the associated STAT-3 signaling pathway contribute to the heightened plastic state observed in sensory neurons contralateral and remote to 1 week unilateral sciatic nerve injury and can be mimicked by bolus injection of interleukin-6 into the subarachnoid space (Dubovy et al., 2018, 2019a, b).
Notably, the temporal biphasic nature of the contralateral FOXO3a response in sensory neurons parallels that which we observed for another stress transcription factor, Luman/CREB3 (Hasmatali et al., 2019) and mirrors the phasic transcriptome changes described for the injured neurons (Li et al., 2015). These changes coupled with the changes remote to injury support the existence of humoral factors that exert systemic transcriptome changes that broadly influence the stress response to injury. However, the fact that the temporal patterns of change in injured versus uninjured contralateral neurons with respect to FOXO3a did not parallel each other support that different factors also contribute to the response in these two populations of neurons.
Conclusion and significance
Cellular stress and trauma has a profound effect on cell phenotype, as epitomized by DRG neurons that regenerate after injury. Transcription factor regulation during this compromised state is essential to the activation of repair and regeneration programs. The impact of peripheral nerve axotomy on FOXO3a and its localization supports that its absence during the initiation and early phases of regeneration may likely benefit the injured neuron while its rising expression at more protracted 1 week time point suggests it may serve a role in the second wave of transcriptional events associated with regeneration of the injured neuron and potentially the delayed cell death.
The alterations in FOXO3a expression and localization observed contralaterally to injury in uninjured neurons mirror the biphasic pattern observed for Luman/CREB3, another stress-associated transcription factor that also correlates with an increased plasticity in the contralateral uninjured neurons (Hasmatali et al., 2019). This suggests that select systemic factors are induced that effect parallel transcriptional phases described for the injured neuron, supporting that a global response to injury exists and that uninjured DRG neurons sense these subtle changes. The reality of contralateral and humoral effects of nerve injury illustrates the importance of using na?ve animals and not uninjured tissue as controls. The identity of the underlying mechanism(s) responsible for contralateral and remote alterations in FOXO3a and other transcription factors is still in question.
Author contributions:JCDH, HN, BHJ, VM, and VMKV participated in the conception, design, writing, and interpretation of the research presented. JCDH and JMJ conducted the surgeries and western blot experiments; JCDH, JDG, and JMJ processed tissue for immunofluorescence and conducted image and data analysis. VM and VMKV are co-senior authors as JCDH was co-supervised by them. All authors approved the final version of this paper.
Conflicts of interest:The authors have no duality or conflicts of interest to declare.
Financial support:This work was supported by Canadian Institutes of Health Research (CIHR) grants #74747 and #14238 (both to VMKV) and by a Natural Sciences and Science and Engineering Research Council (NSERC) of Canada grant (to VM). JCDH was supported by University of Saskatchewan College of Graduate and Postdoctoral Studies Scholarships.
Institutional review board statement:Approval to conduct these studies was obtained from the University of Saskatchewan Animal Research Ethiics Board (protocol #19920164).
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
 中國(guó)神經(jīng)再生研究(英文版)2020年12期
中國(guó)神經(jīng)再生研究(英文版)2020年12期
- 中國(guó)神經(jīng)再生研究(英文版)的其它文章
- Activation of β2 adrenergic receptors promotes adult hippocampal neurogenesis
- Neuroprotective mechanisms of DNA methyltransferase in a mouse hippocampal neuronal cell line after hypoxic preconditioning
- Lycium barbarum polysaccharides related RAGE and Aβ levels in the retina of mice with acute ocular hypertension and promote maintenance of blood retinal barrier
- Insulin-like growth factor 1 partially rescues early developmental defects caused by SHANK2 knockdown in human neurons
- Enriched environment enhances histone acetylation of NMDA receptor in the hippocampus and improves cognitive dysfunction in aged mice
- Pentraxin 3 contributes to neurogenesis after traumatic brain injury in mice
