Visible Light-Induced 3D Bioprinting Technologies and Corresponding Bioink Materials for Tissue Engineering: A Review
Zizhuo Zheng, David Eglin, Mauro Alini, Geoff R. Rihards, Ling Qin, Yuxiao Lai,e,*
a Centre for Translational Medicine Research and Development, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen 518055, China
b Key Laboratory of Health Informatics, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen 518055, China
c AO Research Institute, Davos CH-7270, Switzerland
d Musculoskeletal Research Laboratory, Department of Orthopedics and Traumatology, The Chinese University of Hong Kong, Hong Kong 999077, China
e Guangdong Engineering Laboratory of Biomaterials Additive Manufacturing, Shenzhen 518055, China
ABSTRACT
Keywords:Medical additive manufacturing Bioink Tissue engineering 3D bioprinting
1. Introduction
With an alarming increase in the incidence of end-stage failure of vital organs,there is an urgent need for an innovative therapeutic approach that could effectively repair and restore damaged organs. In addition, the shortage of organs from optimal donors and matching difficulty are challenges confronting the field of organ transplantation.Recent notable achievements in tissue engineering for regenerating damaged tissue have gained considerable attention among transplant clinicians and researchers.Tissue engineering is recognized as a possible means to address the increasing demand for living organs and the limitations of living organs[1–4].Cells, scaffolds, and biological/biochemical factors are generally referred to as essential elements of the ‘‘building blocks” of tissue engineering-based regenerative medicine strategies[5–7].An ideal bioactive scaffold for tissue engineering would provide a platform to support the interaction among cells, bioactive factors, and surrounding tissue [4]. In addition, scaffolds provide the physical support for cells and control the release of factors.
Charles W. Hull first proposed the concept of 3D printing technologies in 1986 [8]. Three-dimensional (3D) printing is a manufacturing process for constructing objects from computer-aided design models [5]. In contrast to traditional manufacturing, for example, casting and forging processes, 3D printing produces an object or scaffold by adding materials layer by layer and is one of the additive manufacturing technologies [1,3,5]. Bioprinting is becoming an increasingly attractive technology for designing①the microstructure of scaffolds and control cells and ②the distribution of bioactive factors,and thereby,fulfilling the demand for regenerated tissues. The printing material, cell, and printing equipment/method are considered the most important factors in the application of this technology.
According to the American Society for Testing and Materials standard (F2792), 3D printing technologies are classified into photopolymerization, material jetting, material extrusion, powder bed fusion, binder jetting, sheet lamination, and direct energy deposition [9]. In terms of cell viability and printing capability,the printing methods based on photopolymerization exhibit many advantages over other types of bioprinting methods,such as rapid curing at room temperature,high printing fidelity,and gentle reaction process. The printing structure and speed can be controlled conveniently by adjusting the light intensity, exposure time, and illuminated area [7]. Four out of the various bioprinting methods are widely applied to photo-cured bioprinting: inkjet, extrusion,stereolithography, and digital light process.
A bioink is a printing precursor in bioprinting and is typically based on thermosensitive or photopolymerization materials that contain cells[10].It functions as a cell carrier,ensures precise positioning, and plays an important role in the protection of the cells during the printing process and that of the microenvironment formed by the material after printing. Among the many printing materials, hydrogel is a class of 3D network polymers formed through chemical bonds or physical forces. These can swell in water, but not dissolve in it. A few hydrogels display a permeable structure that is similar to the natural extracellular matrix (ECM).This structure provides a remarkable 3D microenvironment for cell proliferation[11–15].Given these properties,many types of hydrogels can be applied to various areas of tissue engineering. Two types of crosslinking occur between polymer chains: chemical and physical crosslinking. The various crosslinking schemes affect the gelation kinetics and properties of hydrogels differently.Physically crosslinked hydrogels rely mostly on intermolecular van der Waals forces,hydrogen bonds,and other weak interaction forces. Chemically crosslinked hydrogels are formed by covalent bonds and are relatively stronger than physically crosslinked hydrogels [11]. Among the chemical crosslinking methods,photopolymerization has attracted considerable attention owing to its unique characteristics [16–18].
Photopolymerization is a simple,clean,and convenient method for achieving covalently crosslinked hydrogels. Photopolymerization can effectively control the formation and structure of hydrogels spatially and temporally. At present, photopolymerization is implemented largely using ultraviolet (UV) light. This may cause cell damage during exposure [19]. In contrast, when UV light is replaced with visible light, the hydrogel system achieves higher cell compatibility and wider application prospects. In addition,visible light has a higher penetration depth, which results in a more uniform hydrogel structure [20]. Visible light crosslinkable hydrogels have been widely researched and applied in many fields such as tissue engineering [21], 3D cell encapsulation [22], and drug delivery [23].
In this review, we briefly discuss the operating principles and specialities of 3D bioprinting technologies and devices that can be applied to visible light-induced bioprinting systems (Table 1[20,21,24–33]). Then, we systematically summarize visible lightcrosslinkable bioink, including the crosslinking mechanisms and visible light initiators, and highlight their biomedical applications.Finally, existing challenges in the bioprinting and visible light crosslinkable hydrogels are discussed, and future prospects and development directions are proposed.
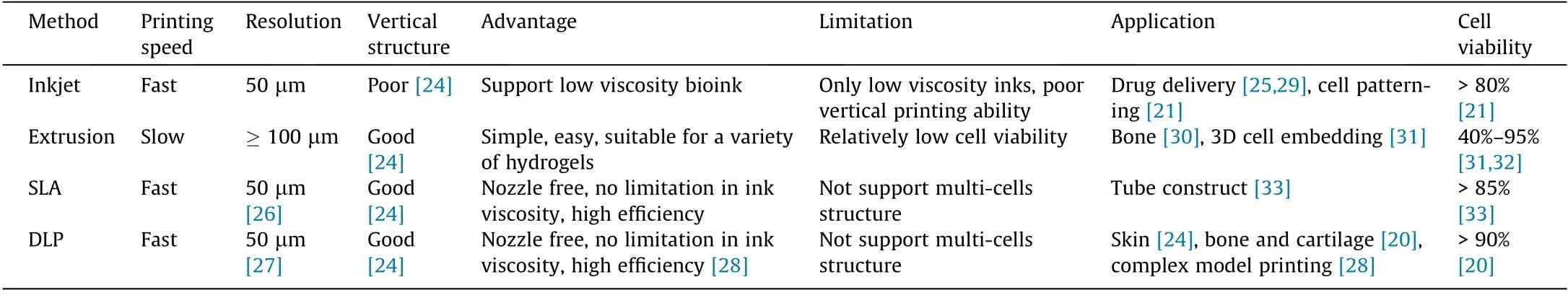
Table 1 Visible light-induced bioprinting methods.
2. Photo-induced 3D bioprinting methods
2.1. Inkjet-based bioprinting
Inkjet-based bioprinting originated from commercial 2D inkjet printing,which drops and deposits a cell-laden bioink into a predefined area to form a preset shape[34].The droplet,which is ejected from the nozzle to the substrate, is typically produced via thermal or piezoelectric actuation, as shown in Fig. 1 [24]. Thermal actuation generates droplets through heating elements that overheat the bioink in 2 μs, such that a high temperature (e.g., 300 °C) will not affect cell viability[9].Piezoelectric actuation adjusts the voltage rapidly to force the bioink to expel a droplet through the piezoelectric material. The gelation of bioink by physical and chemical processes can occur simultaneously with the printing process to guarantee printing fidelity. With the low volume of droplets (10–50 μm in diameter) and high throughput (up to 10 000 droplets per minite), inkjet-based printing ensures a high printing resolution (lower than 50 μm) and printing speed [24]. In addition, the cell viability after printing can exceed 80%. However, a drawback of inkjet-based bioprinting is that its application is limited to low-viscosity bioink because a high viscosity bioink tends to clog the nozzle and cause high shear stress [35]. Consequently, inkjetbased bioprinting limits the options of bioink materials and cell concentrations. Furthermore, it is challenging to form large and complex 3D structures. Acosta-Vélez et al. [25] developed a drug tablet that can be fabricated in 30 s by inkjet printing under exposure to visible light. The visible light system was used rather than UV because the latter affects drug stability [25].
2.2. Extrusion-based bioprinting

Fig. 1. Schematic diagram of inkjet-based bioprinting. Reproduced from Ref. [24]with permission of Elsevier, ? 2018.
Extrusion-based printing is one of the most common additive manufacturing methods used for fabricating scaffolds. The extrusion is controlled by pneumatic, piston-driven, and screw-driven systems[36].Unlike the case of inkjet-based printing,the pressure of the extrusion process can be conveniently controlled, and the viscosity of bioink can be within a wider range (30–6 × 107MPa.s).That is, the selection of materials can be more diverse, albeit with limitation in terms of viable cell support [37]. In accordance with the fundamental theory of the extrusion process, the major drawback of extrusion-based printing is the limited resolution and low printing speed,because of the size of the needles.The resolution of extrusion printing by current bioprinting applications can attain 100 μm [9]. The location of the optical device becomes critical when a photocrosslinkable bioink is applied to extrusion-based bioprinting. The photocuring process could be performed before(pre-crosslink), after (post-crosslink), or during (in-situ-crosslink)extrusion,as shown in Fig.2[38].Ouyang et al.[38]illustrated that pre-crosslinking resulted in high and inconsistent extrusion forces,heterogeneous printed structures, and low cell viability (about 47%). Although post-crosslinking could improve the cell viability and lower the extrusion force,the bioink flowed prior to stabilization and could not maintain the filament structure. The hydrogel could crosslink before deposition (in-situ-crosslink with UV or visible light) when the needle was replaced with a photo-permeable capillary. This yielded a high printing fidelity and relatively high cell viability (over 95%) after printing [38]. Through adjustments of the hydrogel concentrations, post-crosslink could achieve higher fidelity under visible light than under UV in extrusion-based bioprinting, and also ensure a high cell viability(over 90%) [39].
2.3. Stereolithography and digital light process
Stereolithography (SLA) and digital light processing (DLP) have similar molding mechanisms.
SLA is one of the printing methods that use digital micromirror arrays to control the light intensity of each pixel for the printing areas [6]. During SLA printing, a laser light is applied to a liquid photosensitive material in a point-by-point manner to form a solidified layer. After the solidification of the first layer, the platform rises by a defined height, and a second layer is photocrosslinked. This is repeated until the complete shape is printed(Fig.3(a)).SLA does not require extrusion through a nozzle and is faster,more accurate,and has a higher resolution(<100 μm)than extrusion-based printing[40].In general,SLA bioprinting uses UV light as its light source.It induces cell damage during bioprinting, thereby limiting its use. Wang et al. [26] developed a visible light-induced SLA-based bioprinting process and used it with an eosin Y(EY)-based photoinitiator to fabricate a polyethylene glycol diacrylate (PEGDA) and gelatin methacrylate (GelMA) composite hydrogel. They achieved a resolution of 50 μm and high cell viability (85%) for at least five days [26].
DLP bioprinting is similar to the SLA-based printing method,except that it uses a projector to project light onto photopolymerized materials for curing the layer image rather than a point [41–43] (Fig. 3(b)). The print speed of DLP is higher than that of SLA,particularly when printing larger objects. However, the printable area is reduced in comparison with that of SLA because of the constraints imposed by the project area and resolution of the digital light mirrors. Consequently, only small objects are generally printed. Lim et al. [39] explored the resolution of 3D DLP printing of silk fibroin(SF)hydrogel.They attained a resolution of 66 μm in the X-direction and 146 μm in the Z-direction. This indicates its capability of printing complex structures (e.g., Eiffel Tower) with high precision [39]. Lim et al. [39] developed a visible lightinduced DLP system.It achieved a resolution of 50 μm and cell viability of over 90%[27].DLP is a highly efficient method of layer-bylayer printing.Kelly et al.[44]presented a new method for manufacturing by rotating a photopolymer in a dynamically evolving light field(Fig.3(c)).It is based on the DLP method.This method is scalable to larger print volumes and is faster by several orders of magnitude than the common DLP method, under a wider range of conditions [44]. Bernal et al. [28] used visible light (405 nm)-induced volumetric bioprinting (Fig. 3(d)) to print complex centimeter-scale architectures(including anatomically correct trabecular bone models with embedded angiogenic sprouts and meniscal grafts).They achieved a high cell viability(>85%) in seconds to tens of seconds [28]. These methods are summarized in Table 1.

Fig. 2. Schematic diagram of three extrusion-based bioprinting. Reproduced from Ref. [38] with permission of Wiley, ? 2017.
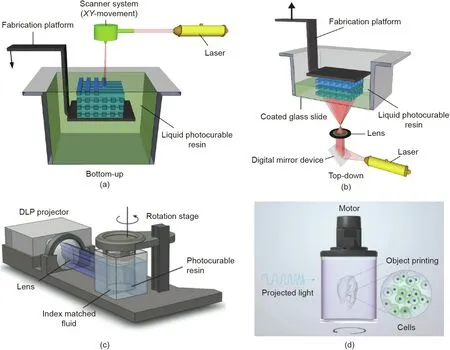
Fig.3. Schematic diagram of(a)SLA and(b)DLP bioprinting method.(c)Schematic of the computed axial lithography system.(d)Volumetric bioprinting process showing the cell-laden gel-resin reservoir connected to a rotating platform. (a, b) Reproduced from Ref. [10] with permission of Elsevier, ? 2012; (c) reproduced from Ref. [44] with permission of Science, ? 2019; (d) reproduced from Ref. [28] with permission of Wiley, ? 2019.
3. Visible light crosslinkable materials
3.1. Visible light initiators
Most of the photocrosslinkable bioinks require the presence of photoinitiators. The type of photoinitiator and the duration of exposure to visible light can affect the cell viability and photoinitiation efficiency. Therefore, the selection of a visible light initiator requires the consideration of its absorption spectrum, water solubility, capability to generate free radicals, and stability.
Visible light initiators can be classified into free radical and cationic photoinitiators based on the polymerized active species.However,cationic photoinitiators cannot be applied in the biomedical field because of the protonic acid produced when polymerization is initiated [7,16]. Therefore, visible light crosslinkable hydrogels rely mostly on visible light-initiated radical polymerization. Free radical photoinitiators can be divided into type I(one-component pyrolysis) and type II (photosensitizer/co-initiator photoinitiators) [45]. Type I photoinitiators absorb incident photons and divide them into two primary radicals upon exposure to light. However, less options are available in the visible region for a type I photoinitiator, and lithium phenyl-2,4,6-trimethylbenzoyl phosphinate (LAP) is commonly used [46]. In contrast, there are considerably more and diverse alternatives for type II photoinitiators,which extract hydrogen from the co-initiator to generate secondary radicals. At present, the ruthenium pyridine complex, EY,and camphorquinone (CQ) are attracting significant attention and being widely applied in tissue engineering. In a visible light crosslinkable hydrogel system, the cytotoxicity and absorption spectrum of the initiator are particularly important for the encapsulated cells. The commonly used visible light initiators are listed in Table 2 [39,46–56].
The CyQuant Direct Cell Proliferation Assay method reveals that the viability of human primary renal proximal tubule epithelial cells (hRPTECs) decreases marginally as the LAP concentration increases. However, it can still satisfy the biocompatibility standard [51]. In an early work, Lin et al. [57] introduced LAPinitiated GelMA hydrogel-encapsulated human bone marrowderived mesenchymal stem cells (MSCs) that exhibit long-term viability and proliferation(over 90 d)and good integrity.Although LAP can absorb the energy of near-UV blue light (405 nm) to produce free radicals,the cost of producing a bioprinting device with a similar blue light is high. Moreover, this type of device is not remarkably superior to the current UV bioprinting system.Furthermore, such strong near-UV blue light is hazardous to mammalian cells and disruptive to cellular processes [51]. CQ, fluorescein,and riboflavin (RF) have similar absorption spectra (between 400 and 500 nm) [52]. The methacrylated glycol chitosan (MeGC)hydrogels initiated by these types of initiators are tested. The results show that the hydrogel initiated by RF has the highest mechanical strength and lowest cytotoxicity. Moreover, thegelation time and cell exhibit a negative correlation[52].Donnelly et al.[54]developed an RF-initiated tyramine-substituted hyaluronate (HA-Tyr) hydrogel to coat TC-28a2 chondrocytes. It showed over 99% cells to be alive after one day.

Table 2 Types of visible light initiators.
Among visible light initiators, EY has many more advantages than others [2]. EY is highly water-soluble, and has an absorption peak at approximately 515 nm and low cytotoxicity [51]. EY and LAP exhibit a similar cytocompatibility for hepatic progenitor HepaRG cells. It is noteworthy that in contrast to LAP, the gelatin hydrogel initiated by EY marginally increases the degree of hepatic gene expression [58]. Gwon et al. [59] demonstrated that human adipose-derived MSCs grow and proliferate effectively in hyaluronic acid (HA) hydrogel mortified with heparin (cell viability of 95%).Furthermore,the hydrogel can support 3D adipogenic differentiation of adipose-derived MSCs[59].Kerscher et al.[60]demonstrated that low-density GelMA hydrogels can be formed in 1 min by EY and that they facilitate high efficiency cardiac differentiation.On Day 8 of differentiation, the hydrogel initiated spontaneous contractions in conjunction with synchronicity, frequency,velocity, and appropriate temporal variations in the cardiac gene expression [60].
Thetris(2,2?-bipyridyl)dichlororuthenium(II)hexahydrate([Ru(II)(bpy)3]2+)/sodium persulfate (SPS) system also displays unique strength. [Ru(II)(bpy)3]2+/SPS can alleviate the effect of oxygen inhibition during the polymerization, which affects the fidelity of 3D bioprinting in the vertical direction[39].3D bioprinting is widely adopted for printing complex structures that are to be implanted in vivo, wherein it is challenging to maintain the structure in the vertical direction and ensure precision in the horizontal direction. A few studies have revealed that the print fidelity of 3D bioprinting and the photopolymerized hydrogel structure are directly affected by oxygen inhibition. The presence of oxygen affects free radicals because these can react with oxygen and be converted into peroxyl radicals, which cannot react with unsaturated bonds. Meanwhile, peroxyl radicals reduce the number of protons in the system. This results in the formation of either hydroperoxides or alcohols that hinder the formation of covalent crosslinks. These reactions cause an incomplete or insufficient formation of hydrogels, thereby affecting the stack between layers and the printing fidelity in the vertical direction. To solve this problem, Lim et al. [39] applied the visible light +[Ru(II)(bpy)3]2+/SPS system. Unlike the UV + I2959 system, this system alleviates the effects of oxygen inhibition on porous biofabricated constructs (Fig. 4 [61]) and maintains a cell viability of over 85%in 21 d[39].Al-Abboodi et al.[55]developed a gelatinhydroxyphenylpropionic acid (Gtn-HPA) conjugate hydrogel initiated by[Ru(II)(bpy)3]2+/SPS.It shows good cell viability(over 85%).
3.2. Mechanisms of photopolymerization and gelation
There are two types of photopolymerization: photoinitiatorfreepolymerizationandphotoinitiatorpolymerization.Photoinitiator-free polymerization is directly initiated by UV light.Farkas et al.[6]developed a type of photoinitiator-free 3D scaffold.It is fabricated by excimer laser photocuring under light with a wavelength of 248 or 308 nm[6].It shows higher cell viability than those initiated by photoinitiators. However, this type of polymerization requires energy higher than that of the covalent bond of the monomer. Furthermore, it is challenging to satisfy this requirement in the visible range. Therefore, it is unlikely to be applied in the field of visible light-induced polymerization.Polymerization under visible light requires an initiator. Three types of gel mechanisms have been widely used in studies: free radical-initiated chain polymerization,thiol–ene‘‘click”reaction,and photo radical coupling reaction. The details of the gelation mechanisms are described below.
3.2.1. Free radical-initiated chain polymerization
The development and advancement of synthetic chemistry has enabled the modification and synthesis of functionalized monomers and macromolecular chains through various methods.Furthermore, photocrosslinkable bioinks can be prepared through free-radical polymerization (FRP). The process of FRP has three reaction stages, as shown in Fig. 5: chain initiation, propagation,and termination[14]. After a radical is generated via the exposure of an initiator to light,the radical reacts with a double bond to generate a new radical. This reacts further with a double bond on the monomer or forms oligomers, and propagates further until termination [7].
Owing to the cytoxicity of the methacrylate monomers,bioinks amenable to FRP are produced by introducing a limited amount of methacryloyl groups using methacrylic anhydride [62], glycerol methacrylate [63], or methacryloyl chloride [24], into natural or synthetic macromolecular chains, and then selecting a suitable photoinitiators to produce water-based photocrosslinkable bioinks. The mechanisms of FRP and gelation are illustrated in Fig. 5.

Fig. 4. Difference between UV- and visible light-polymerized GelMA/collagen constructs. Vis: visible light; Ru: [Ru(II)(bpy)3]2+. Reproduced from Ref. [61] with permission of American Chemical Society, ? 2016.
3.2.2. Thiol–ene ‘‘click” reaction
The thiol–ene‘‘click”reaction is a fast,highly selective,and versatile method for preparing photocrosslinkable hydrogels.Classical thiol–ene chemistry emerged when Charles Goodyear discovered the vulcanization of natural rubber (poly(cis-isoprene)) by sulfur in the mid-19th century. Since then, the mechanism, kinetic characteristics, and properties of sulfhydryl/vinyl polymerization have been studied extensively[64,65].The radical growth mechanism of sulfhydryl/radical photopolymerization is different from the growth mechanism of the free radical chains of vinyl.Furthermore,the sulfhydryl monomers correspond to crosslinkers [65]. The thiol–ene reaction is unaffected by oxygen inhibition in air and can achieve photopolymerization rapidly [66,67]. Therefore, a lesser amount of photoinitiator is used.Furthermore,the formation of thioether bonds can enhance the strength of materials.
After the initiator is activated,protons are abstracted from sulfhydryl groups to form thiyl radicals. These then react with vinyl bonds. The reaction forms a thioether bond and another carboncentered radical that can generate another thiyl radical. The thiol–ene reaction propagates until the limiting moiety is depleted[64]. The reaction with an electron-rich vinyl monomer such as norbornene [68], acrylate, methacrylate, styrene, or conjugated diene [13,40] involves the homopolymerization reaction of vinyl monomers and the copolymerization reaction between the thiol and vinyl groups [65] (Fig. 6).
3.2.3. Photo radical coupling reaction
This type of reaction generally requires the presence of phenolic hydroxyl groups such as Tyr.Furthermore,ruthenium(Ru(II))or EY is commonly used as the visible light initiator. Different initiators display different initiation mechanism during the reaction.[Ru(II)(bpy)3]2+is photo-oxidized into [Ru(III)(bpy)3]3+by visible light. Then, the activated Ru(III) extracts an electron from the phenolic hydroxyl group (Fig. 7(a)). This yields a radical species that can then attack a wide variety of other groups [69], as shown in Fig. 7(b). However, the ground-state EY absorbs a photon is transformed to the first excited singlet state (1EO). It is then converted to a long-lived triplet state (3EO*) by intersystem crossing.Energy is transferred in the presence of oxygen to form singlet oxygen(1O2) [70]. Then, the singlet oxygen reacts with phenolic hydroxyl groups to yield radical species that sustain the crosslinking, as shown in Fig. 7(c). The commonly used strategy to achieve this reaction is to modify the polymer with molecules containing a phenolic hydroxyl group.Sakai et al.[33]successfully developed a type of bioink by modifying alginate with TYR. This ink could be gelled using a normal desktop lamp in 10 s [33].

Fig. 5. Mechanism of FRP and gelation. hv: photon energy.

Fig. 6. Mechanism of thiol–ene ‘‘click” reaction. hv: photon energy.

Fig.7. The mechanism of photo radical coupling reaction.(a)Initiation mechanism of ruthenium;(b)initiation mechanism of EY;(c)the mechanism of photo radical coupling reaction.
3.3. Visible light crosslinkable materials
Photopolymerized materials are primarily categorized into nature-derived and synthetic materials.The most common method to achieve photopolymerization capability is to modify the specific side or terminal groups with compounds that contain a double bond, such as acrylate, methacrylate, styrene, conjugated diene,and Tyr. The common types of photopolymerized materials and modified methods are provided in Table 3 [23,27,33,46,51,55,71–85].
3.3.1. Nature-derived materials
Cells are reportedly incubated in the ECM,which is made up of complex structural and functional macromolecules. Natural materials are derived from organisms’ polysaccharides and proteins.Most of the nature-derived materials such as gelatin and collagen have superior cell response and cell adhesion,and can be degraded in vivo. In addition, nature-derived materials are inexpensive and renewable. However, these have a few limitations, for example,high degradation rate, complex purification process, and poor mechanical properties.
Gelatin is an animal protein that is isolated from animal tissues and prepared through the thermal denaturation of collagen[6,85],which is a heterogeneous aggregation of polypeptides that contain 18 amino acids [86]. Considering the gelatin construct, gelatin exhibits the potential to be modified with functional monomers without a significant reduction in its cytocompatibility. Lin et al.[85] introduced degradable gelatin hydrogel-encapsulated human bone marrow-derived MSCs. They exhibit long-term viability and proliferation(over 90 d)and good integrity.In 2000,Van Den Bulcke et al. [87] first developed and patented the photopolymerizable gelatin methacryloyl derivative, GelMA. It was obtained through the reaction of primary amines in the side chains of (hydroxy)-lysine and ornithine and methacrylic anhydride [47,48]. The GelMA precursor forms hydrogen bonds between the chains at a low temperature(<25°C) to increase the solution’s viscosity.This facilitates the attainment of the 3D printing process’ viscosity requirement.GelMA has been widely applied for bioprinting either as a standalone material or by being co-crosslinked with other materials to form a hydrogel. Several types of photocrosslinkable gelatin derivatives based on this conjugation method have been developed in addition to GelMA. Mazaki et al. [71] developed a furfurylamine-conjugated gelatin. It could crosslink by visible light,thereby supporting bone marrow-derived stromal cells chondrogenic differentiation in vitro [71].
Chitosan is a polysaccharide that consists of randomly distributed β-(1–4) linked D-glucosamine and N-acetyl-Dglucosamine. It is prepared from the chitin shells of shrimp and other crustaceans, through chemical processing [75]. Given its antifungal and antibacterial activities,chitosan has been approved by the US Food and Drug Administration(FDA)for medical wound dressing. With regard to its chemical properties, the presence of hydrogen bonds decreases chitosan’s solubility in water [88]. The abundant groups in chitosan, such as amidogen, provide many opportunities for modifying region properties. Chitosan can be reacted with methacrylate anhydride or glyceryl methacrylate to form a photopolymerizable chitosan derivative. This derivative can be used for bioactive carriers [23,75] and bioink [89].
HA is a non-sulfated glycosaminoglycan with disaccharide unit repeats of D-glucuronic acid and N-acetyl-D-glucosamine [90,91].HA is distributed widely in connective, epithelial, and neural tissues, typically in an anionic form. Each monomer of HA has sites for modification with reactive groups [92]. Therefore, researchers have refined existing chemistries for synthesizing HA macromer derivatives such as methacryloyl HA [38] and norbornene functionalized HA[77].With regard to its roles in the ECM,HA exhibits high hydrophilicity and considerable cytocompatibility to support cell growth, migration, and differentiation [91]. Gwon et al. [59]demonstrated that human adipose-derived MSCs grow and proliferate well in HA hydrogel mortified with heparin. The production of several types of functional marker and their synergistic effects could be observed during the cell culture [82]. Hinton et al. [93]used methacrylated HA(MeHA),collagen,and other soft materials to test a new extrusion-based bioprinting method(freeform reversible embedding of suspended hydrogels). It displayed substantial potential to be applied in bioprinting natural materials [93].
SF is an insoluble protein that is present in silk produced by silk worms. It has three chains: light, heavy, and glycoprotein P25 chains. The heavy and light chains are linked by disulfide bonds.Moreover, these associate with P25 via noncovalent interactions[94]. Given its nontoxicity, low immunogenicity, and low degradation rate [95], SF can be applied to wound dressing,enzyme immobilization matrix, vascular prosthesis, and structural implant [79]. SF is also applied to bioprinting after modification. Kim et al. [79] developed a modified glycidyl methacrylated SF bioink. It enabled the construction of highly complex organ structures including the heart, vessel, brain,trachea, and ear with remarkable structural stability and reliable biocompatibility [79].

Table 3 Photopolymerized materials characteristics and applications.
Alginate is an anionic polysaccharide that consists of linear copolymers of β-(1–4) linked D-mannuronic acid and β-(1–4)-linked L-guluronic acid units obtained from brown seaweed [81].Alginate’s properties such as the superior biocompatibility, low toxicity, low cost, and convenient gelation ensure its applicability to bioprinting [96]. In general, the bioprinting process of alginate involves the addition of divalent cations(Ca2+,etc.)[97].However,the common alginate hydrogel lost these mechanical properties rapidly during in vitro culture (approximately 40% within nine days). Furthermore, they have inadequate cell adhesive sites [61].If the carboxyl of an alginate monomer reacts with 2-aminoethyl methacrylate (AEMA), the methacrylated alginate could become photocrosslinkable and improve its mechanical properties [80].Norbornene functionalized alginate enables printability at a lower concentration (2 wt%) and maintains a more stable 3D construct than pure ionic crosslinking printing [81].
3.3.2. Synthetic materials
Although synthetic materials have inadequate bioactivity compared with nature-derived materials, their chemical and mechanical properties are reproducible, consistent, and tunable owing to the control over the chemical and biological functional group presentation [15].
Polyethylene glycol (PEG), also called polyethylene oxide or polyoxyethylene,is a linear synthetic polyether of ethylene glycol,which is hydrophilic.The terminal functional groups of PEG and its highly controllable molecular weight enable the modification of the terminal functional groups [98] and its synthesis into fourarms [99] or eight-arms [100], thereby increasing the diversity of materials.The major advantages of the application of PEG to tissue engineering include the adjustable structure and mechanical properties, biocompatibility, hydrophilicity, low cytotoxicity, and nonimmunogenicity [101]. Because PEG is nondegradable and has inadequate adhesion sites for cells, it is generally compounded with other materials or peptides to develop bioinks. Bal et al.[102] used several types of peptides to mortify PEG hydrogel(which is initiated by EY)to observe how the combination of MSCs and ligand in a hydrogel affects the insulin secretion of the pancreatic islets.
Polyvinyl alcohol(PVA)is a hydrophilic linear synthetic ethanol homopolymer. The large number of side hydroxyl groups provide the attachment sites for biomolecules and opportunity for modification. The hydrogels prepared from PVA and its derivatives are widely used because of these adjustable chemical properties [5].Pure PVA hydrogel cannot afford long-term cell growth: The MSC cell viability decreases from 87% (Day 1) to 71% (Day 14). When it is combined with GelMA, the cell viability could be 92% on Day 14 [27].
3.4. Visible light-induced 3D bioprinting applications
3.4.1. Tissue engineering
3D bioprinting is primarily used for tissue engineering and regenerative medicine.The ultimate aim is to form artificial tissue substitutes and further, to construct artificial organs. However, at present,it is not possible to form a fully functional artificial tissue substitute to be used in vivo. Therefore, to achieve this ambitious goal, the leading research studies focus on fabricating models in vitro to mimic the in vivo conditions.
To achieve the fabrication of models in vitro,researchers require a high printing resolution to simulate the complex structures of tissues in vitro. Wang et al. [26] developed a visible light-induced SLA-based bioprinting system to prepare PEGDA and GelMA hydrogels with EY.The resolution of the vertical 3D structure was 50 μm(Fig.8(a)),and the cell viability of NIH 3T3 fibroblast cells is 85%for at least five days[26].The work of Bertlein et al.[56]demonstrated that the visible light + [Ru(II)(bpy)3]2+/SPS system has higher fidelity(Figs.8(b)and(c))and longer-term(three weeks)cell viability than the UV + I2959 system. Lim et al. [27] also developed a cellladen methacrylated PVA(PVAMA)/GelMA bioink for DLP bioprinting. It enabled the bioprinting of complex structures with high resolutions(25–50 μm)(Fig.8(d)).It also enabled the encapsulated cells to survive up to 90% in 14 d [27].
Apart from high resolution, it is important to also consider the state of cell proliferation, adhesion, and differentiation in printed construct.Wang et al.[103]also developed an EY/GelMA hydrogel system that forms a 3D cellular network inside the printed pattern on Day 5(which reveals the potential benefits of the research on cell growth morphology),as shown in Fig.9(a).Sakai et al.[33]revealed that Nanog, Oct-4, and Sox-2 genes were upregulated significantly(two-to three-fold from that on Day 1) after human adipose stem cells (hADSCs) were enclosed in tyrosinized HA/gelatin printing structure for 25 d. This indicated that the hADSCs maintained pluripotency[32].Lim et al.[27]demonstrated that PVAMA/GelMA hydrogel supported the osteogenic and chondrogenic differentiation of MSCs. Ouyang et al. [38] reported a norbornene-modified HA hydrogel for coating MSCs. Furthermore, histological analyses validated the production of both glycosaminoghycan(GAG)and collagen(COL)by encapsulated MSCs after 56 d of chondrogenic culture[104]. Petta et al. [48] recently introduced a double crosslinkable hyaluronan bioink crosslinked through enzymes and visible light.It exhibited flexible shear-thinning properties under low substitution during extrusion-based bioprinting[48].Moreover,it preserved the main structure and properties,thereby enabling human marrow stromal cells(hMSCs),chondrocytes,and human telomerase reverse transcriptase(hTERT)fibroblasts to be cultured and recover their 3D shape[20].
The fabrication of multilayer constructs consisting of different cells and material compositions is a key requirement for mimicking the skin structure. DLP bioprinting can form hydrogelcontaining cells similar to the skin, layer by layer. Kwak et al.[105] developed SF/PEG composite hydrogel as an artificial skin model by visible light-induced DLP. Although it retained a high cell-survival rate in the early stages, a dense keratin layer formed on the hydrogel surface within six weeks, as shown in Fig. 9(b) [105].
The heart is one of the most important organs of humans. The complex structure and interaction of multiple cells determine its function. Kumar et al. [72] used furfuryl-gelatin and RF to print multilayered sheets containing C2C12 myoblasts and STO fibroblasts, to study the interaction between cardiac myocytes and fibroblasts in vivo.During the culture and incubation,the different layers combined together owing to their interaction at the junction, rather than falling apart (Fig. 9(c)) [72]. Kumar et al.[106] also developed a fibrin–gelatin bioink for coculturing and coupling of cardiomyocytes and cardiac fibroblasts. In addition,the immunochemistry data demonstrated the heterocellular coupling between two types of cells via connexin43 adhesion junctions, which is critical for cell interactions [106].

Fig.8. (a)Hydrogel patterns fabricated with visible light-induced SLA.(b)Biofabrication of GelMA/Col hydrogel constructs consisting of completely interconnecting 3D pore network. (c) Percentage (%) variation in thickness of GelMA/Col hydrogels with different initiators. Ru: [Ru(II)(bpy)3]2+. (d) Gyroid construct, showing its complex porous pattern.*:P<0.05. CAD:computer aided design.(a)Reproduced from Ref.[26]with permission of IOP Science,?2015;(b,c)reproduced from Ref.[39] with permission of American Chemical Society, 2016; (d) reproduced from Ref. [27] with permission of IOP Science, ? 2019.
A fully functional artificial organ cannot live without a vascularized network. Bioprinting is an effective method for reconstruct vascular tubes. Sakai et al. [33] used [Ru(II)(bpy)3]2+/SPS induced Tyr-modified alginate bioink to print constructs that contain a smooth helical lumen with a diameter of 1 mm. It supplies a solution to construct a complex 3D cell-culture structure containing a vascular network in vitro, as shown in Fig. 9(d) [33].
3.4.2. Drug delivery
3D bioprinting, particularly inkjet-based printing, has been used in drug delivery for therapeutic applications. 3D bioprinting offers a viable alternative to traditional tablet manufacturing techniques:Personalized dosage forms customized to the genomic and pathophysiological profile are fabricated. Moreover, it is convenient to design the tablet’s shape by bioprinting, so that the drug release can be controlled. Pharmaceutical tablets were made from UV-crosslinked PEGDA and N-vinylpyrrolidone (NVP) using 3D inkjet printing to control the release of carvedilol, a drug used to treat hypertension and heart failure[107].However, UV can affect the stability of active pharmaceutical ingredients. Visible lightinduced inkjet bioprinting is a more effective here. Acosta-Vélez et al. [29] used inkjet bioprinting to develop a visible light crosslinkable norbornene-modified HA tablet containing hydrophilic ropinirole, to treat Parkinson’s disease and restless legs.Furthermore, ropinirole released 60% within 15 min under acidic condition, which is suitable for oral medicines [29]. Acosta-Vélez et al. [25] developed a PEGDA tablet containing naproxen cured by EY, which controls the release based on the percent of PEGDA in the formulation and the light exposure time for curing the bioinks [25].
4. Conclusion, challenge, and outlook
The expansive field of photopolymerized hydrogel has been researched extensively. In this review, we presented the present status of visible light-curing 3D bioprinting methods and photopolymerized hydrogel initiated by visible light. We summarized the types of initiators and their activation mechanisms.Direct and indirect light-induced strategies ranging from radical polymerization to thiol–ene ‘‘click” reaction were investigated. We also reviewed several common biomedical applications of visible light crosslinkable hydrogels in tissue engineering in recent years.Nonetheless, visible light-induced 3D bioprinting systems and the corresponding hydrogels have many more potential areas of application.
During the past several years, considerable progress has been achieved in 3D bioprinting. Given its development potential and application diversity, light-cured 3D bioprinting has been widely researched and is rapidly expanding. The evolution trend of light-cured 3D bioprinting is remarkable. A few common challenges in bioprinting must be addressed. These are with regard to ①printing device, particularly the printing resolution, printing fidelity, and microstructure reproduction; ②cell viability, which involves cellular nutrition and oxygen supply;and ③bioink property,including the physical strength and biocompatibility.Furthermore, in the area of visible light-induced 3D bioprinting, we must also address the challenge of the photopolymerization speed and print structure fidelity. These can be developed by improving the property of the photopolymerized hydrogels.
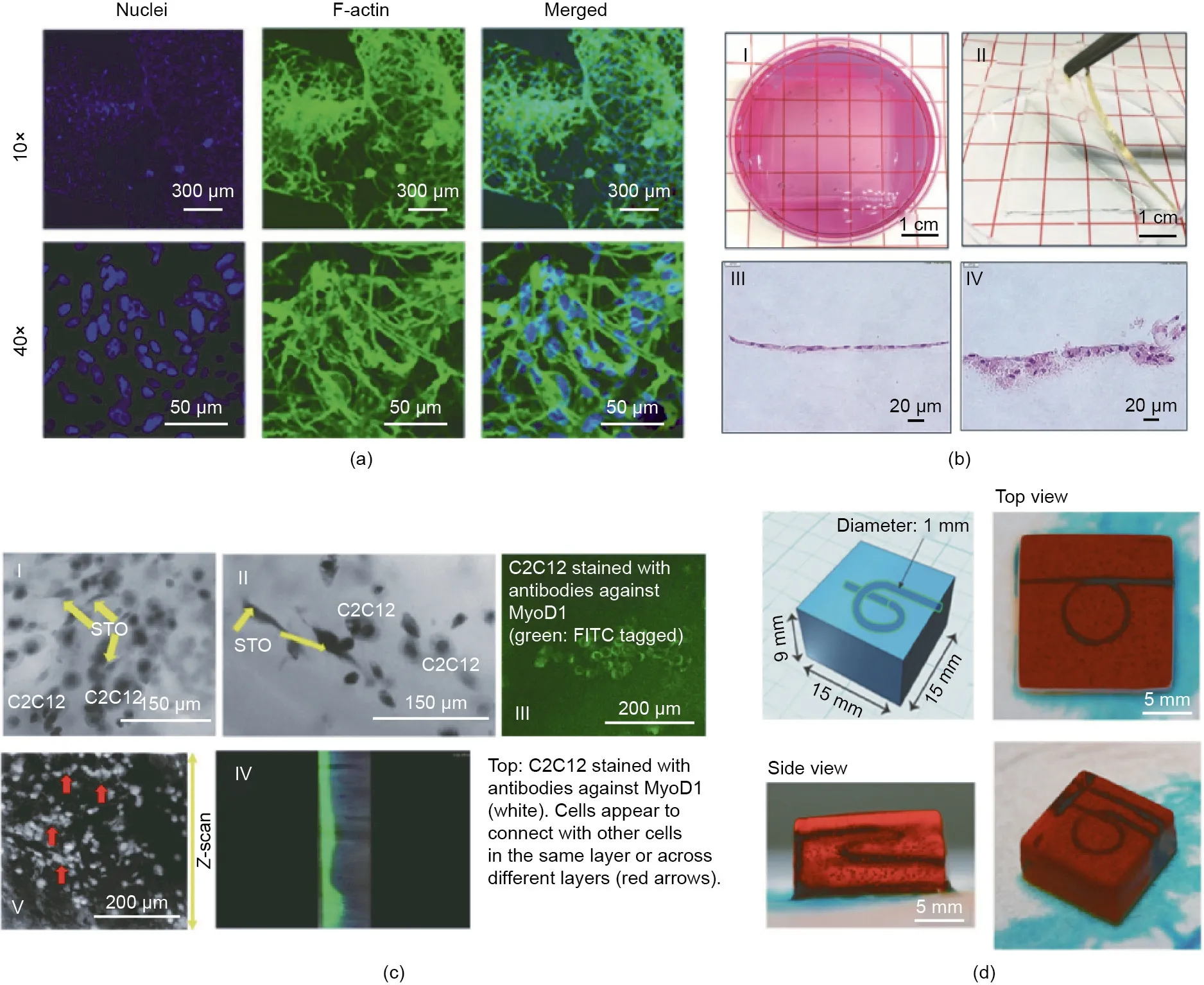
Fig.9. (a)Confocal fluorescence microscopy images of a junction in 3D-bioprinted cellular networks.(b)Formation of keratin layer on 3D-printed SF/PEG hydrogel containing fibroblast cells.(c)STO fibroblasts cocultured with C2C12 myoblasts cells.MyoD1:myogenic regulatory protein.(d)Blueprint and printed rectangular prism-shaped hydrogel containing a perfusable helical lumen(1 mm in diameter)structure.FITC:fluorescein 5-isothiocyanate.(a)Reproduced from Ref.[103]with permission of American Chemical Society,?2018;(b)reproduced from Ref.[105]with permission of Elsevier,?2019;(c)reproduced from Ref.[94]with permission of Wiley,?2019;(d)reproduced from Ref.[33] with permission of American Chemical Society, ? 2018.
Visible light crosslinkable materials exhibit better properties and higher application potential than the photopolymerized hydrogel induced by UV. Although visible light exhibits a lower cytotoxicity than UV light, it has limitations. The activation of the common visible-light initiators generally requires the presence of co-initiators and co-monomers. For example, EY activation requires triethanolamine as a co-initiator and NVP as a comonomer.Because these are required at relatively high concentrations and because of the co-initiator’s cytotoxicity, their application is limited. Research should be performed to overcome this disadvantage by improving the gelation efficiency without increasing the cytotoxicity.One of the methods to solve this problem is to improve the light intensity or mortify the chains with more multifunctional groups. After these challenges are overcome successfully, visible light-induced 3D bioprinting can be integrated effectively into tissue engineering.
Considerable progress has been achieved in 3D bioprinting and tissue engineering in terms of methods and materials.Visible light crosslinkable hydrogels can be photopolymerized as rapidly as UV crosslinkable hydrogels to achieve the appropriate mechanical strength and the desired construct in a spatiotemporal manner.Thereby, these have emerged as versatile biomaterial platforms for 3D bioprinting and tissue engineering. Recent advancements have imparted numerous advantages to visible light crosslinkable hydrogels, such as high cytocompatibility with different types of cells, tunable structure for strength, and cheaper crosslinking device. Moreover, there are many potential application areas for visible light-induced bioprinting, such as disease models and drug screening. The dynamic behavior of cell communication in 3D space can be observed more conveniently in vitro by 3D bioprinting than by culturing in a Petri dish.Hydrogel mimics the composition of ECM to accurately simulate dynamic variations in vivo as well as the function of natural tissues [78]. In addition, it is more convenient to design and adjust the microstructure of a photopolymerized hydrogel in a spatial layout. Overall, visible light-induced bioprinting has high value for future regenerative and biomedical engineering.
Acknowledgements
This work was supported by the Key-Area Research and Development Program of Guangdong Province (2019B010941001), the Shenzhen Double Chain Project for Innovation and Development Industry supported by the Bureau of Industry and Information Technology of Shenzhen (201908141541), Shenzhen Fundamental Research Foundation (GJHZ20170314154845576 and GJHS201703 14161106706).
Compliance with ethics guidelines
Zizhuo Zheng, David Eglin, Mauro Alini, Geoff R Richards, Ling Qin, and Yuxiao Lai declare that they have no conflict of interest or financial conflicts to disclose.
- Engineering的其它文章
- Temporal Profiles of Antibody Responses, Cytokines, and Survival of COVID-19 Patients: A Retrospective Cohort
- The East–West Divide in Response to COVID-19
- COVID-19 Vaccine Allocation: Modeling Health Outcomes and Equity Implications of Alternative Strategies
- Facilities for Centralized Isolation and Quarantine for the Observation and Treatment of Patients with COVID-19
- Next Steps for Efficacy Evaluation in Clinical Trials of COVID-19 Vaccines
- Non-Communicable Diseases During the COVID-19 Pandemic and Beyond

